Note: Descriptions are shown in the official language in which they were submitted.
l~g~4~
Title: CHEMICAL HEATING PAD WITH DIFFERING AIR-ADMITTING
PERFORATION SETS FOR DIFFERENT HEAT--GENERATION
LEVELS
: Field of the Invention
~ hls in~ention is~related generally to heatlng pads
and, more particularly, to self-con~ained chemical heating :
pads for applying heat to the human body.
: ~ .
:
- :~
.
' ': '
,.
~z9g~
1 Background of the I~Yen5iQn
Chemical heating pads of the type activated by
exposure of a chemical mixture to atmospheric air reaching
the mixture through openings in an envelope which contains
the mixture are well known and have been known for many
years.
Numerous patents have been granted for improvements
in such chemical heating pads over the course of many
decades, including the follo~ing United States patents:
Patent Nos. 1,434,576 ~Wertheimer); 1,609,958 (Perrault);
1,620,581 (Smith); 3,301,250 (Glasser); 3,976,049
(Yamashita et al.); 4,106,478 (Higashijima); 4,282,005
(Sato et al.); 4,366,804 (Abe); 4,516,564 (Koiso et al.);
and 4,573,447 (Thrash et al.).
A typical example is Patent No. 3,976,0~9, which
discloses a warming pad having an exothermic composition
including iron powder, a chloride or sulfate salt/ carbon
powder, and water in a two-layered bag made of an
air-permeable cloth layer inside a ~ilm layer with aeration
holes. The entire pad is contained in an impermeable
envelope which is opened to allow removal of the pad. This
allows atmospheric air to pass through the aeration holes
and permeable layer of the bag, which exposes the
composition to air (specifically its oxygen) to begin the
exothermic reaction. This device is typical of such
heating pads.
While there have been many developments in the field
of chemical heating pads, there remain significant problems
and shortcomings with heating pads of this general type.
For example, such pads are often too hot or not hot enough
for the intended purpose.
It is known that the amount of heat produced, the
rate of heat generation, and achievable temperatures are
de-pendent on, ~n~Q~_alL~, the chemical composition of the
mixture inside the pad, the size and number of holes in the
pad exposed to the atmosphere, and the thickness of the
material of the pad. These factors often have been
lZ9904(;~
considered in the prior art in seeking to provide chemical
heating pads meeting particular requirements.
Qne object of some developments of the prior art has
been to achieve constant and predictable heat conditions on
the major surfaces of an air-dependent chemical heating
pad. In certain other cases the object has been to have
heat emitted from only one side of such a heating pad, with
the second side shielded or insulated to prevent heat
emission. These objects contrast sharply with a principal
object of this invention, that is, to achieve diEferent
heat-generation levels and different useful heat conditions
when opposite sides of a pad are applied to the body or
other surface to be heated.
When chemical heating pads are used on human skin for
various purposes, they are frequently either too hot or not
hot enough for the skin of the users. That sensitivities
to topically-applied heat vary greatly in this way is
established. It is also known, in the field of therapeutic
heat treatment, that heat sensitivity varies not only from
person to person but according to the location on the body
of the person being treated. For any person, some areas of
skin are better able to receive applications of heat.
The inability of prior air-dependent chemical heating
pads to adequately satisfy varying sensitivities of users
is well known, and has limited the use of such heating
pads. Pads with only a single level of heat generation and
heat transfer or only one heat-transfer surface are simply
not suitable for therapeutic use unless special
accommodations are made. There is a long-standing and
clear need for an improved self-contained heating pad.
Objects of the_ In~n~i~n
It is an object of this invention to provide an
improved chemical heating pad overcoming some of the
problems and shortcomings of heating pads of the prior artO
~nother object of this invention is to provide an
improved self-contained chemical heating pad which may be
~Z~19040
1 used by people with widely varying sensitivities to heat
applied to the skin.
Another object of this invention is to provide an
improved chemical heating pad of the air-dependent type
having two heat-generation levels.
Another object of this invention is to provide an
improved chemical heating pad with significantly different
but useful heat conditions on its opposite surfaces as each
is applied against the body of a user~
These and other important objects will be apparent
from the descriptions which follow.
Summary of the I~ntiQn
This invention is an improved chemical heating pad of
the air-dependent type, which overcomes certain problems
20 and shortcomings of prior art devic~s, including those
mentioned above. The heating pad of this invention is of
the type having a particulate ~hemical mixture which is
exothermically reactive in the presence of air and first
25 and second opposed panels forming an envelope which
contains the mixture while admi~ting air.
In the heating pad of this invention, the first panel
has a first body-contact surface ~its outer surface) and a
30 first set of air-admitting perforations which extend from
the first body-contact surface to inside the envelope. The
second panel has a second body-contact surface (its outer
surface) and a second set of air-admitting perforations
35 which extend from the second body-contact surface to inside
the envelope. The second set of perforations differs from
the first set of perforations, such that two different heat
conditions develop and may be applied to the human body by
40 the two body-contact surfaces.
With the first body-contact surface applied to the
skin, the second set of perforations, which begin on the
second body-contact surface, are available to admit air to
the contained reactive mixture in a particular way, and as
a result a particular heat generation occurs within the pad
~2g904~
1 and is applied to the skin through the first body-contact
surface.
Likewise, with the second body-contact surface
applied to the skin, the first set of perforations, which
begin on the first body-contact surface, are available to
admit air to the contained reactive mixture in a different
way, with the result that a different level of heat
generation occurs within the pad and is applied to the skin
through the second body-contact surface~
In each case, the set of perforations of the panel
opposite the panel which is in contact with the skin play
15 an important role in determining the level of the
temperatures applied and the amount of heat transfer
occurring.
In highly preferred embodiments, each of the first
20 and second panels, except for the perforations, is
substantially air- and moisture-impermeable. The
perforations are made in air- and moisture-impermeable
material. The panels, at least one and preferably both of
25 them, are laminates, including at least one layer of the
air- and moisture-impermeable material, as already
- described. Such laminate preferably also includes a layer
of air- and moisture-permeable material9 Such air- and
30 moisture-permeable material is preferably an outer layer to
form one of the body-contact surfaces, giving lt an
excellent tactile quality.
In preferred embodiments, the chemical mixture is
35 preferably a moist particulate mixture of carbon powder,
iron powder, vermiculite, and a salt-water solution which
is selected and included in amounts appropriate to provide
an exothermic reaction in air.
4~ Some preferred heating pads in accordance with this
invention have their chemical mixtures, their first and
second panels, and their differing perforation sets
selected to provide temperatures, on the surfaces to be
applied to the body, within a range o~ about 57.5 - 65
degrees C. at the first body-contact surface and within
.
.
129~
1 a range of about 50 - 57.5 degrees C. at the second
body-contact surface. This invention is readily capable of
providing controlled heat to meet these preferred
temperature range requirements.
The differing sets of air-admitting perforations
allow the differing heat-generation and heat-transfer
characteristics which are at the heart of this invention.
Such differin~ perforation sets can differ in various ways,
in size, number, shape, arrangement, length~ and/or other
ways, in each case allowing a predetermined extent and/or
character of air admission to achieve the desired different
15 heat characteristics.
In one preferred structure of this invention, the
perforations of the first set are of greater size than the
perforations of the second set. This allows greater air
20 flow through the first panel than through the second panel,
which contributes to the different heat conditions.
In another preferred structure, the first set of
perforations has a greater number of perforations than the
25 second set of perforations. This allows better access of
air to the chemical mixture, thereby contributing to the
different heat conditions and doing so independently of the
relative extent of air flow by means of the second set of
30 perforations.
In certain preferred embodiments of this in~ention~
the first and second panels may dif~er in thickness. This
can contribute to the different heat conditions.
The objects of this invention are achieved by the
heating pad as described aboveO The heating pad of this
invention provides a predetermined and controlled higher
level of intensity of heat at one body-contact surface of
the heating pad and a predetermined and controlled lower
level o~ heat at the ot~er body-contact surface~
The heating pad of this invention, prior to use, is
kept in an air- and moisture-impermeable envelope. When it
is time for use, the pad is removed from such air- and
moisture-impermeable envelope. To initiate the exothermic
1.;~99~
reaction quickly, the pad is shaken or massaged once or
twice. Durin~ its use, the amount of both heat generation
and heat transfer will depend on which body-contact surface
is applied to the skin. In large measure, the amount of
both heat generation and heat transfer will ~epend on which
body contact surface is no~ applied to the skin, so that it
is exposed to the atmosphere.
A person to whose body the pad will be applied,
whether a hospital patient, athlete, or other individual
requiriny heat, can select the side of the heating pad for
application to the skin or body member to be treated
15 according to the individual comfort level. The choice may
be made by a nurse or other medical personnel treating the
person, with or without consultation, depending on the
therapeutic requirements.
For a practical therapeutic pad in accordance with
this invention, it has been found that the body-contact
surface with a greater heat transfer heat should be at a
temperature of from about 57.5 - 65 degrees C. for contact
25 with the body, while the other body-contact surface with a
lower heat transfer should be maintained at a temperature
of from about 5~ - 57.5 degrees ~. for contact with the
body. This allows a skin temperature of from about 4Q - 45
30 degrees C. to be attained regardless of which side of the
heating pad is used. The choice of which side to a~pply to
the skin of a particular patient will depend on whether his
or her tolerance level is good because of good circulation
and/or limited fatty tissue, or pGor because of poor
circulation and/or excess fatty tissue.
It will be understood that heat levels can be
controlled within relatively narrow ranges by proper
40 selection of the chemical composition to be placed in the
envelope, by the number and size of the perforations in the
first and second panels, and by proper selection of
materials. In selecting materials and perforation
45 characteristics, use can be made of instruments such as a
Gurley air flow tester, and/or empirical results may be
~29g~
1 collected by making pads and adjusting pad characteristics.
The devices of the present invention, therefore, are
well adapted ~or therapeutic heat application to patients.
The different levels of heat at the opposed body-contact
surfaces can accommodate either the individual sensitivity
of a person to heat, or the sensitivity of a particular
part of his or her body.
Description of_$h~ Drawings~
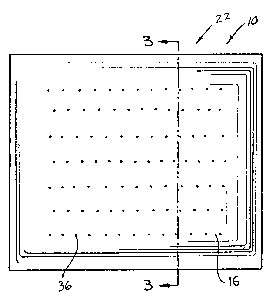
FIGURE 1 is a front elevation of a heating pad in
accordance with this invention.
FIGURE 2 is a rear elevation of FIGURE 1.
FIGURE 3 is a sectional view of the heating pad of
FIGURE 1 taken along section 3-3, as indicated in FIGURE 1,
in a gas- and moisture-impermeable container.
FIGURE 4 is an enlarged fragmentary view of FIGU~E 3.
FIGURE 5 is a front elevation of another heating pad
in accordance with this invention.
FIGURE 6 is a rear elevation of FIGURE 5.
FIGURE 7 is a sectional view of the heating pad of
FIGURE 5 taken along section 7-7, as indicated in FIGURE 5,
again in a gas- and moisture-impermeable container.
FIGURE 8 is a partially-magnified sectional view of
another heating pad, a highly preferred embodiment of this
invention, again in a a gas- and moisture-impermeable
container.
nptailed Descrip~;onS of Preferred Embodimen~
Three embodiments of the invention are shown in the
drawings, including a heating pad 10 in FIGURES 1-4, a
heating pad 20 in FIGURES 5-7, and a heating pad 30 in
FIGURE 8. Throughout the drawings, like numbers are used
to identify similar elementsO
Heating pad 10 shown in FIGURES 1-4, as shown in
FIGURES 3 and 4, includes a chemical mixture 40 contained
within an envelope. The envelope has first and second
opposed panels 22 and 24 forming the containment space for
1 chemical mixture 40. Opposed panels 22 and 24 each have
two separate layers, inner layers formed by bag 12 and
outer layers 14 and 16.
The inner layer in each case is part of a bag 12 made
of an air- and moisture-permeable material such as a
non-woven or woven cloth. One preferred material is a
polyester non-woven material. The material of bag 12,
while in itself capable of passing air at a high rate~
10 contains particulate chemical mixture 40 very well.
Bag 12 is between outer layers 14 and 16. Outer
layers 14 and 16 are formed of sheets of a material which,
apart ~rom the perforations extending through them, are
gas- and moisture-impermeable. Outer layers 14 and 16 each
provide a body-contact surface. When one body-contact
surface is against the body, the other is exposed to the
atmosphere such that air can pass therethrough.
~ he entire heating pad 10 is stored until time of use
in a gas and moisture-impermeable container 50, which is
illustrated in FIGURE 3.
As illustrated best in FIGURES 1, 2 and 4, outer
layers 14 and 16 contain different sets of air-admitting
perforations. While outer layers have about the same
number of perforations, outer layer 14 has per~orations 34
30 which are larger than the perforations 36 which are in
outer layer 16. Thus, the admission of air to the chemical
mixture 40 is ~aster through panel 22 than it is through
panel 24. Thus, when outer layer l& is against the human
body, the exothermic reaction is faster and hotter, thereby
producing a higher level or intensity of heat than when
heating pad 10 is used by applying outer layer 14 to the
body.
Heating pad 20, shown in FIGURES 5-7, is generally
similar to heating pad 10. However, heating pad 20 has
outer layers 44 and 46 which di~fer ~rom outer layers 14
and 16 of heating pad 10. FIGURES 5 and 6 illustrate that
45 the sets of perorations in outer layers 44 and 46 differ.
While each perforation 64 in outer layer 44 is similar in
~z~9o~
l size to perforations 66 in outer layer 46, there are twice
as many in outer sheet 46 as in outer sheet 44. Thus, when
outer layer 44 is applied to the body, air reaches more
portions of chemical mixture 40 more easily. ~ven if
perforations 64 were enough larger than perforations 44 to
allow the same rate o air passage through both sides of
the pad, the better distribution of air to the reactive
10 mixture would provide more intense heat generation.
Outer layer 46 is substantially thicker than outer
layer 44. This contributes to the different heat
conditions available through selective contact with the
15 opposite panels of heating pad 10. Such difference in
panel thickness has the effect as well of changing the
characteristics of the air-admitting perforations extending
therethrough. In one preferred embodiment, the thickness
20 of outer layer 44 is about 0.007 inch while the thic~ness
of outer layer 46 is 0.013 inch.
Heating pad 30, shown in FIGURE 8, is a highly
preferred embodiment of this invention having first and
25 second panels 72 and 74 which are laminates; that is, each
panel 72 and 74 has two inner and outer layers which are
adhered together all across their common surfaces. Each
laminate is a composite of a non-woven polyester cloth
30 outer layer 87 and a polyethylene film inner layer 88.
As shown in the magnified portion of FIGURE 8, in
heating pad 30 perforations 89 extend straight throu~h both
layers 87 and 88, unlike the air-admitting perforations (or
35 passages) of heating pads 10 and 20 which include the
perforations in the outer layers and the interstices of the
inner bag. Perforations 89 in panel 72 are of one size
and/or pattern, while the perforations (not shown) in panel
4~ 72 are or another size and or pattern. Such differing sets
of perforations provide the desired different heat
intensities and heat transfer characteristics.
As with heating pads 10 and 20, heating pad 30 is
45 stored until time of use in a gas and moisture-impermeable
container 50.
'lZ99()4~
11
~ he chemical mixture in the heating pads shown in the
drawings include an intermediate having 30% by weight of
vermiculite, 55% by weight of a 10% sodium chloride
solution in water, and 15% by weight o carban having of
fine particle size, suoh intermediate combined on a 50/50
weight ratio with iron powder of fine particle size.
The embodiments of FIGURES 1-7 may be made by
10 carrying out the following steps in sequence:
1. A 10% sodium chloride solution is prepared by
mixing sodium chloride in tap water or deionized water
until it is dissolved.
2. The sodium chloride solution is added to a vessel
containing the vermiculite with gentle stirring action to
achieve uniformity without crushing the vermiculite.
3. The carbon is added to the vermiculite/sodium
20 chloride solution mixture and blended to uniformity, thus
completing preparation of the intermediate.
4. Bag 12 is prepared by heat-sealing an air- and
moisture-permeable non-woven cloth laminate on three edges
25 to form a pouch.
5. The intermediate is loaded~into the pouch.
6. The iron po~der is then loaded into the pouch
which alxeady contains the intermediateO
7. The fourth edge of the pouch is then heat sealed
and the sealed pouch is then shaken to obtain uniformity of
the chemical mixture.
8. Air- and moisture-impermeable materials are
35 selected or formed, having~the desired thicknesses and
having perforations (formed by punching, laser cutting, or
mechanical cutting) as desired. The previously-sealed
pouch is then sandwiched between layers of such differing
40 air- and moisture-impermeable materials, which are then
heat~sealed along their com n edges.
9. The heating pad is then placed into an air- and
moisture-impermeable bag which is heat-sealed along its
common edges, sealing it such that the chemical mixture
will not react until desired.
.
3040
12
1In the embodiment of FIGURE 8, the chemical mixture
is added directly to an envelope prepared ~rom the
laminates, having layers and perforations as described
above, and the envelope then sealed. The heating pad is
then packaged as described in step 9 above.
The heating pads in accordance with ~his invention
may be made using a variety o other production methods.
~cceptable method would be apparent to those skilled in the
art who are familiar with this invention.
The chemical mixture may be varied in a number of
ways.
15Iron powder is preferred because it reacts readily
with atmospheric oxygen in the presence o~ moisture to
generate heat. And, because it is dense it is a good
thermal conductor. The fineness o the powder can be
varied to change the rate of the reaction. Other reactive
metal powders, such as magnesium, zinc, and aluminum, can
be utilized.
The carbon powder of the reaction mixture is use~ul
because of its large surface area to weight ratio. The
carbon contains a network of holes and channels, enabling
the carbon to absorb atmospheric oxygen in large amounts to
supply the oxygen for the oxidation reaction. The
30 oxygen-absorbing capacity is greatly increased when the
carbon is slightly wet, as it is when mixed with the sodium
chloride solution. The carbon powder, however, can be
replaced by other particulate materials such as talc.
Sodium chloride is utilized in order to catalyze the
oxidation of iron. It is particularly desirable in that it
is readily available, inexpensive, and the toxicity is
low. The sodium chloride, however, can be replaced by
40 other suitable chlorides~and sulfates, such as ferric
sulfate, potassium sulfate, sodium sulfate, and magnesium
sulfate, potassium chloride, calcium chloride, and
magnesium chloride.
45Additionally, the ratios of the components of ~he
chemical mixture can be varied substantially in order to
~Z99~0
l make either a hotter or cooler reaction mixture. It is
understood that, as a general rule, the greater the amount
of metal powder the hotter the reaction. All of these
characteristics are known to those skilled in the art.
Formula modifications will generally be within the
followin~ parameters~ sodium chloride in an amount of about
0.5 - 30 parts by weight per 100 parts of iron powder;
carbon in an amount of about 2.5 - 400 parts by weight per
100 parts o~ iron powder; and water in an amount of~about
10 - 250 parts by weight per 100 parts of iron. The amount
of vermiculate can be varied greatly. Variation of any or
all ingredients in quantity, particle size or grade will
affect the rate of reaction and, thus, the temperatures
achieved and the duration of the reaction.
The air- and moisture-permeable bag 12, instead of
20 the preferred polyester material mentioned above, can be
made of other synthetic fiber cloths or of natural
materials such as cotton. The air and moisture-impermeable
layer can be polyethylene, as noted abovel or can be a wide
25 variety of other materials, such as polypropylene or nylon
film. Polyethylene is preferred for its heat-sealing ease.
Laminates may be in many different forms. The
laminate as described above represents a highly preferred
30 improvement. It provides an excellent tactile quality to
the body-contact surfaces. And, we have discovered that
the laminates as described contains the chemical mixture
very well even though the perforations extend entirely
35 through them-
Other acceptable materials for laminates include asuitable non-woven or woven material with a film layer,
such as polyethylene, polypropylene, polyvinylidene
40 chloride, or the like, or a metal foil or a metalized cloth
which is impermeable except for its discrete per~orations.
Acceptable laminates will be well-known to one skilled in
the art who are familiar with this invention.
Perforations can be made in many different ways,
including cutting and punching. It has been found that the
lZ9~0~
14
l holes can ~e made of superior uniform size by ~he use of
laser beams to burn or form the holes. In some cases it
can be preferable to arrange the holes in a thin area or
strip of the air- and moisture-impermeahle layer as opposed
to having the holes distributed throughout the layer. By
having the holes arranged in a strip, it is possible to
create a greenhouse effect which can provide for a
conservation of the moisture within the chemical mixture,
increasing the life of the chemical mixture. Acce~ptable
modifications are within the ability o~ those skilled in
the art who are familiar with this invention.
While the principles of this invention have been
described in connection with specific embodiments, it
should be understood clearly that these descriptions are
made only by way of example and are not intended to limit
20 the scope of the invention.
:
~`
~; :