Note: Descriptions are shown in the official language in which they were submitted.
~3003~i
9024
PendergraEt
"SULFUR RECOVERY PROCESS USING METAL OXIDE
ABSORBENT_WITH_IMPROVED PURGE"
FIELD OF THE INVENTION
The invention relates to the removal of sulfur
and sulfur compounds from gaseous streams containing such
15 compounds. In one aspect, the invention relates to the
removal of sulfur compounds including H2S (hydrogen sul-
fide) and SO2 (sulfur dioxide) from Claus plant tail gas.
In another aspect, the invention relates to the use of
solid high surface area contact materials (absorbents),
20 for example, ZnO-based (zinc oxide-based) absorbents, for
absorbing sulfur compounds such as SO2 and H2S. In a fur-
ther aspect, the invention relates to preventing increased
S2 emissions from appearing in plant emissions when
treating freshly regenerated ZnO absorbent with a reducing
25 gas stream under conditions for reducing the time
required.
SETTING OF THE INVENTION
A developing area of sulfur recovery technology
is that of tail gas cleanup, that is, of removing trace
30 quantities of sulfur compounds from gaseous effluent
streams (tail gas) of Claus process sulfur recovery
plants. Tailgas may contain substantial amounts of sulfur
compounds. Tailgas from Claus or extended Claus plants
(having at least one Claus low temperature adsorption
35 reactor) typically can contain about 0.5-10% of the sulfur
present in feed to the plant as elemental sulfur, H2S,
SO2, COS (carbonyl sulfide), CS2 (carbon disulfide), and
the like. Tailgas cleanup processes remove at least part
of such residual sulfur compounds from Claus tail gas.
~300:~A5
--2--
In prior U. S. Patent ~,533,529, Claus tail ga~
is contacted with ZnO (zinc o.Yide) in an absorber reducing
average overall emission levels from the absorber to less
than 250 ppm sulfur species. It is desirable, however,
5 and necessitated by certain environmental requirements,
that not only average but instantaneous emissions be con-
tinuously maintained at a very low level.
It has been discovered, after ZnS (zinc sulfide)
is regenerated to ZnO, that an increase in SO2 emissions
10 occurs from the absorber upon returning regenerated ZnO to
absorption where it is contacted with reducing gases.
These SO2 emissions interfere with continuously main-
taining instantaneous emissions at a very low level.
Accordingly, there is provided a process capable
15 of diminishing such an increase in SO2 emissions and main-
taining effluent from the absorber at a continuous low
level of emissions.
SU~IP RY OF THE. NV~N r ION
The invention comprises a process for continu-
20 ously removing sulfur compounds, for example, H2S and SO2,from a Claus plant gaseous effluent stream to an extremely
low level. In this process, the sulfur compounds are
removed in the presence of an absorbent based on ZnO as
active absorbent (herein referred to as ZnO or ZnO-based
25 absorbent) to produce a laden, sulfided absorbent (ZnS)
and a purified gaseous stream (absorber effluent) continu-
ously having on the order of 250 ppm or less total resi-
dual H2S and SO2.
; There is provided a new and advantageous combi-
30 nation of steps for preventing higher emissions of sulfur
dioxide from freshly regenerated ZnO absorbent, the ZnO
absorbent having been regenerated in the presence of mole-
cular oxygen and sulfur dioxide, from appearing in effl-
uent from the plant. The invention comprises a new and
35 advantageous combination of-steps for use in a method for
absorbing at least H2S from a stream by sulfidization of
ZnO producing ZnS, the ZnS then being regenerated to ZnO,
producing sulfur dioxide (SO2) in the presence of 2 and
, ~
.,: .
1300:~AS
retu!ned to absorbincJ ~2S from the stream. The new and
advanta~eo~s combination of steps comprises (1) following
regeiner.ltion of ZnS to ZnO, passing the strearn containing
at least H2S in contact with thus regerlerated ZnO, pro-
S ducing effluent comprising SO2 and (2) pa,~ing the e~fl-
u~nt comprising SO2 in contact with ZnO It~ r conditions,
including temperature and compositior~, for rernovirlg SO2 in
the presence of the ZnO.
In accordance with another aspect: o~ th~ inv~n-
10 tion, the invention comprises a new alld advantayeous com-
bination of steps for use in a method for removing at
least sulfur dioxide SO2 from a stream ~omprising SO2 and
reducing species effective for converting SO2 to H2S in
the presence of an effective ZnO absorbent producing ZnS.
15 The ZnS is then regenerated to ZnO, producing SO2, in the
presence of 2~ and then returned to absorbing at least
S2 from the stream. The new and advantageous combination
of steps comprises: (l) following regeneration of ZnS to
ZnO, passing a stream comprising reducing species in con-
20 tact with thus regenerated ZnO, producing effluent com-
prising SO2 therefrom, and (2) passing the effluent com-
prising SO2 in contact with ZnO under conditions including
temperature and gas composition for removing SO2 there-
from.
In accordance with further aspects of the inven-
tion, steps (1) and (2) are continued Eor a period of time
for reducing SO2 levels in the effluent from the plant to
less than about 250 ppm, and more preferably, to less than
about 50 ppm.
In a further aspect, a process for the recovery
of sulfur from a H2S containing gaseous stream comprises
converting H2S to elemental sulfur by the Claus reaction
in a Claus plant comprising a Claus thermal reaction zone
(furnace) and at least one Claus catalytic reaction zone
35 and producing a tail gas comprising significant amounts of
both H2S and SO2. As used herein, significant amounts of
H2S and SO2 means that each is present in excess of
250 ppm. The tail gas can then be treated to remove each
,~ ' ' ' . '
1300:~S
of H2S and SO2 by ren:'ion with ZnO in a ~irst absorptio
zone containing ZnO (functioning as an absorber) in the
presence of reducing species for converting substantially
ail sulfur species in the ta;l gas to H2S, ?ro~ucing ZnS
and absorber effluent. The res~lting ZnS can be regerler--
ated, that is, returned to the active ZnO form of the
absorbent, by introducing 2 (molecular ox~gen) into a
second absorQtion zone (~unctioning as a reg~nerat:or) and
reger.erating the zinc sulfide to ZnO, producing regenera--
10 tion effluent. Regeneration effluent comprising SO2 isreturned to the Claus plant. Followin~ regeneration of
absorbent (converting ZnS to ZnO) in the second absorption
zone, the tail gas is introduced into the second absorp-
tion zone, producing ZnS and absorber effluent which,
15 during an initial emissions period, contains an elevated
level of SO2. Effluent from the second absorption zone is
introduced into the first absorption zone operating under
absorption conditions during the emissions period and the
increased level of SO2 is removed by the ZnO in ~he first
20 absorption zone in the presence of reducing species.
Thus, purging in accordance with the invention is accom-
plished by beginning absorption in a freshly regenerated
absorber zone and passing effluent from the freshly regen-
erated absorber zone to another absorption zone where the
25 elevated levels of SO2 are removed to an acceptable level.
After the emissions period, the first absorption zone can
be removed from absorption function and regenerated by
introducing 2 thereinto and regenerating the ZnS to ZnO,
producing regeneration effluent. The process is addition-
30 ally characterized by the fact that the purge periodduring which effluent from a freshly regenerated absorber
is provided to a downstream absorber on absorption, lasts
for a period of time effective for removing at least 10~
of an increase in SO2 emissions from the freshly regener-
35 ated absorber during an emissions period at the start ofabsorption following interchanging of the absorber and the
regenerator.
~3~0345
The invention accordin~ly co~prises the pro-
cesses and systems, together with their steps, parts, and
interrela~ionships which are exemplified in the present
disclosure, and tne scope of which will be indicated in
5 the appended cla;ms.
BRIEF D~SC~IPTION OF _HE DRAWINGS
FIGURE 1 shows schematically apparatus for prac-
ticing the invented process.
FIGURG` 2 shows graphically an increase ir- SO~
10 emissions occurring where ~reshly regenerated absorbent is
not purged before absorption with an effective reducing
gas.
FIGURE 3 shows graphically that such an increase
in SO2 emissions as shown in FIGURE 2 can be eliminated by
15 purging before absorption with an effective reducing gas.
DETAILED DESCRIPTION OF THE INVENTION
In accordance with the invention, SO2 emissions
are prevented from appearing in effluent from a sulfur
recovery plant by steps following regeneration of ZnS to
20 ZnO. The steps comprise operating an absorber containing
freshly regenerated ZnO under conditions, including tem-
perature and gas composition, for causing SO2 emissions to
occur from the freshly regenerated absorbent. The effl-
uent from such zone containing the SO2 is then provided to
25 a second ZnO absorber zone operating under conditions
effective for removing such SO2 in the presence of ZnO and
effective reducing species. Thus, the SO2 emissions are
prevented from appearing in emissions from the plant by
providing them during an emissions period to another ZnO
30 absorber downstream thereof. Following the emissions
period, the ZnS in the downstream absorber zone can be
regenerated by introducing dilute molecular oxygen ther-
einto, producing effluent comprising SO2, which is
returned to a Claus plant from which the tail gas is der-
35 ived and in which the SO2 is converted to sulfur and
- removed from the gas-in-process.
Sulfur is recovered from an H2S-containing
stream by introducing the stream into a Claus plant com-
:
.~
: ::
~30034!;
prising a thermal r~ tion zone (Claus fur,l.~ce~ and atleast one Claus ca~alytlc reaction zone. The C~aus
thermal reaction zone can be, for example, a Claus muF~le
furnace, a fire tube (tunnel) furnace, or the like. Gen-
5 erally, the Claus thermal reaction zone functions for con--
verting a portion of H2S, preferably about 1/3, to SO~ for
thermal or catalytic Claus reaction with H2S to form ele-
mental sulfur.
In the Claus furnace, the H2S-containing gas and
10 oxidant can be reacted at a temperature generally in the
range of about 1800-2600F. The effluent from the Claus
thermal reaction zcne can be cooled, for example, in a
waste heat boiler, and optionally passed through a sulfur
condenser to condense and remove liquid sulfur.
The gaseous effluent can then be fed into a
Claus catalytic reaction zone operated above the sulfur
dewpoint having an inlet temperature in the range, for
example, o~ about 350-650F. In the Claus high tempera-
ture catalytic reactor, sulfur is formed by the Claus
20 reaction (shown below) in the presence of an effective
Claus reaction-promoting catalyst such as alumina or
bauxite:
2 H2S + SO2 -~ 3/2 S + 2 H2O
t 25
Gas containing elemental sulfur vapor can be continuously
removed from the reactor and provided to a sulfur con-
denser where sulfur is condensed and removed as a liquid.
Gaseous effluent from the sulfur condenser can be
30 reheated, if desired, and passed to further high tempera-
ture Claus reactors and associated sulfur condensers as is
known in the art. The effluent gas from the final sulfur
condenser is then the Claus plant tail gas. Where a Claus
low-temperature adsorption zone is used, it may or may not
35 be followed by a sulfur condenser. Thus, adsorber effl-
uent may be the Claus plant tail gas.
Preferably, the Claus plant tail gas is from a
Claus plant which includes at least one Claus catalytic
1300:~45
--7--
reactor operated untlf~lr collditions, includ;ng temperature,
effective for depositing a pceponderance Ol- t~e formed
sulfur on Claus catalyst therein. Such a Claus low tem-
perature adsorption zone can be broadly operated in the
5 range of from about 160 to about 330~1, preferably in the
range of from about 260-300 F.
The operation of such Claus plar,Ls having Claus
furnaces, Claus high temperature reactors, and Claus low
temperature adsorption reactors is well kno~n in th~ art
10 and need not be further described here. See, for example,
U.S. Patents 4,044,114; 4,426,369; 4,430,317; 4,473,541;
4,482,532; 4,483,844; 4,507,275; 4,508,69~, and numerous
others.
The tail gas from such Claus plants comprises
15 H2S, SO2, organic sulfides, and reducing species such as
H2 and CO. Tailgas from plants having only Claus high
temperature reactors can contain H2S in the range of about
0.4 to about 4 mol%, SO2 in the range of about 0.2 to
about 2 mol%, water in the range of about 20 to about
20 50 mol% (typically 30-40 mol%), as well as organic sul-
fides such as COS and CS2, and elemental sulfur. Where
the tail gas is from a plant having one or more Claus low
temperature adsorption reactors, the tail gas may have
equivalent of about 0.4 mol%, preferably about 0.2 mol%,
25 or less single sul~ur species.
Use of at least one Claus low-temperature
adsorption reactor is preferable in part because such
reactors remove significant amounts of organic sulfides,
such as COS, CS2, and the like from the gas in process.
30 These organic sulfides are not removed by sulfur recovery
processes such as the IFP process described in DeZael, et
al., U. S. Patent 4,044,114 (1977) which forms elemental
sulfur in the presence of polyethylene glycol and sodium
benzoate. See, e.g., Kohl and Riesenfeld, Gas Purifica-
35 tion, pages 491-493 (3d Ed. 1979).
For the same reason, it is also preferred to
operate at least one Claus high temperature reactor so
that the effluent has a temperature in the range from
' ~`
, : :
~30034~;
about 550 to 700F, preferably fr(~)m anout 600 to 650E to
diminish the amount o~ organic sulfides in the effl~lent.
See, e.g., Kunkel, et al., U.S. Patent 4,035,474 (1977).
Both H2S and SO2, as well as organic sulfides,
5 can be concurrently removed in the absorber containing ZnO
in the presence of reducing species for reducing the SO2
and other sulfur species to H2S. It is preferre~ to
operate the Claus plant so that about a 2:1 ratio of
H2S:SO2 is maintained in the Claus plant tail gas to max-
10 imize sulfur recovery in the Claus plant and to millimizethe amount of sulfur remaining in the Claus plant tail gas
to be remove~ by the ZnO absorbers. Such ratio can be
maintained by control systems well known in the art and
need not be further described here. By reducing the
15 organic sulfide and other sulfur content in the feed to
the ZnO absorbers, the volume of regeneration effluent
returned to the Claus plant can be reduced or diminished.
An effect of operating at about a 2:1 ratio, however, is
that quantities of both H2S and SO2 are present in the
20 Claus plant tail gas, i.e., more than about 250 ppm of
each of H2S and SO2.
The reducing species, for example, H2 and/or CO,
required for conversion of sulfur compounds in the tail
gas to H2S can be obtained from any convenient source
25 including that present in the tail gas as H2, or available
from a donor such as CO, which can react with water to
yield H2. H2 is preferred, whether contained in the tail
gas or internally generated or provided from an outside
source.
The Claus plant tail gas can contain sufficient
reducing species where the Claus plant is appropriately
operated. For most Claus plants, by operating the Claus
furnace so that slightly less air is utilized than that
~; required for producing Claus plant tail gas having a 2:1
; 35 H2S:SO2 ratio and by insuring that the tail gas leaving
the final sulfur condenser of the Claus plant has a low
level of residual elemental sulfur, the Claus plant tail
gas will contain sufficient reducing species. By further
~';
.,
' `
` '
~3~034S
_9_
reducirlg the amour-t of o~idant introd~lced into the Claus
t furnace or by other methods which will be apparent to per-
sons skilled i!l the art, the amount of reducing species
can be further increased if desired.
S The Claus plant tail gas having su~ficient
reducing species to reduce all sulfur compollnds therein to
H2S can be heated, for example, directly by mf~ans o~
direct fired heaters, or indirectly by heat exchange, for
example, with other process streams such as absorber effl-
10 uent, to produce a heated Claus plant tail gas efluent
stream having a temperature effective for removal of each
of H2S and SO2 in the presence of a solid part;culate
preferably high surface area (for example, pellets, extru-
dates, and the like) ZnO absorbent effective for such
lS removal. This removal of each of H2S and SO2 is consid-
ered to proceed by hydrogenation of sulfur compounds
present in the tail gas to H2S in the presence of ZnO, ZnO
in this respect acting as a catalyst, followed by absorp-
tion of the thus-formed H2S by the ZnO by sulfiding the
20 ZnO to ZnS, the ZnO acting as an absorbent. Preferably,
the Claus plant tail gas is heated to above about 1000QF.
As illustrated in EXAMP~E I below, by operating at these
absorber temperatures, a hydrogenation reactor is not
required before removal of sulfur compounds other than H2S
25 in the absorber. When operating at temperatures above
about 1000F, H2S emissions and the reduction of ZnO to Zn
vapor under a reducing environment can set a practical
upper limit on the absorption temperature which will be
used. Currently for these reasons it may be appropriate
30 that the upper limit during absorption be about 1200F.
Higher temperatures can also be used. Absorber operation
above about 1000F is preferred because such higher tem-
peratures favor higher absorption capacity of the absorber
and the hydrogenation reactor can be eliminated. Also,
35 since absorption and regeneration will then be conducted
at approximately the same inlet temperature (1000-1200~F),
temperature stress on equipment can be reduced. As a
result, there will be no significant heating anù cooling
: .
' ' .
1300345
--10--
periods. Hence, the time available for regeneration will
be increased and the rate of regeneration effluent
returned to the Claus plant can be decreased.
While a first absorption zone is functioning as
5 an absorber, a second absorption zone can be functioning
as a regenerator.
As u~ed herein, and in the claims, the terms
"absorbent", "ZnO", "ZnO absorbent", and the like ~hall
mean an absorbent effective for removal of both H S and
10 SO in the presence of reducing species. A major portion
of the active absorbent, for example, fifty percent or
more, is in the form of ZnO which is the active form. The
absorbent can also contain binders, strengtheners, and
support materials, for example, alumina (Al o ), calcium
15 oxide (CaO) and the like. Zinc sulfide and zinc sulfate
can be used as starting materials and treated with heat
and/or oxygen to produce an active ZnO sorbent. Other
~uitable starting material~ can al~o be used. The ZnO
absorbent i~ effective for absorbing H2S by underqoing
20 ~ulfidization to produce a laden (sulfided) absorbent;
~imultaneously, if desired, hydrogenation of other sulfur
compounds to H2S followed by such absorption can occur.
Preferably, the ZnO absorbent is capable of a high level
o removal of sulfur compounds and is relatively insensi-
25 tive to water vapor.
Particularly preferred are ZnO absorbents whichare thermally stable, regenerable, and capable of
absorbing substantial amounts of sulfur compounds. An
acceptable absorbent is United Catalysts, Inc., G72D*
30 Sulfur Removal Catalyst, available from United Catalysts,
Inc., Louisville, KY, having the following chemical compo-
sition and physical properties:
CHEMICAL COMPOSITION
wt~ Trace Metal Impurities wt~
ZnO........... 90.0 +5% Pb..................... <0.15
Carbon........ ~O. 20 Sn..................... ~0. 005
~ Sulfur........ <0.15 As..................... ~0.005
`~ ~ Chlorides..... <0.02 Hg..................... ~0.005
* G72D is a trademark.
~:~00345
A12O3......... 3-7 Fe.............. ~...... <0.1
CaO........... 0.5--3.0 Cd...................... <0.005
PH~S r CAL PRO~'.E~'LI~S
Form Pellets
Size 3/16 in.
Bulk Density 65 +5 lbs~ft3
Surface Area 35 m2/g mini~T~lu
Pore Voiume 0.25-0.35 cc,/g
10 Crush Strength 15 lbs minimum avera~e
Representative chemical reactions considered to
occur during absorption, regeneration and purging are
shown below:
During Absorption:
H2S + ZnO ~ ZnS + H2O (l)
20 COS + ZnO -~ ZnS + CO2 (2)
CS2 + 2ZnO -~ 2ZnS + CO2 (3)
S2 + 3H2 ~~ H2S + 2H2O (4)
H2S + Sulfated Absorbent -~ SO2
+ ZnO Absorbent (5)
30 During absorption, H2S, COS and CS2 in the stream can
react with ZnO to form ZnS as shown in Eqs. (l) to (3).
S2 can react directly with H2 to form H2S as shown by
Eq. (4), and the resulting H2S can then react with ZnO.
COS and CS2 may also be hydrogenated and/or hydrolyzed to
35 H2S before absorption by ZnO. When elements in the absor-
bent such as zinc, calcium, aluminum, or other elements
become sulfated during regeneration, SO2 may be produced
during absorption as indicated by Eq. (5) due to the pres-
1300345
-12-
ence of effective reducing species in the absorber feed.
Sulfation is reversed by purging the regenerated absorbent
with effective reducing species before returning regener-
ated ZnO to absorption and providing the produced SO2 to a
5 downstream ZnO absorption zone.
DU ring Regeneration:
ZnS + 3/2 2 ~ ZnO + SO2 (6)
Absorbent + SO2 + 2 ' Sulfated Absorbent (7)
Regeneration of sulfided absorbent is effected by oxi-
dizing ZnS to ZnO as shown by Eq. (6). Absorbent sulfa-
15 tion can also occur, as shown by Eq. (7) during regenera-
tion in the presence of 2 and SO2. Temperature rise
during regeneration can suffice if unchecked to destroy
both the physical integrity and the chemical activity of
the absorbent as well as to exceed metallurgical limits of
20 preferred materials of construction. Consequently, tem-
perature rise during regeneration is preferably controlled
to less than about 1500F.
During Purging:
Sulfated Absorbent + H2 ~ Absorbent + SO2 + H20 (8)
Sulfated Absorbent + CO ~ Absorbent + SO2 + CO2 (9)
Sulfated Absorbent + H2S ~ Absorbent + SO2 + H2O (10)
Purging in accordance with the invention is
accomplished by introducing Claus plant tail gas feed to
an absorption zone into the freshly regenerated reactor
35 while another ZnO absorber is downstream operating under
absorption conditions for removing SO2 produced. Such
Claus plant tail gas contains H2, CO, and H2S and there-
- fore contains the effective reducing species for causing
S2 emissions.
1~0034S
-13-
Reduction of the sulfated absGrbent will occur
at temperatures above about 1000F in the pr-sence of H2,
CO or other reducing species such as H2S. Re~uction of
the sulfated absorbent is not effected at lower tempera- -
5 tures such as 900P or lower or in the absence Oe such
effective reducing species.
Methane is not effective in reasonable periods
of time under process conditions for causing the p~oduc-
tion of SO2 ~rom freshly regenerated ZnO. Further, an
10 inert gas will not cause such SO2 emissions to occur.
Upon switching to absorption, however, the freshly regen-
erated, sulfated ZnO absorbent will be contacted with a
stream containing the effective reducing species (~2' CO,
and H2S) and SO2 emissions will occur. Accordingly, for
15 causing SO2 emissions, during the purging, it is essential
that effective reducing species be present and that the
temperature be greater than about 1000F, but preferably
not much greater than about 1200F since significant
losses of zinc can occur above that temperature in the
20 presence of reducing species. Nevertheless, higher tem-
peratures can be used.
The absorber zone containing ZnO can comprise at
least a first absorption zone (functioning as an absorber)
and a second absorption zone (functioning as regenerator)
25 and the process can comprise contacting H2S with absorbent
in the absorber to remove it and other sulfur species pro-
ducing a laden absorbent and absorber effluent lean in
sulfur species. Absorption can be continued for a period
of time (absorption period), preferably less than that
30 required for H2S breakthrough from the absorber. For
practical purposes, H2S breakthrough can be defined as
occurring when the H2S concentration in the absorber effl-
uent stream reaches a preset low value, such as for
example, 50 ppm H2S. As shown in EXAMPLE I and II below,
; 35 breakthrough time and absorption capacity for an absorber
increase with increasing absorber temperature. Concur-
rently with absorption in the absorber, laden absorbent in
the regenerator can be regenerated by introducing a regen-
'
: `~
:
~:
~: :
,~
1300345
-14-
eration stream ce~rising dilute 2 thereinto at a
temperature effective for converting laden sulfided absor-
bent to active absorbent. Regeneration effluent corn-
prising SO2 is returned from the regenerator to the Claus
5 plant, for example, to the thermal reaction zone or to a
downstream Claus catalytic reaction zone. Thereafter, the
tail gas can be introduced into the second absorption zone
functioning as absorber and the first absorption zone can
for a period, receive effluent from the second absorption
10 zone. During this purge period which is characterized by
increased emissions of SO2 from the freshly regenerated
ahsorbent, contacting of freshly regenerated ZnO with the
tail gas which comprises H2S, H2, and Co, causes the pro-
duction of SO2 which can be removed by the downstream
15 first absorption zone operating as an absorber. There~
after, 2 can be introduced into the first absorption zone
for regeneration, and the process can be continued and
repeated for the regenerated ZnO in the first absorption
zone.
During regeneration, a temperature rise of about
145F occurs for each mol percent of oxygen consumed in
converting ZnS back to ZnO. To avoid exceeding metallur-
gical limits of nonrefractory lined vessels and to main-
tain absorbent physical and chemical integrity during
25 regeneration, a maximum of about 3.5 mol% oxygen can be
used during regeneration when the regeneration stream is
introduced at about 1000F, and a maximum of about
2.75 mol% 2 when the regeneration stream is introduced at
about 1100F. Thus, preferably oxygen is introduced
30 during regeneration at a concentration of about 0.4 or
less to about 3.5 mol%, more preferably at about 1 to
about 2.75 mol%. Due to the exothermic nature of the
regeneration reaction, suitable methods for diluting the
oxygen can be used. Where metallurgical limits are not
35 controlling, the maximum temperature during regeneration
can be as high as about 2100F.
Suitable methods for diluting the oxygen during
regeneration include the following: (1) a portion of the
.
: ' ' '
~ :`
~ ~' ' ' .
130034S
regenerator effluent can be recycled back to the
regenerator to dilute 2 in the regeneration stream; (2) a
portion of absorber ef~luent can be used to dilute 2 in
the regeneration stream.
When method (1) is employed, the SO2 level
during regeneration in the regenerator is higher than when
method (2) is used since SO2 produced during regeneration
is recycled to the regenerator. Reference to EXAMPLE VI
indicates that higher SO2 levels during regeneration
10 favors sulfation of the absorbent. It has also ~een found
when method (1) is used that SO2 emissions are larger upon
returning to absorption, and/or that a lo~ger purge time
can be required to eliminate an increase in SO2 e~lssions
following return to absorption. Accordingly, method (2)
15 is preferred for diluting the 2 to a suitable concentra-
tion for regeneration. However, in accordance with the
invention, absorber feed is used for producing SO2 from
freshly regenerated ZnO absorbent in one absorber while
another ZnO absorber is downstream operating under absorp-
20 tion conditions for removing the produced SO2. Hencemethod (1) can also be used since the higher level of
reducing compounds present in the absorber feed will
shorten the purge period in accordance with the invention.
Further, by using absorber feed for purging, purging and
25 the start of absorption for an absorber occur concur-
rently.
The flow rate during regeneration is preferably
a rate sufficient to complete regeneration and purging as
described herein of a ZnO absorber during effective
~- 30 absorption in another ZnO absorber. In this way, only two
absorption zones will be required. Some time can also be
allowed for the contingency of process upsets (slack
time). Preferably, the flow rate during regeneration is
such that the period during which regeneration is occur-
35 ring is equal to the period during which absorption is
occurring less the period required for purging as herein
set forth and such slack time.
~'
., ~, .
.'`.~`.~ ' .
.,, ~ ~ .
1300~45
-16-
As indicated, regeneration effluent comprising
S2 is returned from the regenerator to the Claus plant
for cc,nversion oi the SO2 to elemental sulfur which is
removed fro~! the gas in process. Dilution of the 2 using
5 absorber effluent minimiæes purge time and/or magnitude of
the SO2 emissions at the start of absorption but can
result in a large volu~e of regeneration ef~luent being
returned to the Claus plant. This has the undesired
result of increasing the size of the Claus plant and
10 equipment downstream of the locus wher~ the regeneration
effluent is reintroduced resulting in signiEicant cost
increases.
The rate of regenerator effluent returned to the
Claus plant can, as indicated, be reduced by recycling
15 regenerator effluent as diluent back to the regenerator.
Absorber effluent can be used for diluent and
the rate of regeneration effluent returned to the Claus
plant can be minimized (1) where the Claus plant comprises
at least one Claus low-temperature adsorption reactor,
20 (2) where 2 introduced during the regeneration period
when ZnS is being converted to ZnO is in an amount about
equal to the stoichiometric amount for such conversion,
that is, about 3/2 moles 2 for each mole of ZnS to be
regenerated, and (3) where the rate of absorber effluent
25 diluent introduced during the regeneration is such that
the rate of regeneration effluent during the regeneration
period is less than the rate of regeneration effluent
returned in the absence of treating in a Claus low-
temperature adsorption reactor prior to treatment in a
30 ZnO-containing absorber. As absorber effluent typically
comprises residual H2 and CO, the amount of 2 introduced
can further include about the stoichiometric amount
required for combusting H2 and CO to water and CO2. When
using such regeneration, further benefit can be realized
35 by purging in accordance with the invention since using
full stream purging can shorten purge time requirements,
permitting a longer regeneration period and further
reducing the rate of regeneration effluent recycle to the
Claus plant.
,~ .
:~ :
1300345
--17-
By us~ of a l~w-t~mp~rat~re Clalls acl~;orption
reactor, the absorption rate for a ZnO absorb~r i~
decreased since the sul~ur content of the fee~ to the
absorber is reduced, allowing 2 to be introduced to the
5 regenerator at a lower rate. By introducing 2 during the
regeneration period in a total amount effective for oxi-
dizing Zn~ to ZnO and, as appropriate, also ~or combusting
any residual H2 and CO to H2O and CO2, the total volume oE
2 is minimized. This permits the rate of absorber effl-
10 uent introduced as diluent into the regenerator duringregeneration to be such that the volume of regeneration
effluent returned to the Claus plant during regeneration
can oe reduced in comparison with the volume where a Claus
low-temperature adsorption reactor is not used. Further,
15 as indicated, purging in accordance with the invention can
further reduce the rate of return of regeneration effluent
to the Claus plant.
Regeneration can be preferably continued until
substantially all of the sulfided absorbent is regener-
20 ated, for example, until ZnS is substantially reconvertedto ZnO. Completion of regeneration can be conveniently
determined by monitoring 2 or SO2 content or temperature
of the regenerator effluent stream. Preferably, an 2
analyzer is employed downstream of the regenerator to det-
25 ermine the presence of 2 in the regenerator effluent,which is an indication of completion of regeneration.
As will be appreciated by those skilled in the
art from the foregoing discussion, materials of construc-
tion for the valves, vessels, and piping for the process
30 according to the invention can require special attention.
The material preferably has the capability of withstanding
high temperatures, for example, in the range of about
800F to about 1500F or higher while being repeatedly
exposed to reducing and oxidizing atmospheres in the pres-
35 ence of sulfur compounds.
Following regeneration, prior to returning theregenerated absorbent for use during the absorption cycle,
the regenerated absorbent is treated (purged) by passing a
:
1300345
reducing stream in contact with the regenerated, albeit
sulfated absorbent (see Examples VI-VII), while another
ZnO absorber is downstream receiving the produced SO2
under conditions for removing SO2 from the gaseous stream.
S The purging can be conducted for a period of time effec-
tive for reducing by at least 10% a temporary increase in
S2 emissions otherwise occurring from the plant when
freshly regenerated ZnO absorbent is returned to absorp-
tion without such purging with a reducing gas while
10 another ZnO absorber is downstream operating under effe~-
tive absorption conditions. Preferably, the time is
effective for reducing SO2 emissions to below about
250 ppm at all times. Most preferably, the time is effec-
tive for substantially eliminating the increase in SO2
15 emissions, that is, for reducing the increase in SO2 emis-
sions above the usual level during absorption by 90% or
more from the level occurring where such a reducing gas
purge is not used prior to returning to absorption.
The effective purge time can be readily deter-
20 mined by one skilled in the art by monitoring SO2 emis-
sions from a freshly regenerated absorber during at least
the increased SO2 emissions period at the start of absorp-
tion function, while another absorber is downstream oper-
; ating under absorption conditions for removing SO2 there-
25 from, until the SO2 emissions from the freshly regenerated
absorber are reduced to a desired level, for example, to
below about 250 ppm SO2, preferably to below about 50 ppm
SO2. Thereafter the downstream absorber can be removed
from absorption function and can be regenerated by intro-
30 ducing a dilute oxygen gas thereinto and the effluent fromthe now purged, freshly regenerated absorber can be disc-
harged to the atmosphere or, preferably, a portion of that
stream can be used as diluent for oxygen during regenera-
tion. Generally it is expected that the purge period in
35 accordance with the invention will be in the range of
about 0.3 to about 3 hours, preferably in the range of
;~ about 0.5 to about 1 hour.
,
'~'~,
~,
,;~; ~ .
` :~
~300345
--19--
Preferably, the purge stream can comprise at
least a portion of absorber feed. Most preferably, the
purge can be effected by using the entire absorber feed,
that is, the entire tail gas stream.
Thus, it will be appreciated that purging in
accordance with the invention uses a portion o~ th~
absorber feed and preferably the entire absorber fee~.
During the purging period, the ZnO absorbers would be
placed in series on absorption, with the freshly regener-
10 ated ZnO being in the absorber in the first position.
Purging and the beginning of absorption occur concur-
rently. The effluent from the first absorber is fed to
the other absorber to absorb the increase in SO2 emis-
sions. The effluent from the second absorber can then be
15 vented to the atmosphere, thereby eliminating the recycle
stream during operation in the purge mode or, alterna-
tively, a slip stream of the effluent from the downs~ream
absorption zone can be recycled to the Claus plant. The
purge time in accordance with the invention can be greatly
20 reduced where the entire absorber feed is used because of
the larger flow rate of reducing compounds flowing through
the freshly regenerated ZnO absorber. Reducing the purge
time can allow an increase in the regeneration time and
thereby allow a decrease in the recycle rate of e~fluent
25 during regeneration to the Claus plant, reducing the size
of the Claus and the ZnO absorber portions of the plant.
The invention will be further understood by the
EXAMPLES which are set forth below.
EXAMPLE I - ABSORPTION: EFFECT OF TEMPERATURE
The effect of temperature on H2S breakthrough is
studied using a laboratory catalyst holder/reactor made
from type 304 stainless steel tubing 2" (inch) diameter
(O.D.) x 0.068" thick wall, 27" long overall. Calculated
catalyst volume for 18" depth is 805 ml (milliliters), and
35 the catalyst is supported by a 20 mesh stainless steel
screen. Catalyst used is G72D*Sulfur Removal Catalyst
described above. The reactor is wrapped by six heaters
(22 gauge nichrome wire) for preventing radial heat loss,
*G72D is a trademark.
, .
~,
1300:~45
-20-
and is insulated with fi'oerglass. The total flow rate for
absorption is 10 l./min (liters/min) and for regeneration
5 l./min. The reactor is placed in a large Blue M~ oven,
available f~o~l Blue M Electric Company, Blue Isiand, IL.
5 All gas flow through the catalyst bed is downflow. Provi-
sions for side draw of gas samples are available near the
reactor a~is each 1.5" of catalyst depth.
The effect o~ reaction temperatures on H2S
breakthrough time during absorption is illustrated by
10 introducing a feed gas having the followiny composition
into the reactor inlet:
H2S 0.8 mol%
S2 0 4 mol%
CO 1.0 mol~
H2O 30.0 mol~
N2 45.8 mol~
H2 2.0 mol%
C2 20.0 mol%
The feed gas is introduced at 850F, at 1000F, and at
1150F. Breakthrough, defined for purposes of these runs
as 50 ppm H2S in the absorber effluent, and H2S concentra-
tion in the effluent gas at equilibrium, are determined.
25 Results are set forth in the following Table IA.
TABLE IA
Combined SO2 and
H2S Concentration Absorption Capacity
Time (Hrs) for (Dry Basis) mols absorbed/
Run Temp. Breakthrough at Equilibrium wt~ mols sorbent
1 850F (Immed. SO2 -- --- --
Breakthrough)
~ 2 1000F 25.5 hrs <10 ppm 33~ 0.84
; 3 1150F 27.5 hrs <20 ppm 36% 0.92
The results indicate that higher temperatures
favor increased absorption capacity as indicated by
13003~5
-21-
increased breakthrough times and that lower temperatures
favor lower equilibriurn concentrations of H2S in the
absorber effluellt streams. It is also noted t'nat at
1000F and at 1150F, SO2 present in the inlet stream is
5 substantially completely absorbed or converted to H2S and
absorbed while at 850F, SO2 appears immediately in the
absorber effluent stream. Thus at temperatures at least
abcut 1000F and higher hydrogenation of SO2 to H2S is not
required prior to absorption.
EXAMPLE II - ABSORPTION: EFFECT OF TEMPERATURE
_ _ . . _ ________ __~___ ___ _ _ _
The effect of temperature on H2S breakthrough is
further in~estigated by the following runs using the
apparatus described in EXAMPLE I and using an inlet stream
having the following composition:
H2S1.2 mol%
H2O29.5 mol%
H 1.06 mol%
CO1.01 mol%
C220.39 mol%
N246.83 mol%
This inlet stream can be used to simulate the condition
where SO2 present in a Claus plant eefluent stream is
25 hydrogenated to H2S prior to absorption. Breakthrough
time for various temperatures below 850F are determined
and are shown in Table IIA below:
TABLE IIA
Time (Hrs) for Absorption Capacity
Run Temp. Breakthrough w mols/mol sorbent
4 625F 3 4% 0.10
700F 11 14~ 0.36
6 775F 17 22% 0.46
These results further confirm the dependence of
absorption capacity and breakthrough on absorption temper-
ature.
'
~`
, ~
~, :
13~3~S
-22-
E~XA~?riF Ill - ABSORE'TION: ~FE'ECT OF WATER
The efEect of the presence of water vapor on
sulfur corlpG~ d breakthrough is illustrated in part by
EXA~'PrE I above in which a feed gas stream containiny
5 30.0% water is contacted with a ZnO absorbent and, at
1000E' to 1150F, the sulfur compounds in the effluellt
stream are reduced to 20 ppm or lower.
To further investigate the effect of water on
sulfur compound breakthrough using a metal oxide absor-
10 bent, the apparatus of EXAMPLE I can be used with a zincferrite absorbent containing about 45% iron oxide and
about 55% amorphous silica. About 15% of the 45% iron
oxide is in the form of zinc ferrite. A feed gas having
the following composition is introduced into the reactor
15 inlet at 1000F:
H2S 1.2%
CO 1%
H2 2%
CO21 20% (42%)
H2O1 22% (o~)
N2 53.8%
lCO2 content of inlet stream is increased from 20% to
42% when 22% H2O is eliminated from the feedstream.
After about 5-1/2 hrs, water is eliminated
from the feedstream. The results are shown in Table IIIA
below.
; 35
1300345
-23--
~A4rF IIIA
Time~l2S Concentration
(rlr~s)ln Reactor Ef~luellt
1 663
2.3 733
3.4 ~18
4.1 9~4
5.51 1682
5.7 9
7.1 9
8.6 9
Water eliminated from feeds~ream.
The results indicate that the iron oxide (zinc
lS ferrite) absorbent is sensitive to the presence o water
in the feedstream as compared with the ZnO oE EXA~PLE I.
After water is removed from the feedstream, H2S in the
effluent stream is reduced to 9 ppm. These results indi-
cate that ZnO is less sensitive to water than is iron
20 oxide (zinc ferrite).
EXAMPLE IV - REGENERATION
Regeneration is investigated using the apparatus
described in EXAMPLE I by passing a dilute air stream in
contact with the sul~ided absorbent. The effect of tem-
25 perature on regeneration is investigated. For a dilute
air regeneration stream containing about 5 molO oxygen
having an inlet temperature of about 1000F, the sulfur
recovered as SO2 in the regeneration effluent stream is
only 0.75 mol%. However, when the inlet temperature is
raised to 1150F after 5-1/2 hrs, about 3 mol-O o sulfur
as SO2 appears in the regeneration effluent stream. This
higher regeneration temperature is considered preferred to
overcome the high activation energy required or Reac-
tion (8) above. During regeneration, the concentration of
S2 in the regeneration effluent stream remains above
about 3.5 mol% and the concentration of 2 in the regener-
ation effluent stream remains about 0 mol~, indicating
substantially complete consumption of 2~ for about
;
1300345
-24-
22 hrs. After about 22 hrs, when regeneration is about
complete, 2 starts to breakthrough and SO2 content begins
to decline in the regeneration effluent stream.
EXAMP E V - EFFECT O_ PURGE
Effluent tail gas from a Claus sulfur recovery
plant having two catalytic reactors operated abo~e the
sulfur dewpoint and one Claus low temperature adsorption
reactor on-stream at all times is provided to an absorber
containing ZnO. A portion of absorber effluent is used as
10 a diluent for 2 to a regenerator containing ZnS~ In a
first run, upon completion of reg~neration, the regener-
ator and absorber are interchanged in function. Upon
interchanging the absorbers, an emissions level from the
freshly regenerated catalyst, now functioning as an
15 absorber, of about 350 ppm SO2 is observed. SO2 emissions
decline to less than about 50 ppm in about two (2) hours.
See FIGURE 2. In a second run, upon completion of regen-
eration and prior to interchanging the absorber and the
regenerator, 2 flow into the regenerator is discontinued
20 and the flow of absorber effluent is continued for a
period of about two (2) hours. Upon interchanging the
absorber and regenerator, SO2 emissions from the absorber
are initially less than about 50 ppm and continue at that
low level. See FIGURE 3. This example indicates that
25 discontinuing 2 flow and continuing absorber effluent, or
other reducing gas flow, prior to interchanging an
absorber and a regenerator eliminates a temporary increase
in SO2 emissions above a baseline level otherwise observed
from the absorber after interchanging the two reactors.
EXAMPLE VI - EFFECT OF REGENERATION GAS COMPOSITION ON
PURGE
The effect of SO2 levels during regeneration
upon purge time requirements at the end of regeneration is
; investigated by regenerating sulfided absorbent using
35 regeneration feedstreams having various SO2 levels fol-
lowed by purging with a reducing gas stream having 1.1% H2
and 0.5~ CO at a space velocity of about 1. The results
are set forth in the following table:
~,
.. . .
:,
1300345
Run SO2 in ~c3erler~ition Feed Purge Tim~ (Hours)
_ . . . .. ....... .. . ... . . . .. . . . .
1 0 % 2.0
2 2.9% ~.5
5 3 13.2% >12
The results indicate that the SOz level in the
re~en~ratioll feed greatly affects the purge time and that
10 increased levels of SO2 during regeneration increase the
purge time requirements. The results indicate that the
use of absorber effluent or other reducing gas having
little or no SO2 present at the inlet is advantageous in
reducing purge time.
_AMPLE VII -_EFFECT_OF_REGE ERATION TEM?ERATURE ON
PURGINGjS~BSEQ~ENT A~SORPTION
_ _ _ _ _ _ _ .
Purging runs are made after regeneration
at 900E and 1150F using absorber effluent as the purge
gas. The test results show that by purging at 900F, the
20 increase in SO2 emissions is not removed, whereas by
purging at 1150 F, increased SO2 emissions were not
observed upon returning to absorption. Based upon these
results, it is considered that purging should occur at
temperatures from about 1000 F to about 1200E consistent
25 with the temperatures required for hydrogenation of other
species in the presence of ZnO absorbent as set forth in
Example I above.
EXAMPLE VIII - EFFECT OF Hq ON SOq EMISSIONS
To investigate the effect of H2 on SO2 emis-
30 sions, laden ZnO (ZnS) is regenerated at 1150F with a
regeneration stream having the following inlet composi-
tion:
TABLE VIIIA
2 5 mol%
NH3 720 ppm
C2 85 mol%
H2O 10 mol%
1300;~4S
-26-
After SO2 emissions decrease~ to about 50 ppm,
1 mol% H2 was added. SO2 emissions immediatel~ increased
to about 450 pprn and then decreased with time. (Note:
the NH3 was present to simulate refinery gas in this run;
5 howeverr the presence of NH3 is not considered to affect
the results from the addition of H2 reported he~ein.)
These results indicate that reduciny eq.livalents
such as H2 result in SO2 emissions from a freshly regener-
ated absorbent. Thus, these results indicate tha~ the
10 effect of reducing gases during the purge period is to
cause the production of and allow the removal 3f SO2 from
regenerated sulfated absorbent in the purge effluent
stream prior to return to absorption. SO2 removed during
purge in regeneration effluent is sent to the Claus plant
15 where sulfur is formed and removed from the process. In
this way, SO2 emissions from regenerated absorbent will
not appear as emissions from the plant.
EXAMPLE IX - EFFECT OF HYDROGEN SULFIDE ON REDUCING SO
EMISSIONS
The effect of H2S on reducing SO2 emissions is
investigated by contacting freshly regenerated absorbent
with a stream containing H2S but no SO2. An SO2 emissions
peak of about 100 ppm is observed initially, diminishing
to about 20 ppm after six (6) hours. These results indi-
25 cate that H2S will be effective as a purge gas. It is
noted that H2S will also result in absorbent loading. See
Eq. (3)-
EXAMPLE X - EFFECT OF METHANE ON REDUCING SO EMISSIONS
The effect of methane on reducing SO2 emissions
30 is investigated by contacting absorbent, freshly regener-
ated with a stream comprising about 13% SO2, with methane
for six (6) hours. At the end of the six (6) hours, SO2
emissions are about 2000 ppm. Upon switching to absorp-
tion, with a stream comprising 0.39 mol% H2S, 0.16 mol%
35 SO2, 1.69 mol% H2, and 0.26 mol% CO, SO2 emissions of
about 8000 ppm are observed which decrease to about
1000 ppm in about 7 hours. Mass spectrographic analysis
of the effluent stream during purge with methane indicates
.
, ~
.,~ .
,
~300345
-27-
that methane is not cracked to H2 and CO at regeneration
temperatures of about 1100F. These results indicate that
methane alone is relatively ineffective for purginy to
reduce SO2 e.~issions under process conditions.
S E:.XA~PLE' XI - ANALYSIS OF SUL~IDED_ABSORB~NT
Samples of fresh absorbent and regenerated
absorbent, regeneration having been conducted at ]150F in
the presellce of oxygen and 13% SO2 are analyzed by X-ray
diffraction. The fresh absorberlt is largely crystailine
10 ZnO (zincite). The regenerated absorbent contains ZnO as
the major co~ponent, with minGr concentrations of zinc
oxide sulfate Zn3O (SO4)2, anhydrite CaSO4, and gahnite,
ZnA12O4. These results indicate that sulfated compounds
may be the cause of SO2 emissions when reduced by con-
15 tacting with a reducing gas stream.
The invention will be further described and fur-
ther advantages and applications and equivalents wlll be
apparent to those skilled in the art from the description
20 of FIGURES 1 and 2.
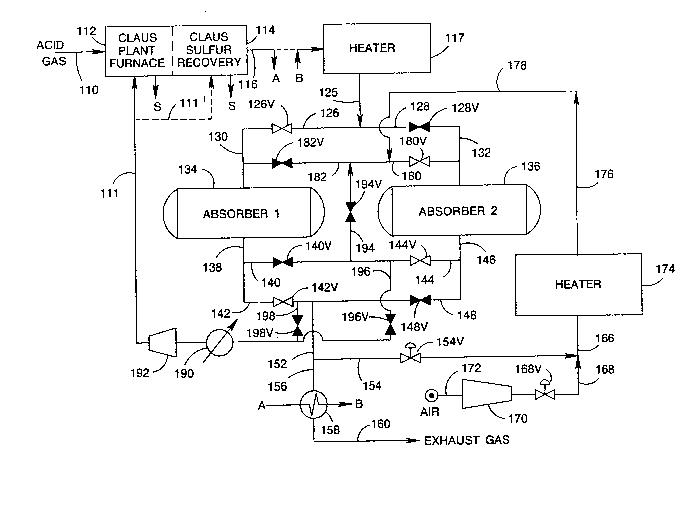
Referring now to the drawings and specifically
to FIGURE 1, FIGURE 1 represents an embodiment of the
invented process in which absorption of H2S by the metal
oxide absorbent can be carried out at a temperature above
25 about 1000F, preferably in the range of about 1000F to
about 1200F.
An acid ~as stream 110 containing H2S is intro-
duced into a Claus plant furnace 112 and combusted, in the
presence of oxygen containing gas, for example, atmos-
30 pheric air (source not shown), and/or SO2 (provided, forexample, via line 111), to produce elemental sulfur, SO2,
and water. The elemental sulfur is recovered and uncon-
verted H2S and SO2 are processed by Claus catalytic sulfur
recovery 114, including at least one Claus catalytic reac-
35 tion zone operated above the sulfur dewpoint and prefer-
ably at least one low-temperature Claus adsorption reac-
tion zone. Elemental sulfur is thus produced and removed,
for example, by sulfur condensers (shown schematically by
,~ ~
130034S
-28-
the arrow S). A Claus plant effluent stream is removed by
line 116 containing sufficient redllcing equivalents for
reduction o~ sul~ur containing compounds remaining therein
to H2S in the hydrogenation zone or in the absorber zone.
The Claus plant effluent stream in line 116 can
then be heated to an effective temperature as described
herein. Pre~erably at least a portion of the heating
requirements can be met by passing the Claus plant effl-
uent stream 116 in direct heat exchange with the absorber
10 effluent stream in line 156, for example, in recuper-
ator 158, as indicated schematically by the line marked A.
Following heating in recuperator 158, the heated Claus
plant effluent stream can be provided by the lines marked
B to heater 117 for further heating to above 1000F, pref-
15 erably in the range of about 1000-1200F. Alternatively,
of course, the Claus plant effluent stream 116 can be pro-
vided directly (as indicated by the dashed line) and can
be heated in heater 117 to a temperature in the range of
about 1000F to about 1200F and introduced by lines 125,
20 126, valve 126V, and line 130 into first absorber 134.
That other provision can be made for heating the Claus
plant effluent stream in accordance with the invention
will be clear to those skilled in this art.
First absorber 134 contains a ZnO absorbent
25 effective to absorb H2S present in the inlet stream to
produce a sulfided absorbent and to produce an absorber
effluent stream 138 containing, for example, less than
about 50 ppm H2S. Simultaneously with absorption in first
absorber 134, after heating to a temperature in the range
30 of 1000F to 1200 F, SO2 present in Claus effluent
stream 116 can be hydrogenated to H2S utilizing reducing
equivalents present in Claus effluent stream 116 and the
resulting H2S can also be absorbed by the absorbent.
The absorber effluent stream 138 can be con-
35 ducted by lines 142, valve 142V, lines 152, 156, heat
recuperator 158, and line 160 for discharge, for example,
to the atmosphere. The heat recuperator 158 provides at
least a portion of the heat required for heating the Claus
,.
~' .
.
~ ''
.~.
: .
130034~
-29-
plant e~]uent stream as described above, or for producing
high press~lre steam. A portion of the absorber e~fluent
stream can be withdrawn from line 152, by way of, for
exarlple, line 154, having valve 154V, for dilution of
5 atmospheric air 172, via compressor 170 and line 168,
having valve 168V, to produce a dilute air reyeneration
strearn 166. During regeneration, valves 154V and 168V
control the recycle rate to the Claus plan~ and the rate
of regeneration.
The regeneration stream 166 can be heated in
heater 174 to regeneration temperatures and can be con-
ducted by lines 176, 178, 180, valve 180V, and line 132 to
second absorber 136 shown on regeneration. The heated
regeneration stream 176 is thus passed in contact with
15 sulfided absorbent in second absorber 136 to produce a
regeneration effluent stream 146 having a reduced 2 con-
tent and an increased SO2 and/or sulfur content.
Stream 146 is conducted by line 144, valve 144V, heat
recuperator 190, compressor 192, and line 111 to the Claus
20 plant furnace 112. Alternatively, the regeneration effl-
uent stream can be introduced into a catalytic zone in the
Claus plant 114 as indicated by dotted line 111'; however,
operation should insure that no free or molecular oxygen
is introduced thereby into the catalytic zone.
Absorption is continued in first absorber 134
until prior to or just before H2S breakthrough occurs in
effluent stream 138 from first absorber 134. Preferably,
the oxygen content and regeneration stream flow rate is
established so that the regeneration time (plus purge and
30 slack time) is equal to absorption time prior to H2Sbreakthrough. H2S breakthrough can be determined by moni-
toring the H2S content of first absorber effluent
stream 138 until H2S content can exceed a predetermined
limit which can be, for example, that suitable to meet
35 emission requirements for discharge of stream 160.
Prior to placing the first absorber on regenera-
tion, purge of the second absorber zone can be effected by
discontinuing 2 flow to the second absorber, for example,
~' .
. ~
~3003~5
-30-
by closing valve 168V, and by stoppiny ~low of absorber
effluent for diluent by closing valve 154V. Then, by
opening valve 128V, the tail gas can be provided to the
second absorption zone. During this period, valves 194V
5 and 182V should be open, and valves 196V and 126V should
be closed. (I~ only partial flow is used ~or pur~e,
valve 126V can be partially closed to control the rate.)
Effluent from the second absorption zone cGntaining SO2
produced during the emissions period can then be provided
10 by line 144 having valve 144V, line 194 having valve 194V,
and line 182 having valve 182V to the first absorption
zone 134 in which the SO2 is removed in the presence of
the ZnO and effective reducing species. Effluent from the
first absorption zone 134 can then by line 138, 142,
15 having valve 142V be exhausted. A portion of the efluent
from the first absorption zone can be provided to the
Claus furnace by line 198, valve 198V, and line 111 to
maintain a steady rate of gas to the Claus plant. Alter-
natively, valve 196V in line 196 could be partially opened
20 to provide a constant rate of recycle to the Claus plant
from the effluent of absorber 136. This method, however,
has the disadvantage of providing a varying SO2 content to
the Claus plant.
Therea~ter, first absorber 134 can be placed on
25 regeneration and second absorber 136 can be placed on
absorption by closing valves 126V (if not already closed),
142V, 180V, 144V, 194V, 196V, and 198V in their respective
lines 126, 142, 180, 144, 194, 196, and 198, and by
opening valves 128V, 182V, 140V, and 148V in the respec-
30 tive lines 128, 182, 140, and 148. Valve 194V in line 194(which can be closed during normal operation) can be uti-
lized to minimize pressure shock during valve switching.
It will be appreciated by those skilled in the
sulfur recovery art that a Claus plant tail gas cleanup
35 process is provided which is not sensitive to water con-
tent in the effluent stream and which is capable of con-
tinuously maintaining low levels of emission while
-~ reducing costs. Other embodiments and applications in the
1300;~45
-31--
spirit of the invention and within the scope of the
appended claims will be apparent to those skillecl in the
art from the description herein.