Note: Descriptions are shown in the official language in which they were submitted.
-- 13~)1472
-- 1 --
EVANESCENT WAVE BACKGROUND ~LUORESCENCE/ABSORBANCE DETECTION
Background of the Invention
.
5 Field of the Invention
The present invention relates to method and apparatus for the
detection and measurement of certain optical characteristics of
biological culture and fermentation media in a bioreactor or the like,
and more particularly, to the measurement of the fluorescence and/or
10 absorbance of a continuous phase in the presence of a discontinuous
phase.
Description of Related Art
Fluorescence of intracellular NADH or NADPH has been shown to be a
15 good indicator of the metabolic state of cells in culture, as well as
serving as an indicator of the concentration of cells in the culture
medium. Several papers have attested to the value of this kind of in
situ measurement for both microbial and yeast culture applications.
See, e.g., W.B. Armiger et al., Analysis and Control of Fed-Batch
20 Fermentations Producing Escherichia coli Using Culture Fluorescence,
Proceedings Biotech 84, Washington, D.C. 1984. Apparatus for performing
these measurements is available from BioChem Technology, Inc., Malvern,
Pennsylvania, as the FluoroMeasureTM System.
From an economic point of view, it would be advantageous to use
25 lower cost complex nutrients, such as molasses or corn steep liquor in
industrially significant cultures. These, however, introduce additional
background fluorescence. If the medium contains a fluorescent component
which does not change during the course of the culture, a background
correction can be made simply by subtracting the reading at time zero
30 from all subsequent readings. In a case where the fluorescence of the
media changes due to use by the cell or in the case where the cells
produce a competing fluorescence, it becomes more difficult to correct
on-line for changes in background. This can be done for a batch culture
by taking serial samples, removing the cells and measuring the
35 fluorescence of the medium. Even more difficult are background
corrections in cases of continuous culture or where nutrients are added
stepwise during the culture.
.~:
13~1472
-- 2 --
For fluorescent media, it would be useful to be able to determine
how the fluorescence of the media is changing during the fermentation,
such that a measurement can be made as to the metabolic state of the
cells and their growth rate. What is needed is a means of effecting the
5 separation of the cell fluorescence from that of soluble materials.
Optical sensors for fermentations or tissue culture are capable of
giving information on intracellular substances and conditions. Such
information would permit a finer control based on actual intracellular
information rather than on the existing on-line sensors, temperature,
- 10 pH, dissolved oxygen, off-gas analysis. It would lead to a better
scaleup and commercialization of products derived from recombinant DNA
and cell fusion technologies. Optical sensors, at present, work best
with media which do not interfere since there is no easy way to correct
for changes in optical background.
The early work with optical sensors for following intracellular
metabolism dates back to 1957, when Duysen and Amesz observed that the
fluorescence of baker's yueast was similar to that of NADH and that the
fluorescence of starved yeast could be enhanced by adding ethanol or
glucose to the suspension. Later, Harrison and Chance built an
20 instrument capable of measuring culture fluorescence in situ and could
monitor aerobic/anaerobic transitions in continuous culture. Using a
similar device, Humphrey and coworkers, and others, have shown that a
fluorometer placed on a fermentor could measure intracellular NADH
changes and might be useful for process control. Zabriskie and Humphrey
25 showed the linear relationship between the logarithm of the fluorescence
of the culture and the logarithm of cell concentration. Ristroph et al.
studied the relationship between culture fluorescence and the growth of
Candida utilis in a fed batch fermentation.
These studies have shown that the concentration in intracellular
30 NADH measured by culture fluorescence in a fermentation is a function of
the number of cells, the energy level within each cell, and the level of
metabolic activity. A mathematical expression which is derived from
these studies is:
F(t) = ~Yf/x(l+m(t]X(t) + E(t)
X(t) is the cell concentration. The term in square brackets is the
.
.
" 13(~472
fluorescence yield, which is made up of an invariant component Yf/x,
which is characteristic of the type of organism and a variable component
m(t), which chabnges in response to shifts in the level of metabolic
activity. The final term, E(t), with which the present invention is
S mainly concerned, is the environmental, or background, fluorescence.
Obviously, if E(t) fluctuates during the fermentation, then it would be
difficult if not impossible to derive information about the cells from
the measured overall fluorescence. Continuous, or batch fed
fermentation or cell cultures only exacerbate the problem. ]n those
techniques, additional variables are introduced without corresponding
information as to concentration.
Almost all of the published studies have used synthetic media where
E(t) is low, or the corrections for E(t) had to be arrived at
empirically, In scaling up fermentations and cell cultures for
commercial production, economic factors may dictate use of the natural
nutrients, like molasses or fetal calf serum, which have a natural
fluorescence and therefore contribute to the background value. When
checking some of the assumptions used in correcting for the background,
I found indications that the background fluorescence of, for instance,
molasses, and the fluorescence of yeast cells do not add linearly. This
pointed up the need for a method for continuously measuring the media
fluorescence background on-line and in real time, i.e. using a sensor or
sensors continuously monitoring the detected variable as the
fermentation or culture is being conducted.
This means that, without physically separating the cells from the
media, a method was needed which caused the media to fluoresce without,
at the same time, causing the cells to nuoresce. In accordance with
the present invention, the evanescent wave phenomenon is used to meet
this need.
13~72
-- 4 --
Brief Descri~tion of the Drawinqs
Fig. 1 i8 a schematic illustration of the evaneacent wave
phenomenon, wherein a light beam 11 traverses a path from left to right
through a wave guide 13 such as an optical fiber.
Fig. 2 is an enlarged view of the circular area 2 within Fig. 1.
Fig. 3 is a schematic illustration of the variation of intensity
of the evanescent wave with distance from the interface between the two
media.
Fig. 4 is a partially schematic illustration of a cross-sectional
elevation of one of the variations of the present invention employing a
metal sheathed optical fiber that may be dipped into a liquid medium.
Fig. 5 ia a cross sectional side elevation of the embodiment shown
in Fig. 4 viewed from a right angle to the view of Fig. 4, taken along
the cross sectional line 5-5.
Fig 6 ia a partially schematic illustration of another embodiment
of the present invention.
Fig. 7 showa the portion of the embodiment of Fig. 6 viewed from a
right angle to the view of Fig. 6, taken along the line 7-7.
Fig. 8 is a partially schematic illustration of a cross-sectional
elevation of a preferred embodiment of the present invention wherein the
optical waveguide Ls a flat plate 1316, and a barrier 1312 confines the
llquid to one ~ide of the waveguide.
Flg. 9 ia an elevation view of the disk 1328 shown in Fig. 8 taken
along the line 9.
Fig. 10 la a croaa aectional aide elevation of the embodiment
ahown Ln Flg. 8 vLewed from a right angle to the view of Fig. 8, taken
along the cros~ sectional line 10-10.
Fig. 11 ia an enlarged view of the area 11 within Fig. 8.
Fig 12 ia a schematic illuatration of the embodiment of Fig. 8
showing the paths of light rays and the steps of procesaing of data.
Fig 13 is a schematic illustration of the contents of the source
and detector houaing of an embodiment alternative to the design shown
housed in element 1331 of Fig 8.
Fig. 14 shows a portion of the embodiment of Fig. 13 viewed from a
right angle to the view of Fig. 13 taken along the line 14.
~.
,~ " ~
13C~1~72
-- 5
Summary of the Invention
When a beam of light is totally reflected from a non-mirrored
interface between two optically transparent media of different
refractive indexes, an evanescent wave phenomenon, such as shown in Fig.
1, exists. The light beam 11 is totally reflected from this kind of
surface, unlike a mirrored surface, and behaves as though it penetrates
for about haLf a wavelength into the less dense medium 12, e.g. an
aqueous medium. Reference numeral 15 identifies the portion of light
beam 11 that is the evanescent wave in the less dense medium 15.
Reference numeral 16 identifies a measuring arrow showing a distance
that is one wavelength of the light beam 11.
Figs. 1 and 2 schematically show that the reflected beam is slightly
displaced from where it would be if reflected from a mirrored suface.
This displacement has been shown experimentally, and it is one of the
proofs of the existence of the evanescent wave. This part of the light
beam has many characteristics of a standing wave parallel to the
surface. Fig. 3 shows how the intensity decreases with distance from
the surface.
In Fig. 3, N is the incident wave, R is the reflected wave e is the
angle of incidence (which is greater than ec, the critical angle. Z is
the distance axis in the rarer medium measured from the interface with
the more dense medium. Eo is the initial magnitude of the electric
field component of the light st zero depth in the rarer medium. dp is
the depth of penetration, defined as the distance required for the
electric field to fall to e 1 of its value at the surface. The value of
dp is directly related to the wave length in the denser medium and is
inversely proportional to the angle of incidence and top the ratio of
refractive indexes of the two media. The greatest strength of the
evanescent wave occurs at the surface, and it decreases exponentially
with distance from the surface.
It can be absorbed by an appropriate colored material, and if the
material is fluorescent, it can excite the material to nuoresce. At
the wave length of interest, 340 nm, the volume in liters swept out by
this evanescent wave over a one square centimeter area would be 1.7 x
10-8 liter. For a 200 um diameter optical fiber 2.5 cm long, the swept
volume would be 1.3 x 10 11 liter.
The present invention accomplishes this separ~tion by using the
i'~-
. ;. .,, : .
. .
13~1~72
characteristics of the evanescent wave which forms in the less dense
medium when light is totally reflected from the interface between two
optically transparent substances of different refractive indexes. It
makes use of my observation that it is unlikely that an intact cell will
be in the volume of fluid swept by the evanescent wave next to the
optical waveguide since the wave penetrates approximately only 1/3 to
V2 of a wavelength into the aqueous layer. The present invention
therefore contemplate measurement of the fluorescence of the medium
without interference from the intracellular fluorescence or from fluo-
rescence of particles in solution.
The same concept is also adapted to measuring the optical
absorbance of the medium independent of cells and particulate material.
This can significantly reduce the complexity of the computer programs
needed to deconvolute the data and thereby make the control of
fermentation and tissue culture easier to achieve.
At the usual concentration of cells in a bioreactor, it is
unlikely, as I said, that a cell would be in this small volume of fluid
at any given time. Also, since, at an excitation wavelength of for
example 280 nm, the evanescent wave only penetrates about 110 to 1~0 nm
into the liquid phase, even if the cell is resting right on the surface
of the optical wave guide, very little of the cell volume (mostly the
cell wall or cell membrane), will interact with the evanescent wave.
Thus, by limiting the volume that can interact with the light wave to
that within the evanescent wave, the present invention provides, in
effect, a separation of the intracellular fluorescence or absorbance
from the fluorescence or absorbance of the media.
It is an object of this invention to facilitate the opening up of
fermentation and cell culture to intracellular optical measurement under
a wider variety of culture conditions because there will be a way to
correct continuously for environmental or background changes on-line in
real time.
It is a further object of the present invention to provide for the
separation of the optical effects of intracellular contents from the
optical effects of the culture medium.
It is a further object of the present invention to follow changes in
the culture medium without interference from changes in the
intracellular contents.
',:
r,~, ~ ,
13~147Z
-- 7 --
It is a further object of the present invention to Eollow chsnges in
the intrscellular contents without interference from changes in the
culture medium.
It is a still further object of the present invention to reduce
5 costs of monitoring the fluorescence of cell cultures.
It is a still further object of the present invention to simplify
and shorten the time for scaleup or change of media in a fermentation,
since separate, offline empirical measurements of background or
environmental fluorescence will not have to be made. By a single test
10 run, culture conditions might be brought to a preliminary optimization
by appropriate additions of media components.
It is a still further object of the present invention to provide
information about the extent of cell rupture, whether due to shear
forces or other mechanical or chemical causes. In accordance with the
15 present invention, the effect of stirring forces on cell integrity could
be measured on-line in real time, allowing corrections to be made during
a fermentation run rather than after the run when data shall been
subsequently analyzed.
It is a still further object of the present invention to permit the
20 use of optical absorbance methods for following other non-fluorescent
intracellular materials, since the ability to correct for background
would provide the equivalent of a continuous dual beam
spectrophotometer.
It is a still further object of the present invention to provide for
25 the better control of the concentration of individual nutrients in the
culture media through the improved ability to follow specific changes of
optically differentiable materials in the medium.
It is a still further object of the present invention to
significantly improve the yield of fermentation and tissue cultures,
30 reduce the time and cost of scaleup, allow for a more precise control
based on the state of the intracellular metabolism, and speed up the
commercialization of new recombinant DNA and cell fusion technologies
through more efficient fermentation and tissue culture techniques.
,,~ .
1301~'7Z
Deta ed Description
The embodiment of the invention shown in Figs. 4 and 5 provides a
relatively simple means of determining the background fluorescence of a
solution containing particulate matter by dipping into the liquid a
5 dipstick 40 which comprises a metal housing 41 surrounding an optic
fiber 44 through which an evanescent wave of light is used to excite the
fluorescence of the solution. The housing 41 defines a chamber 47, into
which the solution to be tested passes through apertures 46 in the
housing 41.
On the portion of the optic fiber 44 wiLhin the chamber 47, the
opaque sheath 42 and transparent cladding 43 have been removed, exposing
the fiber 44 directly to the solution within the chamber 47.
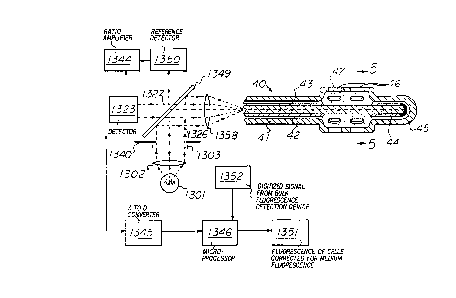
To illuminate the fiber 44, there is provided a light source 1301
of either visible or invisible light, such as an incandescent lamp or
15 laser, an excitation beam lens 1302, and an excitation beam filter 1303,
arranged as show n in Fig. 4.
The radiation from the light source 1301 passes through the lens
1302, where it is collimated, and then passes through the filter 1303,
where it is filtered into a monochromatic excitation beam 1326 of
20 desired wavelength (schematically illustrated by dashed lines with
arrows) and passes through an aperture in plate 1340.
The excitation beam 1326 thereupon encounters a dichroic mirror
1349 adapted to reflect the wavelength of light represented by the
excitation beam 1326 into the excitation-emission beam lens 1358, where
25 the beam 1326 is decollimated and directed into the proximal end of
optic fiber 44, which may extend from the metal housing 41 if desired.
As stated above, the excitation beam 1326 travels through optic fiber 44
until it reaches the distal end, where it encounters a light trap 45,
e g. of black silicone rubber.
The evanescent wave portion of the excitation beam 1326 traveling
down the optic fiber 44 encounters the molecules of solute within
chamber 47 that are immediately adjacent the fiber 44 (i.e. within about
1/3 to 1/2 wavelength as discussed above) and, to the extent that it is
susceptible, excites the solute to emit fluorescence.
Such fluorescence passes into the optic fiber 44 and travels to its
proximal end as the emission beam 1327, where it e:cits the optic fiber
44, encounters the excitation-emission beam lens 1358 and is collimated.
.1,, ' :
13~1472
9 _
The beam 1327 then passes through the dichroic mirror 1349, which has
been fashioned to pass the wavelength of the light emitted by the
fluorescence of the solute to be measured.
The emission beam then impinges upon an electronic detection system
5 1323 such as a photomultiplier tube in counting mode. The detection
system 1323 is selectively responsive to the wavelength of the emission
radiation because a filter or monochromator is incorporated therein.
The electronic detection system 1323 generates 8 signal that is sent to
a lock-in ratio amplifier 1344. A reference detector 1350, which
10 detects the intensity of the excitation beam 1326, also generates a
signal that is sent to the lock-in ratio amplifier 1344, where the two
signals are processed conventionally to compensate for variations in the
intensity of the excitation beam 1326.
The signal from the lock-in ratio amplifier 1344 is transmitted to
15 an analog-to-digital convertor 1345, which generates a signal fed to a
digital display panel or signal processor 1346. Desir~bly an additional
digital signal 1352 from a conventional device reading bulk fluorescence - -(of the solution and any particulate matter in it) is generated and
amilarly fed to the display panel or processor 1346. The signal 1352
20 may, for example, be from a FluoromeasureTM fluorometer (BioChem
Technologies, Inc., Malvern, Pa). When the data in signal 1345 is
subtracted from the data in signal 1352, the resulting data sent to
element 1351 describes the fluorescence of the particulate matter, inas-
much as fluctuations in the fluorescence of the solute have been
25 subtracted from the fluorescence of the bulk.
An alternative embodiment shown in Figs. 6 and 7 utilizes an optic
fiber 62 within a housing 61 defining a chamber 67 having a pair of
ports 72, 73. The slurry to be subjected to optical measurement in
30 accordance with the present invention may be introduced through inlet
port 72 and exhausted through outlet port 73. Desirably the chamber 67
is designed so that flow therethrough is essentially laminar.
As in the embodiment previously described (Figs. 4 and 5), light
from a light source 1301 passing through a lens 1302 and filter 1303 is
35 introduced into the end of the fiber optic 62. However, to maximize the
evanescent wave relative to the radiation traveling straight through the
fiber optic 62, a plate 1340A, having an O-shaped aperture, with a
. ~ ,, .
'~
13~1472
- 10 -
filled-in center, may be used rather than one having a circular cutout.
This will block rays from entering the fiber optic 62 along the axis.
In this configuration, a reference detector 1350 is disposed along
a different path from the light source 1301 than that traveled by the
5 excitation beam 1326 so that variations in the intensity of the light
source can be detected and fed to a lock-in ratio amplifier 1344 as i8
conventional.
At the distal end of of the fiber optic 62, an electronic detection
system 1323 is placed to receive light traveling therethrough. The
10 detection system 1323 may be set up to detect the intensity of light of
the wavelength of the emission beam 1326. In that event, it will
generate a signal useful in determining the absorbance of the solute in
solution, free of cellular or other particulate matter. The absorbance
information may be related to the concentration of a solute that is to
15 be monitored, or it may be a background figure which may appropriately
be subtracted from another absorbance reading to provide useful data.
Alternatively the detection system 13Z3 may be set up with an
appropriate filter or monochromator to detect a wavelèngth of radiation
which is emitted by a solute as fluorescence, in which event the resul-
20 ting data will be similar to that generated by the embodiment of theinvention shown in Figs. 4 and 5.
Similarly to the previously described embodiment, the signal from
detection system 1323 is supplied to the lock-in ratio amplifier 1344,
and the output thereof is directed to a display panel or processor 1346,
25 the output of which may, for example, be fed to a chart recorder 1353 as
shown.
Using the evanescent wave phenomenon, the embodiment of the present
invention shown in Figs. 8 to 11 is capable of determining both
3û absorbance and fluorescence in such quick alternating succession as to
provide virtually simultaneous readings. With a flat plate 1316 as the
wave guide, the device housed in detector enclosure 1332 is particularly
adapted to be used in a reactor or fermentation vessel 1312 for real-
time determination of several variables which assist in determining the
35 instantaneous concentration of various components of the contents of the
vessel 1312.
, The detector enclosure 1332 is mounted within a conventional pipe-
~,, .
13~1472
11 -
like maunting port 1362 extending from the reactor vessel wall 1312.
The distal end of mounting port 1362 is threaded to mate with grommet
1333, which secures the enclosure 1332 to the port 1362 and thereby to
the reactor vessel wall 1312.
Oepending on the length of the mounting port 1362 and the depth to
which it is desired that the detector housing 1332 penetrate beyond the
vessel wall 1312 into the reaction mixture 1314, a rubber-like O-ring
1311 is interposed within any of three O-ring grooves 1361 to seal the
retainer sleeve 1354 of housing 1332 watertight within the port 1362.
A screw-threaded wave guide plate mounting sleeve 1336 mates with
the retainer sleeve 1354 and holds the wave guide plate 1316 securely in
place. An insert 1335, which may be one of optionally several lengths,
extends the wave guide plate mounting sleeve 1336 so that the wave guide
plate 1316 extends the deaired distance into the reaction mixture 1314
beyond vessel wall 1312.
A fluorescence enclosure 13ûa extends outwardly from the vessel
wall 1312, screw-threaded to the insert 1335. Enciosed within the
aforesaid elements are optical fiber elements 1305, 1317, 1319 and 1320,
which convey light to and from the wave guide plate 1316. The optical
flber elements 1305, 1317, 1319 and 1320 pass through fluorescence
enclosure cover 13û7, which is held in placs by a fluorescsnce enclosure
cover retainer bezsl 1309.
The light sourcs 1301, lens 13û2, filter 1303 and aperture 1340 ars
generally as have been described above. As shown schematically in Fig.
12 as well as generally in Fig. a, an excitation beam light chopper
1325 is interposed in the optical path to pass the focused excitation
beam 1326 to the evanescent wave excitation fiber optic 1305 and then to
the direct wave excitation fiber optic 1320 in alternating succsssion.
As shown more particularly in Fig. 9 the excitation beam light chopper
1325 comprises a disk 1328 having mounted thereon a semicircular mirror
13Z9, the other half of the disk 1328 having an opening 1342
sufficiently wide to allow the excitation beam 1326 to pass through to
the optical fiber 1320.
The chopper disk 1328 is rotated by a shaft 1330. As the disk 1328
rotates, the excitation beam 1326 is directed into two alternate paths.
When the excitation beam 1326 is incident on the mirror 1329, the beam
follows path 1326A directed to optic fiber 1305. Alternately, when the
X~
~3~t147Z
disk 1328 has rotated to a position where the excitation beam 1326 is
incident on the opening 1342, it passes through to the optical fiber
1320.
The optical fiber 1305 carries the excitation beam 1326A from the
5 sealed, light-tight housing 1331, to fluorescence detector enclosure
1332. The optical fiber 1305 typically consists of several individual
fibers completely surrounded by a flexible transparent cladding 1306 and
an opaque flexible sheath 1304. The refractive index of the
transparent cladding 1306 is slightly less than that of the optic fiber
10 1305.
After passing through grommet 1333, the individual optical fibers
of the optic fiber 1305 pass through a mounting block 1355 where they
are spread out, as shown in Fig. 10. Optic fiber mounting block 1355 ia
desirably injection molded of a plastic capable of withstanding
15 sterilization temperature of about 140 C, e.g. polysulfone.
The individual fibers of optical fiber 1305 that are in contact
with the prism 1310 along its oblique suface are cut square to the
longitudinal axis of the fibers. The mounting block 1355 holds the
fiber bundle 1305 such that the light enters at right angles to the
20 oblique surface of the prism 1310. The angle of the oblique surface of
prism 1310 to the side of the prism in contact with the flat plate wave
guide 1316 i9 such as to introduce the light beam 1326A into the flat
plate wave guide 1316 at an angle greater than the critical angle, so
that the light beam 1326A will be confined to the flat plate wave guide
25 and will generate an evanescent wave at the interface of the wave guide
1316 and the reaction medium 1314 in contact with the wave guide.
Prism 1310 is rectangular and has the same refractive index as the
flat plate wave guide 1316. The loss of intensity of excitation beam
1326 during its transition from the optic fiber 1305 to the prism 1310
30 is minimized by having the sides of the prism 131û greater than the
diameter of the optical fiber 1305 and by having the transitional
interface between the optic fiber 1305 and the slanted surface of the
prism 131û covered by a liquid 1324 having same rsfractive index as that
of the prism 1310.
The flat transparent side of the prism 1310 is cemented to the
distal face 1337 of the flat plate wave guide 1316 by utilizing a
transparent cement having a same refractive index as that of prism 1310
,
, .
~3~7~
- 13 -
and flat plate wave guide 1316. The actual angle of the oolique surface
of prism 1310 is a function of the refractive indiceo of the materials
uoed for prism 1310 and the flat plate wave guide 1316. The flat plate
wave guide 1316 is circular in cross section and is typically made of
5 bubble-free and distortion-free material such a~ quartz. The two
parallel faces 1337 and 133~ are optically polished to a high degree and
are truly parallel within the the normal manufacturing tolerances. The
cylindrical side wall of the flat plate wave guide 1316 is significantly
less in height than its diameter.
The flat plate wave guide 1316 i8 sealed along, its side wall by an
opaque seal 1356 of PTFE polymer (e.g. Tsflon*or the like) to prevent
loss of light and also act as a sealent. The frontal side 1333 of the
flat plate wsve guide 1316 is coated with a very thin and hsrd
transparent layer 1315 of material such as Surlyri~(Dupont), deposited
15 diamond etc. The thickness of the wave guide coating 1315 should be such
as to have no effect on the penetration of the evanescent wave 1339 into
the medium 1314. The wave guide coating 1315 is deoirable to
prevent the adherence of cellular products generated by the
particulate matter 1313 or the dirt present in the medium 1314. The
20 wave guide coating 1315 also prevents damage such as acratches to the
frontal side 133a of the flat plate wave guide 1316.
An evanescent wave 1339 is created within the flat plate wave guide
1316 when the excitation beam 1326 is repeatedly reflected between the
two non-mirrored surfaceo 1337 and 1338 reopectively. The evancent
25 wave 1339 w generated penetrates into the medlum 1314 under observation
through the frontal side 133~ of the flat plate wave guide 1316. As ex-
plained above, the evanescent wave only penetrateo a distance up to
about half of a wave bngth of the excitation beam 1326 into the medium
1314 under observation~ The discrete particulate matter 1313 such as
30 cells present in the medium 1314 has virtually no interaction with the
evanescent wave 1339.
There is a prism arrangement 1341 similar to prism 1310 at the
opposite end of the flat plate wave guide L316 along its distal side
1337 as shown in r igs. a and 12. The thickness of the flat plate .vave
35 guide and the distance between the prisms 1310, 1341 is such that the
incident light beam 1326A is refracted an integral number of times and
exits through prism 1341. The ends of the individual fibers of fiber
* Denotes Trade mark.
.
~,
:130~L~72
- 14 -
optic 1317 have been cut square to the longitudinal axis of the fibers.
The mounting block 1355 holds the fiber bundle 131? such that its
longitudinal axis is at right angles to the oblique surface of prism
1341.
As an alternate construction, the flat plate wave guide 1316 and
prisms 1310, 1341 may be fabricated as a single unit. Moreover,
alternatively to the relationship illuatrated herein, wherein the faces
of the prisms 1310, 1341 are raised above the surface of the wave gùide
1316, the oblique faces of the prisms may be recsssed into the surface
1û of the wave guide. As an additional alternative, two diametrically
opposite edges of the wave guide plate may be beveled to serve an
equivalent function to the oblique edges of prisms 1310 and 1341.
Individual fibers of the optical fiber 1317 are attached to an
oblique surface of the prism 1341, the transitional interface con9isting
L5 of a liquid film 1324 having a refractive index close to that of the
flat plate wave guide 1316. The optical fiber 1317 then passes through
the optic fiber mounting block 1355 and its cross section then becoming
circular. The optical fiber 1317 then passes through the grommet 1333
and enters .he source and detection housinq 1331. In direct path of
20 optical fiber 1317 as shown in Fig. 8 there exists a light chopper
assembly 1318 which is constructed similarly to excitation light chopper
1325. Next to the light chopper assembly 1318 in the ssme direction
there is an emission beam filter 1321 which eliminates all the
nonfluorescent light, followed by the emission beam lens 1322 which
25 focusses the emission beam 1327 on an electronic detection system 1323.
Fig. 12 is a schematic representation of the light psth in the
embodiment of the present invention shown in FIg. 8. The light beam
1326 from light source 13û1 is focused by a lens 1302 such that the
30 light is properly coupled to the optical fiber bundle~ 13û5 and 1320.
In coupling light to the optical fibers or wave guides, attention must
be paid to the numerical aperture ("NA") of the fiber optic or wave
guide. It is a matter of matching the lens 1302 to the NA and the
diameter of the fiber or fiber bundle or quartz wave guide.
Care must be taken to prsvent the fiber cladding from acting as a
wave guide. This can be achieved by sheathing the cladding with an
opaque sheath. To get uniform distribution of light, ths lens must
13~)1472
-- 15 --
confine the light uniformly across the input ~ace of the fiber or wave
guide. The light then passes through a slit or diaphragm and thence to
chopper 1325.
Chopper 1325 then, by alternately interposing and removing the
mirror 1329 from the excitation Ught beam 1326, divides the Ught beam
1326 into two psths, 1326A and 13268.
Beam 1326A travels through fiber bundle 1305 to prism 1310, which
couples the light beam properly to flat plate wave guide 1316 such that
the light beam is guided by multiple internal reflection through the
wave guide. The evanescent wave is absorbed by the solution components
able to interact with Ught of the selected wavelength. The evanescent
wave does not optically intersct with or excite to fluorescence the
psrticles 1313 suspended in the reaction medium 1314. Those solution
components able to fluoresce will emit their fluorescence at or nesr the
15- surface of the nat plate wave guide 1316.
A portion of the emitted light will couple into the wave guide and
be transmitted through light psth 1327A to chopper 1318. Light beam
1326B is transmitted via fiber bundle 1320 to the internal surface 1337
of the flat plate wave guide, which acts as a window; since the light
impinges at right angle to the surface, it goes right through and
illuminates the bulk suspension near the flat plate 1316, thus exciting
to fluorescence both the solution 1314 and the particles or cells 1313
suspended therein which are able to Quoresce.
A portion of the emitted fluorescence besm 1327B pssses back
through the flat plate 1316 and enters fiber bundle 1319 and is guided
to chopper 1318. Chopper 1318 is synchronized with chopper 1325 through
synchronizer 1348 such that when chopper 1325 is diverting the Ught
besm over psthway 1326A, chopper 1318 is configured to allow light
through pathway 1327A to go through filter 1321 and lens 1322 to
detector 1323. When chopper 1325 is diverting the Ught beam over
pathway 1326B, chopper 1318 is configured to allow light through pathway
13278 to go through filter 1321 and lens 1322 to detector 1323.
Synchronizer 1348 also serves to synchronize the signal processing
train with the chopper positions such that the signal processor is is
treating the signal as is appropriate to the mode of generation of the
signal, e.g. determination of evanescent wave fluorescence vis-a-vis
determination of bulk fluorescence.
... .. .. . ..
~3~1472
- 16 -
The signal from the det&ctor 1323 and from synchronizer 1348 is
fed, for example, to an AC to OC converter and linearizer 1343, then to
a lock-in ratio amplifier 1344, where the amplitude of the aignal i8
corrected for variations in the amplitude of the excitation beam
detected by reference detector 135û, then to an analog-to-digital con-
verter 1345, then to a digital dlsplay or signal processor 1346 and then
to a digital storage or memory 1347.
if filter 1321 is constructed to pass the emitted fluorescence
wavelength, then the device of the present invention measures
fluorescence. If filter 1321 is constructed to pass the same wavelength
as the excitation beam 1326, then the device measures the optical
absorbance of the solution 1314.
In the event that it is desired that only fluorescence and not
optical absorbance be measured, an alternative embodiment (not shown)
may omit the chopper 1318 of Figs. 8 and 12. In that event, light beam
1327A is merely trapped rather than being guided to frlter 1321 and
detector I323. Fiber optic 1317, in such an embodiment, may be omitted
and replaced with a light trap, or fiber optic 1317 may itself channel
the light away from the flat plate wave guide 1316 as a light trap.
30th the bulk fluorescence excited by light beam 13263 and the solution
fluorescence excited by light beam 1326A generating an evanescent wave at
the surface of the flat plate wave gude 1316 are transmitted over light
path 1327B to fllter 1321. Filter 1321 is ~elected to allow only the
emitted fluorescent wavelength to pass through.
fig. 13 illustrates yet another embodiment where the contents of
housing 1360 are substituted for the contents of housing 1331 of the
embodiment of Fig. 8. This embodiment is suitable for slow speed
chopping of the light beams, for example, a few hertz or even fractions
of a hertz. The chopping device viewed in the direction of arrow 14 i8
shown in Fig 14. A flat plate 1a2 is affixed to a shaft 1a1, which is
attached to a speed reducer clutch 183. The clutch 1a3 is attached to
the shaft of reversible motor 184. A flat mirror 171 i8 attached to
plate 182.
The plats 182 with mirror 171 attached is pivoted to swing between
the position shown in r i9. 13 with solid lines or alternately the posi-
tion shown with dashed lines. A stop 172 limits the travel of mirror
~'~3
- 17
171 as the plate 182 abuts it and acts as a rigid point fixing the
position of the mirror precisely again and again. Mirror 173 is simi-
larly mounted for reciprocation and synchronized with mirror 171,
generally as described with respect to the embodiment of Figs. 8 and
5 12.
List of Illustrated Elements
40 Dipstick
10 41 Fluorescence Detector Metal Housing
42 Optic Fiber Opaque Sheath
43 Optic Fiber Transparent Cladding
44 Optic Fiber
45 Light Trap
15 46 Apertures in Housing
47 Chamber in Housing
61 Housing
62 Optic Fiber
64 Cladding
20 65 Sheath
66 Liquid Medium in Chamber
67 Chambsr
72 Inlet Port
73 Outlet Port
25 171 Swinging Mirror Frame
172 Rubber-cushioned Stops (Restrict travel)
173 Mirror
181 Electric Motor Shaft
182 Counter weight
30 183 Friction clutch to prevent over torquing mirror
184 Low torque reversible electric motor
1301 Light source
1302 Excitation Beam Lens
1303 Excitation Beam Fiter
35 1304 Opaque Sheath of a typical optic fiber
1305 Excitation Fiber Optic--Evanescent Wave
1306 Optic fiber transparent cladding
!
'' ''
130147Z
-- 18 --
1307 Fluorescence Enclosure Cover
1308 Fluorescence Enclosure
1309 Fluorescence Enclosure Cover Retainer Bezel
1310 Excitation Beam Prism
1311 O-ring
1312 Reactor Vessel Wall
1313 Particulate Matter .
1314 Reaction ~edium
1315 Evanescent W ave Guide Coating
1316 Flat Plate Evanescent Wave Guide
1317 Emission Fiber Optic--Evanescent Wave
1318 Emission Beam Light Chopper
1319 Emission Fiber Opti~l)irect Wave
1320 Excitation Fiber Optic--Direct Wave.
1321 Emission Beam Filter
1322 Emission Beam Lens
1323 Electronic Detection System
1324 Liquid Separator between prisms and Excitation fiber optic and
Emission Fiber Optic--Evanescent Wave
1325 Excitation Beam Light Chopper
1326 Excitation Beam
1327 Emission Beam
1328 Disc (Typical Design) for Light Choppers
1329 Light Chopper ~irror
1330 Light Chopper Motor Shaft
1331 Source ~ Detector Housing
1332 Fluorescence/Absorbence Detector Enclosure
1333 Grommet
1334 Fluorescence Detector Retainer Flange
1335 Insert (which may be of various sizes) for wave guide plate
position adjustment
1336 Wave Guide Plate ~qounting Sleeve
1337 Distal Side of the Wave Guide Plate
1338 Frontal Side of the Wave Guide Plate
1339 Evanescent Wave
1340 Plate with Aperture
1341 Emission Beam Prism
:~
~ ' . ' . .
.
~3V~47Z
-- 19 --
1342 Opening in Light Chopper Disk
1343 AC to DC Convertor snd Linearizer
1344 Lock-ln Ratio Amplifier
1345 Analog to Digital Convertor
5 1346 Digital Display Panel or Signal Processor
1347 Digital Memory Storage .
1348 Chopper Synchronizer
1349 Dichroic Mirror
1350 Reference Detector
10 1351 Pluorescence of cells corrected for medium fluorescence
1352 Digitized Signal from Bulk Fluorescence Detection Device
1353 Strip Chart Recorder
1354 Retainer Sleeve
1355 Optic Fiber Mounting Block
15 1356 Wave Guide Edge Seal
1358 Emissio~Excitation 8eam Lens
1360 Source snd Detector Housing
1361 O-ring groove
1362 Mounting Port
~' ; s ~,1 !
,