Note: Descriptions are shown in the official language in which they were submitted.
~ 13~(~87~
This inven-tion relates to barium titanate
based dielectric compositions and, more particularly,
relates to stoichiome-tric, dispersible, submicron
barium ti-tanate or coforms, with very narrow particle
size distributions.
The high dielectric constant and strength of
barium titanate make it an especially desirable
material from which capacitors, condensers, and other
electronic components can be fabricated. Especially
attractive is the fact that barium titanate's
electrical properties can be controlled within a wide
range by means of mixed crystal formation and doping.
The very simple cubic perovskite structure
exhibited by barium titanate is the high temperature
crystal form for many mixed oxides of the ABO3 type.
This crystal structure consists of a regular array of
corner-sharing oxygen octahedra with smaller titanium
(IV) cations occupying the central octahèdral B site
and barium (II) cations filling the interstices
between octahedra in the larger 12-coordinated
A-sites. This crystal structure is of particular
significance since it is amenable to a plethora of
multiple cation substitutions at bo-th the A and B
sites so that many more complex ferrolectric compounds
can be easily produced.
Barium titanate's relatively simple lattice
structure is characterized by the TiO6-octahedra
which, because of their high polarizability,
essentially determine the dielectric properties of the
structure. The high polarizabiIity is due to the fact
that the small Ti(IV) ions have relatively more space
within the oxygen octahedra. This cubic unit cell,
however, is stable only above the Curie poin-t
temperature of about 130C. Below 130C, the Ti(IV)
ions occupy off-center positions. This transition to
- ' ~
'
the off-center position results in a change in crystal
structure from cubic to tetragonal between
temperatures of 5C and 130C, to orthorhombic between
-90C and 5C and finally to rhombohedral at
temperatures less than -90C. Needless to say, the
dielectric constant and strength also decreases
relative to these temperature and crystal s-tructure
changes.
The dielectric constant of barium titanate
cerarnic has a strong temperature dependence and
exhibits a pronounced, maximum dielectric constant at
or around the Curie point. In view of the temperature
dependence of the dielectric constant and its
relatively low value at room temperature, pure BaTiO3
is rarely used in the production of commercial
dielect:ric compositions. E~ence, in practice,
additives are employed to upgrade the dielectric
properties of barium -titanate. For example, i-t is
known in -the art that the Curie temperature can be
shi~-ted to lower temperatures and broadened by
effecting a par-tial substitution by strontium and/or
calcium for barium and by zirconium and/or tin for
titanium, thereby resulting in materials with a
maximum dielectric constant of 10,000 to 15,000 at
room temperature. Alternative1y, the Curie
temperature can be increased by a partial substitution
of lead (II) for barium. Additlonally, the
substitution of small amounts of other metallic ions
of suitable size but with valencies which are
different to those of barium and titanium, as
summarized in B. Jaffee, W. R. Cook, Jr. and H. Jaffe,
"Piezoelectric Ceramics", Academic Press, N.Y. 1971,
can cause profound changes in the nature of the
~ielectr1c ~roperties.
- 2 -
.
.- :
~ ~3~ V
In commercial practice, barium titanate
based dielectric powders are produced either by
blending the required pure titanates, zirconates,
stannates and dopants or by directly producing the
desired dielectric powder by a high temperature solid
state reaction of an in-timate mixture of the
appropriate stoichiometric amounts of the oxides or
oxide precurors (e.g., carbonates, hydroxides or
nitrates) of barium, calcium, titanium, etc. The pure
titanates, zirconates, stannates, ete. are also,
typically, produced by a high temperature solid phase
reaction process. In such caleination proeesses the
required reactants are wet milled to accomplish the
formation of an intimate mixture. The resulting
slurry is dried and caleined at elevated temperatures,
ranging from about 700 to 1200C, to attain the
desired solid state reaetions. Thereafter, the
ealeine is remilled to produee a dispersible powder
for use in making green bodies.
Although the barium titanate based
dieleetxic formulations produced by solid phase
reaetions are aeeeptable for many eleetrieal
applieations, they do suffer from several
disadvantages. Firstly, the milling step serves as a
souree of eontaminants whieh ean adversely affect
electrical properties. Compositional inhomogeneities
on a mieroseale can lead to the formation of
undesirable phases, sueh as barium orthotitanate,
which ean give rise to moisture sensitive properties.
Moreover, during ealeination substantial partiele
growth and interparticle sintering can occur. As a
consequence, the milled produet eonsists of
irregularly shaped fraetured aggregates which have a
wide particle size distribution ranging from about 0.2
up to 10 micronsO Published studies have shown that
green bodies formed from sueh aggregated powders with
3 -
~.. ,;,;.,,. - - . ~
' . :
`
87~
broad aggregate size distributions require elevated
sintering temperatures and give sintered bodies with
broad grain size distributions. In the production of
complex dielectric bodies, however, such as monolithic
multilayer capacitors, there is a substantial economic
advantage to employing lower sin-tering temperatures
rather than higher sintering temperatures, since the
percentage of lower cost silver in the
silver-palladium electrode can be increased as the
sintering temperature is reduced.
As is known in the art, the capacitance of a
dielectric layer is inversely proportional to its
thickness. In current multilayer capacitors, the
dielectric layer thickness is of the order of 25
microns. Although very desirable, this value cannot
be substantially reduced because as layer thickness is
decreased the number of defects in the dielectric
film, such as pin holes, increases. The defects
adversely affect the performance of the capacitor.
One major source of such defects is the presence of
undispersed aggregates having sizes comparable with
the film thickness. During sintering, because of the
presence of such aggregates, non-uniform shrinkage
occurs and pin holes are formed. Hence, utilization
of barium titanate based dielectric formulations
formed by solid state reactions significantly
increases the overall manufactur.ing cost of monolithic
multilayer capacitors.
In view of the limitations of the product
rendered by conventional solid state reaction
: processes~, the prior art has developed several other
methods for producing barium titanate. These methods
nclude the thermal decomposition of barium titanyl
oxalate and barium titanyl citrate and the high
: 35 temperature oxidation of atomized solutions of either
barium and titanium alcoholates dissolved in alcohol
-- 4 --
.
~ ~ .
,
~3~1~B~i~
or barium and titanium lactates dissolved in water.
In addition, barium titanate has been produced from
molten salts, by hydrolysis of barium and titanium
alkoxides dissolved in alcohol and by the reaction of
barium hydroxide with titania both hydrothermally and
in aqueous media. Because the product morphologies
derived from some of these processes approach those
desired here, the prior art has attempted to produce
barium titanate based compositions with the same
methods used to produce pure barium titanate. For
example, B. J. Mulder discloses in an article entitled
"Preparation of BaTiO3 and Other Ceramic Powders by
Coprecipitation of Citrates in an Alcohol", Ceramic
Bulltein, 49, No. 11, 1970, pages 990-993, that Batio3
based compositions or coforms can be prepared by a
coprecipitation process. In this process aqueous
solutions of TI(IV), Zr(IV) and~or Sn(IV) citrates and
formates of Ba(II), Mg(II), CatII), Sr(II) and/or
Pb(II) are sprayed into alcohol to effect
coprecipitation. The precipitates are decomposed by
calcination in a stream of air diluted with N2 at
700-800C to give globular and rod shaped particles
having an average size of 3 to 10 microns.
Barium titanate based coforms have been
prepared by precipltation and subsequent calcination
of mixed alkali metal and/or Pb(II) titanyl and/or
zirconyl oxalates as disclosed by Gallagher et al. in
an article entitled "Preparation of Semi-Conducting
Titanates by Chemical Methods", J. Amer. Ceramics
30 Soc., 46, No. 8, 1963 pages 359-365. These workers
demonstrated that BaTiO3 based compositions in which
Ba is replaced by Sr or Pb in the range oE 0 to 50
mole percent or in which TitIV) is replaced by Zr(IV)
in the range of 0 to 20 mole percent may be produced.
-- 5
.~.. . .... .. .. . .
`"` ~.3C3~
Faxon et al. discloses in U.S. Patent No.
3,637,531 that BaTiO3 based coforms can be synthesized
by heating a solution of a titanium chelate or a
ti-tanium alkoxide, an alkaline earth salt and a
lanthanide salt to form a semisolid mass. The mass is
then calcined to produce the desired titanate coform.
In each of the prior art references cited
above, however, calcination is employed to synthesize
the particles of the barium titanate based coforms.
For reasons already noted this elevated temperature
operation produces aggregated products which after
comminution give smaller aggregate ~ragments with wide
size distributions.
The prior art has also attempted to
circumvent the disadvantages of conventionally
prepared BaTiO3 powders by synthesizing a mixed
alkaline earth titanate-zirconate composition through
a molten sal-t reaction. Such a process is disclosed
in U.S. Patent No. 4,293,534 to ~rendt. In the
practice of this process -titania or zirconia or
mixtures thereof and barium oxide, strontium oxide or
mixtures thereof are mixed with alkali metal
hydroxides and heated to temperatures suffi.cient to
melt the hydroxide solvent. The reactants dissolve in
the molten solvent and precipitate as an alkaline
earth titanate, zirconate or a solid solution having
the general formula BaxSr(l x)TiyZr(l y)O3. The
products are characterized as chemically homogeneous,
relatively monodisperse,. submicron crystallites. This
- 30 method is limited, however, in that it can only
produce Sr and/or Zr containing coforms.
:Hydrothermal: processes have also been
described in which coforms are produced. Balduzzi and
: Steinemann in British Patent No. 715,7~2 heated
aqueous slurries of hydrated Tio2 with stoichiometric
amounts of alkaline earth hydroxide to temperatures
-- 6
, ., .~, ~. . :
\ - ~
between 200 and 400C to form mixed alkaline earth
titanates. Although it was stated that products of
any desired size up to about 100 ~lm could be produced,
it is doubtful that, other than in the case of
Sr-containing coforms, products with the morphological
characteristics of this invention co~ld be obtained.
This contention is based on the fact that whereas
Ba(OH)2 is soluble in aqueous media Ca(OH)2, and
Mg(OH)2, especially in the presence of Ba(OH)2, are
relatively insoluble. Accordingly, in the case of
Ca-containing coforms it has been found that under the
experimental conditions of Balduzzi and Steinemann
that BaTiO3 is first formed and then Ca(OH)2 reacts
with the balance of the unreacted titania to form
CaTiO3 during the heating process to 200 to 400C.
Matsushita et al. in European patent publication No.
0141551 published May 15, 1985 demonstrated that dilute
slurries of hydrous titania can be reacted with
Ba(OH)2 and/or Sr(OH)2 by heating to temperatures up
to 110C to produce either BaTiO3 or Sr-containing
coforms. The morphological characteristics of these
coforms appear to be comparable with those of this
invention. The method, however, is again limited to
producing only Sr-containing coforms.
A publication of the Sakai Chemical Industry
Company entitled "Easily Sinterable BaTiO3 Powder" by
Abe et al. discloses a hydrothermal process for
synthesizing a barium titanate based coform with the
formula BaTi(l-x)snxo3 In this process a 0.6M
Ti(l_x)SnxO2 slurry, prepared by neutralizing an
aqueous solution of SnOC12 and TiC14, is mixed with
0.9M Ba(OH)2 and subjected to a hydrothermal treatment
at 200C for at least five hours. Although not
explicitly delineated, Abe et al. imply the slurry was
heated to temperature. Although no description of the
coform morphology was indicated, the BaTiO3 product
- 7 -
.,, .. ~ , .
produced by the same process had a surface area of 11
m2/g, a particle size of 0.1 ~m and appeared to be
dispersible. Presumably the Sn-containing coforms
have comparable morphologies and are thus comparable
with those of this invention. However, Abe et al. is
limited in that it teaches only that Sn(IV) can be
synthesized into a barium titanate coform. Perhaps,
by analogy, it does suggest the use of other
tetravalent cations such as Zr(IV) and possibly the
use of divalent Sr(II), since, like Ba(OH)2, Sr(OH)2
is quite soluble in aqueous media. However, the
process of Abe et al. cannot be used for substitution
of divalent Curie point shifters such as Pb and Ca for
the divalent Ba.
Hence, there is absent in the prior art any
coforms of barium titanate which include calcium
and/or lead or multiple divalent and tetravalent
cation substitutions which are stoichiometric,
dispersible, spherical, and submicron with narrow
particle size distributions.
The present invention includes a wide
variety of dispersible coforms of barium titanate
which are substantially spherical, stoichiometric, and
submicron with narrow particle size distributions.
Most importantly, the barium titanate based dielectric
compositions according to the present invention
include those coforms having a partial substitution by
divalent lead and/or calcium for the divalent barium
as well as coforms in which the divalent barium is
partially replaced by lead, calcium and strontium and
the tetravalent titanium is partially replaced by tin,
zirconium and hafnium.
In one important embodiment of the present
invention, the barium titanate~ based coform is
represented by the general formula
(l-x-xl-xll)pbxcax~srxllTi(l-y-yl-yll)snyzrylHf ,,O3
- 8 -
..". ~,
'"
)8~'0
where x, x' and x'' represent the mole fractions of
the divalent cations and have independent values
ranging from 0 -to 0.3 and the sum x + x' + x'' has a
value ranging from 0 to 0.4, while y, y' and y''
represent the mole fractions of the tetravalent
cations and have independent values ranging from 0 to
0.3 and the sum of y + y' + y'' has a value ranging
from 0 to 0.~.
In another important embodiment of the
present invention, the barium titanate coform is
represented by the general formula
Ba(l_xl)caxlTi(l-y-yl y,,)SnyZry,HF ,,O3 wherein
calcium is partially substituted for the divalent
barium cation and in another important embodiment of
the invention the barium titanate dielectric
composition is represented by the general formula
Ba Pb Ti , " Sn Zr ,Hf ,,O , wherein lead
tl-x) x (l-y-y -y ) y y y 3
is substituted for the divalent barium. In each of
the latter embodiments, the independent values for the
mole fractions x, x', x'' and y, y', y'' and
consistent with those already cited for the more
complex coEorm having the general formula
(l-x-xl-x~l)pbxcaxlsrxlli(l-y-y~-yll)snyzr ,Hf ,,O3.
Notwithstanding the chemical composition of
the coform, each of the barium titanate based coforms
of the present invention possess the same unique
chemical and physical properties. The barium titanate
based dielectric formulations are stoichiometric such
that the divalent to tetravalent mole ratio of the
vafyingly composed coforms is 1.000 + 0.015 regardless
of the number and mole percent of any divalent and
tetravalent cation substitutions. Non-stoichiometric
compositions, where the divalent to tetravalent cation
: mole ratio of the varyingly composed coforms is in the
range of 0.9 to 1.1, can also be produced~ The mean
: primary particle size of the barium titanate based
g
3~
coforms is in the range of 0.05 to 0.4 microns.
Moreover, the mean particle size determined by image
analysis is comparable to the mean particle size
determined by sedimentation demonstrating that the
coforms are dispersible. The size dis-tribution curve
of the coform particles has a ~uartile ratio less than
or equal -to 1.5 which establishes that the barium
titanate based coforms have a narrow particle size
distribution. Additionally significant is the fact
that any of the dispersible, submicron barium titanate
based dielec-tric compositions of the present invention
can be produced by a single, general hydrothermal
process.
Accordingly, it is a primary object of the
present invention to provide a dispersible, submicron
barium titanate coform with a narrow particle size
distribution.
It is another object of the present
invention to provide a wide variety of compositions of
such BaTiO3 based coforms having primary particle
sizes which can be controlled in the size range of
0.05 up to about 0.4 ~m.
It is another object of the present
invention to provide a wide variety of coforms which
are syn-thesizable by a single general hydrothermal
process.
It is another object of the present
invention to provide a stoichiometric barium titanate
based coform which is substantially free of mill
media.
It is another object of the present
invention to provide a coform of barium titanate
containing a variety of additives which shifts and/or
broadens the Curie point to the desired temperature
regions and reduces the temperature dependence of the
dielectric compositions so formed.
-- 10 --
, . , ~, . . . .
` ' ~ ,, '
.
.
~30~70
It is another object of the present
invention to provide dispersible BaTiO3 based
dielectric compositions which can be used to give
dielectric layers of reduced thickness which are
substantially defect free.
It is still a further object of the present
invention to provide a barium titanate based
dielectric formulation which uniformly sinters to a
high density at considerably less than conventional
temperatures.
These and other details and advantages of
the invention will be described in connection with the
accompanying drawings in which:
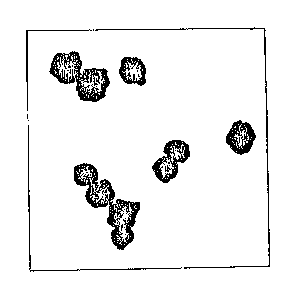
Fig. l is a transmission electron micrograph
15 at 50,000x magnification of a stoichiometric,
dispersible, submicron complex coform according to the
present invention having the general formula
0.856 0.097Ca0.074Ti0.830zr0 099Sn0 0713; and
Fig. 2 is a transmission electron micrograph
20 at 50,000x magnification of pure barium titanate
powder which exhibits a morphology substantially
similar to the morphology of the complex coform of
Fig.l.
At the outset, the invention is described in
its broadest overall aspects, with a more detailed
description following. The preferred embodiment of
the present invention is a coform of the general type
(l-x-xl-x~l)pbxcaxlsrxllTl~l , Sn Zr ~f O
wherein x, x' and x'' represent the mole fractions of
the divalent cations and have independent values
ranging from 0 to 0.3 and, more preferably, from 0 to
0.2 and the sum x + x' + x'' can have values ranging
from 0 to 0.4 and more preferably from 0 to 0.3, y, y'
and y'' represent the mole fractions of the tetra-
valent cations and have independent values ranging
.
13~0870
from 0 to 0.3 and, more preferably, from 0 to 0.25 andthe sum of y + y' + y'' have values ranging from 0 to
0.~ and, more preferably, from 0 to 0.3.
When the sums of (x + x' -t x'') and (y + y'
+ y'') both equal zero the coform simply constitutes
barium titanate powder. When x = x'' = y = y' = y'' =
0 and x' is greater -than 0, the resulting product is a
barium titanate based coform where x' mole fractions
of Ba(II) in BaTiO3 have been replaced by Ca(II) to
give a product with the nominal formula
Ba(l x,)Cax,TiO3. Conversely, when x' = x'' = y = y'
= y'' = 0 and x is greater than zero, the coform has
the composition Ba(l X)PbxTiO3.
Since the values of x, x', x'', y, y', and
y'' can each adopt a wide range of values (within the
cited limits), many combinations of coforms with a
large range of compositions can be prepared.
Regardless of which composition is formed, however,
each of the barium titanate based coforms is uniquely
characterized by its high purity, fine submicron size
and narrow particle size distribution.
Preferably, the fine, dispersible submicron
powder of the present invention consists of a barium
titanate coform having both a tetravalent and a
divalent metal ion substitution of between zero and 30
mole percent. The divalent barium ion can be
partially replaced by either lead, calcium, strontium
or mixtures thereof. Conversely, the -tetravalent
titanium ion can be partially replaced by tin,
zirconium, hafnium or mixtures thereof. Hence, the
barium titanate based dielectric compositions of the
present invention include simple coforms of barium
lead titanate or barium strontium titanate as well as
more complex coforms including barium lead stannate
titanate and barium lead strontium stannate zirconate
; titanate. Of course, the sel~ection of the divalent
~ 12 -
~L~00870
and/or tetravalent cation replacement and the mole
percent of the substitution is dependent upon whether
the Curie temperature is desired to be raised or
lowered as well as by whethex the Curie peak is
desired to be broadened or shifted. Regardless of
which of the wide variety of barium titanate based
compositions is formed, however, the barium titanate
coEorms according to the present invention are still
uniquely identified by the aforementioned
morphological and chemical characteristics. Hence,
both the simple as well as the complex coforms of
barium titanate consist of substantially spherical,
dispersible particles having a primary particle size
in the range of 0.05 and 0.4 microns with narrow size
distributions and a divalent to tetravalent mole ratio
of 1.000 + 0.015, even when both the divalent and
tetravalent ions have been replaced by one or more
other ions.
The narrow particle size distribution and
submicron slze of the barium -titanate based dielectric
compositions make the coforms of the present invention
particularly attractive for fur-ther application in the
production of complex dielectric bodies. Prior
studies have established that green bodies formed from
unaggregated powders with narrow size distributions
will sinter at reduced temperatures and give sintered
bodies with a narrow grain size distribution. The
economic advantage of employing a dielectric
formulation with a lower sintering temperature is
obvious since the percentage of lower cost silver in
the silver-palladium alloy can be increased as the
~sintering temperature is reduced. In addition, since
these BaTiO3 based dielectric compositions are all
dlspersible and have few aggregates exceeding a size
of l micron, they can be employed in the formation of
dielectric films of reduced thickness. Hence, the
- 13 -
~30(:~870
spherical, unaggregated, submicron and narrowlydistribu-ted barium ti-tanate dielectric coform powder
of the present invention should be particularly well
suited for use in complex dielectric applications
requiring sintering.
In most dielectric applications, the
preferred products are those in which the variability
in primary particle composition is relatively small.
In some circumstances, however, compositional
inhomogeneities are an advantage. In these instances,
the availability of products with varying primary
particle size can be utilized to produce a dispersion
of two or more powders with differing compositions
having either comparable numbers of primary particles
or substantially different numbers of primary
particles. Such dispersions give green bodies, and
hence sintered bodies, with controlled degrees of
microinhomogeneities. In such applications, the
compositional inhomogeneity may be inherent in the
barium titanate coform selected or, instead, may
result from a small amount of a barium titanate coform
with a selected composition being added to a barium
titana-te dispersion in order to achieve the desired
compositional inhomogeneity. Since either divalent
barium and/or tetravalent titanium deficient coforms
can be formed according to this invention, the barium
titanate based compositions of the present invention
are also well suited for applications where
compositional inhomogeneities are advantageous.
The preferred approach for producing the
barium titanate based coforms is to heat slurries
con-taining the hydrous tetravalent oxides wi~h
~selected divalent oxides or hydroxides. After
formation of the divalent titanates, the slurry still
contains substantial quantitites of hydrous TiO2
and/or hydrous SnO2, ZrO2 or HfO2. The slurry
- 14 -
: ,.
:
~L3~087~
temperature and concentration are then adjusted and a
stoichiometric excess of Ba(OH)2 solution is then
added under isothermal conditions. In order to
ensure the complete conversion of the tetravalent
oxides to their corresponding oxyanions, the slurry is
preferably taken -to a final, higher heat treatment.
The primary particle size and size
distribution of the present invention are achieved
whether the barium titanate coform is simply ~aTiO3 or
instead is the more complex coform having the formula
l-x-xl-xllPbxcaxlsrxllTil-y-yl-~llzrysnylHf ,,o3.
This becomes readily apparent from the transmission
electron micrograph of the complex coform
0.856 0.097Cao.074Tio 830Zr0 099Sn0 0713 in Fig
1 which shows the presence of predominantly single,
substantially spherical primary particles, although a
few firmly bound doublets and triplets are also
present. The primary particle size of this coform is
0.18 microns with a narrow size distribution. A
comparison of the complex barium titanate based coform
of Fig. 1 with the transmission electron micrograph
of pure barium titanate in Fig. 2 indicates that the
morphologies of the barium titanate based compositions
are very similar. Note that in both micrographs the
particles are substantially spherical,unaggregated,
submicron and uniformly sized. It may also be noted
that the divalent to tetravalent cation mole ratio in
this product, 1.027, is somewhat larger than the value
1.000 + 0.015 specified for stoichiometric products.
This ratio can easily be reduced to the specified
range by minor varia-tions in the synthesis conditions
wlthout affecting morphology.
In order to evaluate the physical and
chemical properties of the barium titanate based
coforms according to the present invention, a variety
of laboratory tests were performed. Image analysis
- 15 -
,.. ~. ~. : .
~3ao~70
was used to determine product primary particle size
and primary particle size distribution. 500 to 1000
par-ticles were sized in a plurality of TEM fields in
order to determine the equivalent spherical diameters
of the primary particles. Two or more touching
particles were visually disaggregated and the sizes of
the individual primary particles were measured. The
e~uivalent spherical diameters were used to compute
the cumulative mass percent distribution as a function
of primary particle size. The median particle size,
by weight, was taken to be the primary particle size
of the sample. The quartile ratio, QR, defined as the
upper quartile diameter (by weight) divided by the
lower quartile diameter, was taken as the measure of
the width of the distribution. Monodisperse products
have a QR value of 1 and, for our testing purposes,
products with QR values ranging from l.0 to about 1.5
were classified as having narrow size distributions,
those with QR values ranging from 1.5 to about 2.0
were classiied as having fairly narrow
distributions, while those with values substantially
greater than 2.0 were classified as having broad size
dis-tributions. The quartile ratio of the barium
titanate coforms of the present invention was
determined to be between 1.0 to 1.5, indicating that
the primary particles have a narrow size distribution.
Surface areas were calculated from the
coform's primary particles and were found to be
consistent with the surface areas determined by
nitrogen adsorption, indicating that the prirnary
par-ticles are essentially nonporous. In cases where
the N2 surface area substantially exceeded the TEM
surface area, it was found that the difference could
be readily accounted for by the presence of unreacted
. .
high surface area hydrous o~ides.
~ - 16 -
-
' ' . ~.
' .~.... .
'. ' ' ' ~
13Q(~87~
Since the coforms of the present invention
have a narrow size distribution, average primary
particle size was readily determined by sizing 20 to
30 particles. It was found that the relationship D=6
S, where D is particle diameter (microns)p is density
(g/cc) and S is N2 surface area (m2tg), could be used
to obtain a good measure of the coform primary
particle size. According to this formula it was found
that the barium titanate based coforms have a primary
particle size in the range between 0.05 and 0.4
microns, regardless of which coform composition was
tested.
Product dispersibility of the coforms was
assessed by comparing the primary particle sizes and
size distributions determined by image analyses with
the comparable values determined by sedimentation
procedures. The sedimentation process gives the
particle Stokes diameter which, roughly, corresponds
to the equivalent spherical diameter. Two
sedimentation methods, the Joyce Loebl Disc Centrifuge
(Vickers Instruments, Ltd., London, U.K.) and the
Micromeritics Sedigraph (Norcross, Georgia) were
employed to determine cumulative mass percent
distributions in terms of Stokes diameters from which
the median Stokes diameters and the QR values were
calculated.
In determining particle size by
sedimentation, the powders were dispersed by a 15 to
30~ minutes sonification in either water containing
30 0.~08g/L sodium tripolyphosphate at pH 10 or in
sopropanal contalnlng 0.08 ~ or 0~12 weight percent
Emphos PS~21A (Witco Organics~ Division, 520 Madison
Ave., New York)~
Since particle siz~e ~de~ermined by image
~analysis and by sedimentation depend on different
pr~inciples, ~an exact correspondence in size by these
- 17 -
:
8~
two methods was not always obtained. Moreover, as
already noted, in image analysis touching particles
are visually disaggregated. In the sedimentation
process bound or flocculated particles act as single
entities. These entities arise because of the
existence of some bonding (e.g., necking) between the
primary particles to give cemented aggregates which
cannot be readily broken down during the sonification
process and because of less than optimum dispersion
stability which leads to some flocculation. Thus, the
QR values determined by sedimentation, as expected,
were somewhat larger than those found by image
analysis.
In the barium titanate based coforms of the
present invention, the primary particle size
determined by image analysis was in reasonable
agreement with the primary particle size determined by
sedimentation. The median particle size determined
varied by no more than a factor of two. This
demonstrates that the coforms are dispersible.
Two additional measures were used to assess
dispersibility. In the first method, the mass
fraction of the product having a Stokes diameter
greater than one micron was used as a measure of the
amount of hard-to-disperse aggregates. In the second
method, a product was classified as being dispersible
if the bulk of the primary particles in the TEM's were
present as single particles. When substantial necking
was observed the product was classlfled as aggregated.
In each of these testsj the barium titanate based
; coforms were again classified as dispersible.
Product composition and stoichiometry of the
:: :
coforms was determined by elemental analysis using
inductively coupled plasma spectroscopy after sample
~ 35 dissolution. The precision of the analyses was about
; ~ ~1%. The mole ratio of divalent cations to
:
- 18 -
... ...
~ ' :
-tetravalent cations of the coforms, regardless of the
number or mole weight percent of the divalent and
tetravalent cation substitutions, was 1.000 + 0.015.
This ratio indicates that the barium titanate coforms
of the present invention are stoichiometric.
The unique properties of the barium titanate
based coforms are further illustrated by the following
non-limiting examples.
Reagent grade chemicals or their equivalents
were used throughout the Examples. The reagent grade
Ba(OH)2 8H2o employed contained l mole percent Sr.
Experiments have shown that Sr(II) is more readily
incorporated than Ba(II) in the coform. For this
reason all coforms described here contain Sr(II).
This cation represents about l mole percent of the
total divalent cation content of the coform. For
simplicity, the Sr(II) mole fraction has been included
in the Ba(II) mole fraction. Ba(OH)2 and/or Sr(OH)2
solutions, maintained at 70-100C, were filtered prior
to use to remove any carbonates present. CaCO3 was
calcined at 800C to give CaO. The latter compound
when contacted with water gives Ca(OH)2. Pb(OH)2 was
prepared by neutralizing a Pb(NO3)2 solution with
aqueous NH3. The washed hydroxide wet cake was used
in subsequent experiments.
Hydrous oxides of TlO2, SnO2 and ZrO2 were
prepared by neutralizing aqueous solutions of their
respective chlorides with aqueous NH3 at ambient
temperatures. The products were filtered off and
washed until chloride-free (as determined by AgNO3)
~filtrates were obtained. The surface areas of the
hyd~ous oxides, detexmined after drying at 110C, were
about 380, 290 and 150 m /g for TiO2, SnO2 and Zr2,
respecti,vely. In addition coprecipitates of hydrous
- 1 9
,....
~~~
13C~O~
TiO2 and ZrO2 or hydrous TiO2 and SnO2 were prepared
by neutralizing aqueous solutions of the chlorides of
Ti(IV) and Sn(IV) or Ti(IV) and Zr~IV).
All experiments were performed in a 2 liter
Autoclave. To prevent product contamination a]l
wetted parts of the autoclave were coated with Teflon
and every effort was made to exclude CO2 from all
parts of the system. Ba(OH)2 or Ba(OH)2 and Sr(OH)2
solutions were introduced into the autoclave either by
means of a high pressure pump or by rapidly
discharging a solution of the hydroxide or hydroxides,
contained in a heated bomb, in-to the autoclave by
means of high pressure nitrogen. The contents of the
autoclave were stirred at 1500 RPM throughout the
synthesis process.
Example 1
A calcium containing coform was prepared by
hydrothermal treatmen-t of 0.64 L of a slurry
containing 0.20 moles of hydrous TiO2 and 0.04 moles
20 of Ca(OH)2 to 200C. The slurry was coo]ed and 0.46 L
of 0.41M Ba(OH)2 was added to the slurry at 120C.
The resulting slurry temperature was raised to 150C
and held there for 60 minutes. The sample was
filtered and the divalent cation concentrations in the
filtrates were determined. The filter cake was dried
and its surface area, nominal stoichiometry and
morphological characteristics was determined.
- 20 -
87~
Divalent/
Filtrate Cation Mole Ratio Tetravalent
g/L in Solids Cation N Area
... __ 2
Ba Ca Ca: Ba: Sr: Ti Mole Ratio m /g
2.62 0.446 0.127 0.842 0.019 1.00 0.988 12.0
Primary Particle Size
Size(TEM) Distributlon
0.15 micron Narrow
x~ e 2
A lead containing coform was prepared by
hydrothermal treatment of 0.64 L of a slurry
containing 0.2 moles of hydrous TiO2 and 0.04 moles
PbO. 0.46 L of Ba(OH)2 was added to the slurry at
150C. The slurry was held at 150C for 60 minutes and
then raised to an elevated temperature for complete
conversion o the tetravalent oxides to the perovskite
structures. The slurry was sampled and characterized.
The results obtained are as follows:
Divalent/
Z0 Filtrate Cation Mole Ratio Tetravalent
g/Lin Solids Cation Area
.... _ - ,~
_ Pb Ba: Pb: Sr: Ti: Mole Ratio m /g_
: ~ 10.6 2.74 0.810:0.173;0.024:1.000 1.007 11.5
~ .
: : Primary Particle Size
~ 25 Size (TEM) Distribution
.. . .. _ _ , .. .
~0.07 micron Narrow
- 21 -
', ' ' ' '' ' '
~3W~
Example 3
Complex coforms are formed in which the
Ba(II) and Ti(IV) in BaTiO3 are partially replaced by
one or more divalent and tetravalent cations. A
preheated Ba(OH)2 solution was introduced into
slurries heated to 150C or 120C containing the
tetravalent hydrous oxides and presynthesized
perovskites of Pb(II) and/or Ca(II). After holding at
temperature for about 20 to 30 minutes, the slurries
were raised to a final temperature to ensure that the
tetravalent hydrous oxides converted to stoichiometric
perovskites. The resulting slurry was characterized
with the following results:
.
~ : :
::::: : ~ ::; : : :
- ~ : .
: ~ ,~ ~: :: :
- 22 -
:
~: ~
, . . . , :-
-, . .
.
.. ' ~ : .
~08~7V
o ~ c~
u~
O ~ d' c~ O
) ~ O ~I ~
a) ~ o ~; a~ o o ~0 ~ ~
D ~ o ,~ ,~ o ~ 1~ Z Z u~ O o o o
Cl
o o ,~
~ o o 1`
U~ o o o
o o o
,1 .. .. ..
~1 ~ ~ cs~ a~
o c~ ~ ,~ a~
U~ o ,~ o
.. . . .
o o o
O E~ o ~ ~
,, ooo ,~ ~0 ~ ~ U) I ,1~,~
(~ O ~) d! ~1 ~ ~1 ~I h rl
,~ o 1` ~ ~ ,1-,1 ,1 ~
o - , . (~ E~ E3 E~ 1 ~
,0, O, ,0, ~ '~ ~ : ~
~ (~O~ N O00 h ooo
t ) o o o ,1 ~ . ~ E3
. N . 00 rl ~ H
o oo: 15'i a
m oOo ~ N
' ~
2 3 -
'
: ,
. ~
~3008 ~'0
The quantitative data for samples 2 and 3
corresponds well with the estimated particle size,
size distribu-tion and dispersibility data drawn from
the transmission electron micrographs. Sample 1,
however, as assessed by the QR value is only
moderately dispersible. Nevertheless, the
sediment~tion data indicates that less than 5 weight
percent of the material is present as aggregates
having a size greater than 1 micron.
It can therefore be seen from the preceding
examples and disclosure, that the coforms of barium
titanate encompassed by the present invention include
those dielectric compositions containing calcium
and/or lead or multiple replacements for either or
both of the divalent barium and tetravalent titanium
cations which are uniquely characterized in that they
are spherical, have a primary particle size in the
range from 0.05 to 0.4 microns, a divalent -to
tetravalent mole ratio of 1.000 + 0.015, and a narrow
particle size distribution. No prior art barium
titanate based dielectric compositions which include
calcium, lead or the complex forms disclosed herein
possess these unique morphological and chemical
characteristics.
It is understood that the preceding
description is given merely by way of illustration and
not in limitation of the invention and that various
; ~ modifications may be made thereto without departing
from the spirit of the invention as claimed.
''
. - 24 -
. ' ' ' " ' ' '
. ~ , , .