Note: Descriptions are shown in the official language in which they were submitted.
~3(~Zl3~)5
-- 1 --
LIQUID FILM CQATING OF_IRON-BASED METALS
This invention pertains to apparatus; and
processes for protective coa~ing of iron-containing
metals such as processes and apparatus for the
continuous hot-dip galvanizin~ of iron-based sheet
metal.
The corrosion of iron-based metals can be
mitigated by coating the metal with a protective
metal coating material, i.e.~, an anodic or cathodic
metal such as zinc, tin, aluminum, lead, or mixtures
or alloys thereof. Anodic materials ~uch as zinc
are sacrificial and thereby provide corrosion
protection to the underlying substrate whereas
cathodic materials typically serve as barrier
layers. The deposition of these metals on an
iron-based metal substrate is herein referred to as
a "protective metal coating process". The
protective metal coating process can be conducted by
immersing the substrate into a vessel containing the
molten protective metal coating material for the
coating or by spraying or otherwise applying a
liquid film of the protective metal coating material
on the substrate. These types of processes are
herein referred to as "liquid film coating"
processeS.
Galvanizing (zinc coating) is a widely
practiced process for liquid film coating and is
conventionally practiced by immersing the metal
substrate into a vessel containing molten zinc and
then removing the metal substrate from the vessel to
effect the coating ("hot-dip" process). Typically
in continuous processes for galvanizing sheet metal,
D-15,331
13VZ~g~S
-- 2 --
the sheet metal is removed vertically from the molten zinc
and passed over a tower roll which enables the movement of
the sheet ts be redirected. In these processes, the tower
roll may be positioned about l~ to 80 meters above the
vessel containing the molten zinc. This distance is
selected on the basis of the time required, under the rate
of movement of the sheet, for the zinc coating to solidify
sufficiently so that the zinc does not transfer to the tower
roll. In general, the zinc or other protective metal
coating material contacting the roll may bç molten,
semi-solid, or solid. Even when solid, but while still hot,
the protective metal coating material can transfer to a roll
since the full strength of the coating has not developed.
- That is the coating may be characterized as being in a
1~ plastic state and is subject to being transferred to a roll
surface.
Further information about applying protective metal
coatings can be found in "THE MAKING, SHAPING AND TREATING
OF STEELn, Tenth Edition, 1985, Association of Iron and
Steel Engineers/United States Steel Corporation.
With the currently emphasis on corrosion resistance
in the automotive and other industries, protective metal
coating processes, especially galvanizing, are sought-after
treatments for iron-based materials for fabrication. In
many such applications the protective metal coating must be
painted and the resulting finish must be very smooth to meet
the approval of the ultimate consumer.
D-15,331
13l~?Z80S
-- 3 --
Unfortunately, galvanized finishes are characterized
by crystal structures that provide large relief
patterns, referred to as "spangles", which ar~
difficult to paint without their crystal pat~ern
showing through the paint.
Recently a process modification referred to
as galvannealing has found acceptance in providing
galvanized coatings having a substantial absence of
spangles as well as superior mechanical properties.
In the galvannealing process, the zinc-coated
substrate exiting the molten zinc bath is heated for
a sufficient time to enable a zinc-iron alloy to be
formed. The alloy has a relatively uniform matte
finish, that can readily be painted, providing a
finish o an acceptable quality to a discriminating
consumer.
Difficulties exist when adapting the
conventional galvanizing processes to utilize the
galvannealing technique. As stated earlier, the
distance between the vessel containing the molten
zinc and the tower roll is selected such that the
zinc coating is solidified sufficiently prior to the
contact of the sheet with the tower roll that a
transfer of the zinc to the tower roll surface does
not occur. The installation of an intervening
galvannealing unit results in shortening the
distance that cooling can occur before the sheet
metal contacts the tower roll. If the normal
production speed is maintained, then the zinc does
not sufficiently solidify prior to contacting the
tower roll. This contact has been found to
adversely affect the guality of the finish. For
D-15,331
~3V28~S
instance, deposits of zinc develop on the tower roll
and cause a marring of the sheet metal surface or
even a perforation of the sheet surface.
Proposals to eliminate the deposits-o~ the
tower roll have included cooling the tower roll,
externslly with water or internally with water or a
glycol solution. 8y cooling the tower roll,~it was
thought that the cooler surface would have less
affinity for the zinc and that the chilling would
result in the spalling of any transferred zinc
particles from the roll. This procedure proved to
be unsuccessful. Pick-up still occurred, and
variations in the temperature over the surface of
the roll resulted, in some instances, in buckling or
warping of the sheet metal. Direct spraying of the
galvanized sheet metal also proved to be
unsuccessful due to temperature differentials that
cause the sheet metal to buckle or warp. Another
proposal has been to scrape the surace of the tower
roll with a blade to remove any accumulated zinc.
This approach has not been effective in adequately
removing the zinc and the problems continue. It is
also possible to reduce the rate of production of
the galvanized metal or to modify the eguipment by
further elevating ~he tower roll to allow for
sufficient cooling to prevent zinc transfer. The
first alternative is unattractive due to the reduced
production capacity and the latter alternative
suffers from substantial capital costs in revamping
existing facilities.
The most common practice has been the
periodic maintenance and/or replacement of tower
D-15,331
.
13~Z80S
rolls~ However, because of the locstion of the
tower rolls and the he~t in the vicinity of the
tower rolls due to thelr locstion in the m~ll, this
procedure is difficult, time consuming, ~nd results
in lost production and spotty quality.
Summ~ry of the Invention
By this invention processes and apparatus
have been provided that ensble iron-based metals
having protective metal coatings applied thereon by
a liquld film coating technique to contact rolls
such ss tower rolls, conveyor rolls, guide rolls and
the like while the protective metal cost~ng material
has not yet eooled or solidlfied sufficiently to
avoid transfer of the protective metsl coating
materisl to ordinary roll surfsces wi~hout transfer
of m~terisl so thst acceptsble finishes can be
obtsined i.e., undue amounts of the protective metsl
coatings do not transfer to the rolls. Accordingly,
conventional galvanizing mills can be modified to
lnclude a galvannealing unit yet still use the
ex~sting tower roll configuration nd production
rates when using this invention. This invention is
also useful ln other types of mills.
In accordance with this invention the rolls
to contact the protective metal coating material,
comprise ~ body defining 8 surface for contact with
the protectlve met~l coated metsl which body is
~dapted to be rotated ~round its axis wherein at
least thst portion of the surface intended to
contsct the metal is a ceramic surfsce or barrier
system. The ceramic surfsce or barrier i8 8
refr~ctory oxide ~nd hss a thickness of st least
D-15,331
13(~28~S
about 20 microns. Advantageously, the roll can be
used ln cpparatus for the llquld film coating of
sheet metal in a contlnuous manner.
Thls lnvention fllso relstes to processes
for the liquid film coating of metal in which the
metal ~s contacted with molten protective ~etal
coatlng material to provlde a coating of the
protective metal coating material on the metsl, and
there~fter contacting the metal wlth a roll in
accordance with this inventlon for purposes of
directing the metal in a deslred dlrectlon, said
contact occurring while the protective metal coating
is capable of transfer.
In another aspect of the invention, the
protective metal coated material contacts a
continuous conveyor and the contact surface of the
conveyer is a ceramic surface system. The conveyor
may comprise narrow strips that are substantially
perpendicular to the movement of the conveyor which
strips are movable in respect to one another or a
loose woven mesh.
As stated above, the protective metal
coating may be capable of transfer to a surface when
it is in a liquid or even solid state, i.e. when it
has not yet cooled or solidlfied sufflciently or it
can be said that the protective metal coating is in
a viscous o~ plastlc state.
The mechanism of transfer of the protective
metal coating mater~al to the tower roll is not well
understood and ls probHbly dependent on the specific
composition of both the coating and the surface of
the tower roll. The temperature of the protective
metal coating material in particular is very
D-15,331
13~Z8C~5
important. The protective metal costlng material,
8S it first comes in contact with ~he tower-roll
surfsce ls ususlly below its solldus tempera*ure,
but may be between the solldus and li~uidus
temperstures in some lnstances; i.e., part of the
msterial may be solid and part liquid. In elther
event, the materlal is ~n a highly plsstic or
viscous state snd is easily transferred to the roll
surface. Transfer may occur ~s the result of either
adhesion or abrasion. Adhesive trsnsfer occurs when
a chemicsl bond forms between the protective metal
costing and the tower roll surface which ls stronger
thsn the internal cohesive strength of the coating
or the bond of the coating to its substrste.
Abrasive transfer may occur when an asperity, harder
than the protective metsl coflting, scoops out
coating material. The tendency for sny of these
mechsnisms to operate diminishes BS the temperature
of the coatlng material decreases because the
strength of the coating increases with decreasing
temperature. Once ~ small amount of protective
met~l coating material hss tr~nsferred to the tower
roll surface, additional materisl may build-up on
this transferred material, eventually forming large
lumps which may damage the coated sheet material.
Detailed DescriPtion of the Invention
The rolls of this invention can be used in
a variety of applications in a number of protective `
metal coating processes. The liquid film coating
processes include hot-dip processes and spraying
processes. In hot dip processes, the metal to be
treated is immersed into a vessel containing molten
protectlve metal coating material and is withdrawn
D-15,331
13(~Z~3OS
- 8 -
in a generally upward direction. Most frequently in
continuous processes, the metal is withdrawn
vertically and passes to a tower roll. The metal is
then redirected and passes over various rolls in a
further cooling section after which it may be
subjected to further treatments or packaged for use.
Another type of hot-dip process involves
removing the metal from the vessel to a
substantially horizontal conveyor for transporting
and cooling. This process is often used when
applying the protective metal coating material to
pieces of metal rather than continuous sheets of
metal. The conveyor system may comprise rollers in
accordance with this invention or a continuous
conveyor in accordance with this invention.
In the spraying process, the molten
protective metal coating material is sprayed to
contact the metal substrate. Often when using the
spraying process, the protective metal coating
material solidifies immediately upon contact with
the coo~ler metal substrate. However, in such
situation, this invention can still be useful if the
protective metal coating material is capable of
transfer.
The most commonly used protective metal
coating materials include zinc, aluminum,
aluminum-zinc alloy, and aluminum-silicon alloy
although tin, terne metal (lead and tin), copper and
copper alloys can be applied using the liquid film
coating technique. The me~al substrate is an
iron-based metal and is often cast iron or steel and
has a sufficiently high softening temperature that
D-15,331
13~280S
g _
it is not adversely affected by the temperatures
required for the application of the molten
protective metal coating material. The form o-f the
metal substrate may vary depending upon the ul~imate
need. For instance, the substrate may be in the
form of a continuous sheet,,wire or screen or it
could be in the form of the final product such as a
molded part or a cast article.
The protective metal coating material for
the application of the liquid film to the metal
substrate is at a temperature to provide the desired
rheological properties for forming a coating of the
desired thickness. The temperature range will vary
depending upon the nature of the protective metal
coating material. However, temperatures should be
avoided at which the metal substrate becomes unduly
adversely affected. The nature of the protective
metal coating material can also be affected by the
time of contact with the molten protective metal
coating material in a hot-dip process.
The cooled substrate may be further heat
treated by maintaining the substrate in a heating
zone under temperatures for chemical interaction or
recrystallization. For instance, the heating in
galvannealing p~rmits chemical interactions to occur
between zinc and iron. The ~emperature and duration
of the heating will vary depending on the desired
result.
When the protective metal coating materials
are characterized by spangles such as galvanized
coatings, the liquid ~ilm coating may be contacted
with a nucleating agent which promotes the formation
D-15,331
~3~Z805
- 10 -
of smaller crystsl structures, l.e., mlcrosp~ngles.
For example, cQmmercisl gslvsnizing processes exist
ln which the metsl removed from the molten-zinc ls
sprayed with finely-divlded zinc to provide
nucleation sltes.
The protective metal coatlng mate~rial, when
contacting the rolls ~n accordsnce with this
invention, ls often st B temperature at which the
protective metal coating m~terial has begun to
solidify. In some instsnces, the protective metal
coating material will be semi-solid or in the solid,
but plastic state, and wi$1 be cap~ble of
transferring protective metal coating materlal to an
iron surface upon contact.
At least the portion of the lsteral surface
of the roll that is to contsct the co~ted metal
substr~te ls a refractory oxide having 8 rel tively
low thermal conductivity such as ~lumina, magnesia,
zirconia, chromis, titsnia, silics, and the like and
mixtures thereof. The preferred oxldes exhibit a
good thermal shock resistance. The refractory oxide
often exhibits a thermal conductivity st 100C of
less than about 0.1, preferably less than about
0.01, csl/(sec x cm x C), snd frequently hss a
coefficient of thermal expsnsion of less than about
1 x 10-5 per C. Zlrconi~ surfaces sre often
desir~ble because of the combination of mechanical
strength, shock resistance, and low thermal
conductlvity. Most preferably, the surface is an
yttri2 stsbllized zirconia, i.e., zirconia
containing sbout 6 to 10, ssy, ~bout 8, weight
percent yttris.
D-15,331
13~ S
The Drawinqs
Figure 1 is a schematic depiction of a
cross-section of a hot-dip galvanizing apparatus
having a galvannealing section and a tower rol~ in
accordance with the invention.
Figure 2 is a schematic depiction of a
tower roll in accordance with this invention'.
Figure 3 is a schematic depiction of a
break-away section of the surface of a tower roll in
accordance with this invention.
Figure 4 is a schematic depiction of a
horizontal galvanizing mill using a conveyor in
accordance with this invention.
With reference to Figure 1, vessel 100 is
externally heated and contains molten zinc 102.
Roll 104 is positioned below the surface of the
molten zinc 102 and is adapted to receive sheet
metal 106. Generally the sheet metal has been
pretreated to facilitate the galvanizing process.
These pretreatment processes include annealing,
chemical cleaning (e.g., with sulfuric acid), flame
cleaning or combinations thereof.
The sheet metal 106 passes underneath roll
104 and is directed vertically from vessel 100.
Above vessel 100 and on both sides of the sheet
metal are air knives 108 which serve to remove
excess molten zinc from the sheet metal.
The sheet metal 106 may then passes through
a galvannealing unit 110. The qalvannealing unit
may be gas fired or electrically heated to a
temperature sufficient to enable a zinc and iron
alloy to form. This alloy provides a matte finish
D-15,331
13~Z~1'5
- 12 -
rather than macrospangling associated with zinc
coatings. This zinc and iron alloy generally forms
as a solid. The sheet metal 106 may then contact a
guide roll 112 and then tower roll 114 where-i~ is
redirected horizontally and is typically fed into a
cooling tower section (not depicted) of the mill.
The cooling tower section may contain a number of
rolls for supporting the sheet metal and moving the
sheet metal to further processing. Although the
zinc and iron alloy may be a solid, it can still be
capable of being transferred.
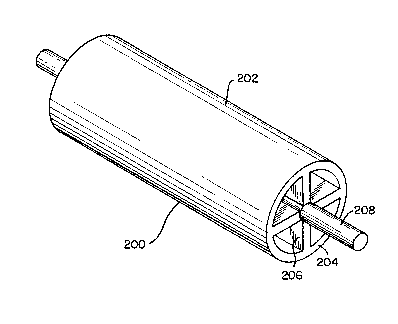
With reference to Figure 2, a tower roll
200 is generally shown. The tower roll has lateral
surface 202, annular support structure 204, and
spokes 206 which terminate at drive shaft 208.
Drive shaft 208 may be adapted for mechanical
communication with a motor for the purposes of
rotating the drive roll at a desired speed to move
the sheet metal. In some mills, however, the tower
roll is not driven.
Figure 3 illustrates an embodiment of the
invention wherein the refractory oxide at the
lateral surface of the tower roll is provided as an
overlay or coating 302 over an intermediate overlay
or coating 304 which improves the bonding and
thermal shock resistance of the refractory oxide
overlay on the tower roll. The intermediate overlay
is shown as being bonded to a metal substructure 306
which can provide the form of the tower roll 200 as
shown in Figure 2.
With reference to Figure 4, iron-based
articles 400 are transported by conveyor 402 having
D-15,331
13~2~3QS
- 13 -
drive roller 404 and end roller 406 into molten zinc
408 contained in vessel 410. Articles are removed
from vessel 410 by conveyor 412 having drive r411er
414 and end roller 416. Both conveyors 402 and 412
are constructed of steel mesh. Articles 400 are
then passed to conveyor 418 having a loose
interlocking, wire mesh structure as depicted in the
inset. The conveyor is fabricated of steel having a
refractory oxide overlay. Conveyor 418 is powered
by drive rolls 420 and 422.
O_er_aYs
The rolls in accordance with this invention
preferably have an overlay of a refractory oxide
material and have a mechanically strong and
relatively inexpensive substructure, e.g., an iron
or steel substructure. The refractory oxide overlay
need not be thick in order to obtain the benefits of
the invention. Often the thickness of the overlay
is about 25 to 700, say, about 50 to 500, microns.
The overlay may be applied in any
convenient manner and commercial services exist for
applying refractory oxide overlays. The refractory
oxide is typically applied through the use of a
thermal spray process such as the plasma or
detonation gun techniques. The refractory oxide,
when applied by the plasma process, is typically
provided in the form of a finely divided powder,
e.g., in the range of about 5 to 100 microns in
average particle size. The application of the
refractory oxide with the plasma process is
desirably sufficient to provide a coating density of
at least about 80 percent, and often at least about
D-15,331
13~B~S
- 14 -
8S to 88 percent. The density is achieved by
adjusting the gas flow, gas composition, amperage,
voltage, torch to work distance and the like as is
commonly practiced in the industry. The specific
parameters that are used will vary with the design
of the plasma torch used for the deposition.
Although plasma spray technigues such as
disclosed in U.S. Patent Numbers 2,858,411 and
3,016,447 and detonation gun techniques such as
disclosed in U.S. Patent Numbers 2,714,563 and
2,950,867 have been mentioned as possible methods of
deposition of the overlays, it should be recognized
that other thermal spray techniques can be used as
well. These include the so-called "high velocity"
plasma and "hypersonic" combustion spray processes
as well as various flame spray processes. These and
similar techni~ues are part of the "thermal spray"
family of deposi~ion technologies. Other
technologies such as physical vapor deposition or
chemical vapor deposition may also be applicable.
The oxide overlay may or may not have an
undercoating. Undercoatings, for instance, composed
of nickel, iron or cobalt based alloy with
resistance to oxidation, ~an often provide enhanced
bond strength and improved thermal shock
resistance. Particularly useful undercoating
materials include nickel-aluminum or nickel-chromium
alloys and the MCrAl and MCrAlY alloys in which M is
nickel, cobalt, iron, or any combination thereof.
Alternative undercoats that may be used consist of a
mixture of metals and oxides, or graded structures
that consist of a first layer of pure metal with
D-15,331
13~)~8~S
continuous or discontinuous additions of oxide with
increasing volume fraction of oxide toward the outer
surface.
The undercoatings can also be applied-using
suitable processes, e.g., the thermal spray process
such as the detonation gun and plasma techniques.
The undercoating, when used, frequently has ~
thickness of at least 20 microns, e.g., between
about 20 to 500, say, about 50 to 250, microns.
When an undercoat is used, it is preferred
that it have sufficient roughness to enhance the
bonding to the refractory oxide overlay. Regardless
of whether the steel superstructure is to be
undercoated its surface should be cleaned and
preferably roughened, e.g., by grit blasting.
Once the refractory oxide is applied, it is
generally desired to finish the surface to produce a
smooth surface. This finishing can be accomplished
by any suitable means such as grinding, belt
sanding, honing, and the like. A surface finish of
less than 20 microinches rms is preferred.
The following examples are provided to
further illustrate the invention and are not
intended ~o be in limitation thereof.
EXAMPLE 1 (comParative)
A tower roll having a diameter of 60 inches
(1.524 meters~ with an 84 inch (2.134 meters) wide
lateral surface and constructed with steel was
overlayed (coated) to a thickness of 75 to 100
microns with a chrome carbide-nichrome overlay
tCr3C2+20(Ni-20Cr)] (prefix numbers refer to
weight percent) applied using a detonation gun. The
D-15,331
13~ S
- 16 -
overlay was finished to 6 to 10 microinches rms.
The tower roll was used in a galvanizing mill having
a galvannealing unit and is similar to that depicted
in Figure 1. The distance between the molte~ zinc
surface in the hot-dip vessel to the tower roll was
about 30 meters and the distance from the top of the
galvannealing unit and the tower roll was about 18
meters. The galvannealing unit was about 3 meters
above the molten zinc surface. Only ambient cooling
was provided between the top of the galvannealing
unit and the tower roll. The galvannealing unit was
not being operated over the entire duration of the
test using this tower roll. Rather, over some
periods of time, the mill was producing the standard
spangled product. After nine days pickup was
visible on the entire roll face in the form of
pinhead size zinc spots with smeared tails in the
direction of strip travel. After an additional
three days of operation, massive buildup on the roll
had occurred. Attempts were made to remove the
buildup using 120 grit al~minum oxide sandpaper with
very little success. The roll face temperature was
measured during operation and found to be about
980F. The roll was removed from service after
about 39 days of operation. This illustrates the
unsatisfactory performance of a state-of-the-art
conventional overlay.
EXAMPLE 2
A steel roll having a 5 inch (12.7 cm.)
diameter and an 84 inch (2.134 meters) lateral
surface was undercoated with a plasma deposited
MCrAlY coating having a composition of
D-15,331
i3~ S
- 17 -
32Ni-21Cr-8Al-O.SY-~alance Co with a thickness of
about 75 microns. An overlay of an yttria-
stabilized zirconia (ZrO2-8Y203) was deposited-
by plasma to a thic~ness of 325 microns. The S
surface was finished to less than 20 microinches rms.
The roll was placed in the same facility as
the tower roll in Example 1 at a position
immediately below the tower roll. The roll was held
against the sheet metal at a force comparable to or
slightly higher than the force of the sheet metal on
the tower roll. When first placing the roll into
service, a tendency to pick-up zinc on the surface
was observed. Even 80, the transferred material did
not appear to agglomerate to such a size that the
quality of the finish on the metal contacting the
surface of the roll was deleteriously affected.
After further use of the roll, zinc no longer
appeared to collect on the roll, and in fact, that
zinc which transferred to the surface of the roll
seemed to be lost. After a period of six months the
roll was removed from service with no evidence of
zinc pickup and little or no wear on the roll face.
There was some grooving at the edge of the strip
because the roll had been performing so successfully
it was used to guide this strip across the tower
roll by applyin~ more pressure to one edge than the
o~her. During this period of service a variety of
strip product was run, including standard-spangled
and galvannealed.
D-15,331