Note: Descriptions are shown in the official language in which they were submitted.
~3~
--1--
PROCESS FOR PREPARING PIGMENTARY
TITANIUM DIOXIDE
This lnvention relates to a process for
preparing pigmentary titanium dioxide from titanium
dioxide-bearing materials. More particularly, it is
desired to provide a simplified and relatively less
expensive process for preparing a TiO2 pigment whereby
the titanium values in titanium dioxide-bearing
materials or ores are not solubilized or converted to a
vaporizable liquid compound, but are separated, through
solid-liquid reactions, from the ore's impurities and
mechanically comminuted to pigmentary size.
The process provides a means of obtaining a
TiO2 pigment which has either a nodular or a~ acicular
particle shape.
- Titanium dioxide (TiO2) is a well-known
opacifying pigment useful in paint and coating
compositions, in plastic materials and as a filler in
paper and other materials. Various known processes for
producing TiO2 include, for example/ conventional
processes commonly referred to as the "sulphate"
process and the "chloride" process.
30,371B-F
~3V;~3~
--2--
The "sulphate" process involves solubilizing
the titanium values in low grade titanium ores, such as
ilmenite or sorelslag, with concentrated sulfuric acid
and meticulously removing ferrous sulfate formed in the
process. This is followed by precipitation, washing,
and calcining to form pigmentary TiO2.
The "chloride" process involves volatilizing,
as tetrahalide, the titanium values in high grade
titanium ores, such as Australian rutile (containing
about.95 percent TiO2) or highly beneficiated ilmenite.
This is followed by purification and oxidation.
The sulphate and chloride processes are very
complex and capital intensive which accounts for the
relatively costly product of TiO2 pigment made by such
processes.
The present invention is therefore directed to
a process for preparing a titanium dioxide pigment
comprising the steps of:
(a) comminuting a titanium bearing material to
a article size of less than 15 microns;
(b) mixing said titanium dioxide bearing
material with an alkali metal compound selected from
alkali metal hydroxide, alkali metal carbonate and
alkali metal oxide prior to, during or after said
comminuting, the ratio by weight of said titanium
dioxide bearing material and said alkali metal compound
being from 100:30 to 100:60;
(c) roasting the mixture of step (b) at a
temperature of from 700C to 950C;
30,371B-F -2-
3~P
2a-
(d) digesting the roasted material of step (c)
in hydrochloric acid for a period of time of from 10 to
120 min; and
(e) calcining the solid product formed in the
digesting step (d) at a temperature of from 800C to
1000C to form a titanium dioxide pigment.
;~
~ 30,371B-F -2a-
.. . .
~3~
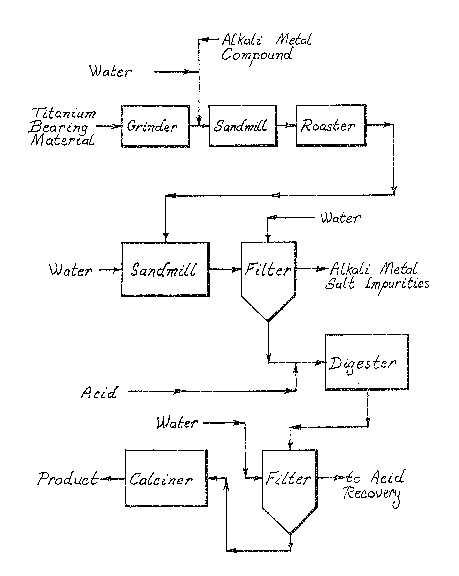
Figure 1 is a schematic flow diagram of one
manner in which the process of the present invention
can be carried out.
The starting material for the process of the
5 present invention is a titanium bearing material, for
example, sorelslag. Various grades of sorelslag may be
used in the present process. For example, the
composition of one typical grade of sorelslag,
expressed as oxides, may consist of approximately 70
weight percent TiO2 and approximately 11 weight pe~cent
FeO as an impurity with the remainder being impurities
including, for example, CaO, MgO, SiO2, Al2O, MnO~ V2O5,
Cr2O3 and other oxides as trace impurities. Another
grade of sorelslag useful in the present process may
consist of approximately 78 weight percent TiO2,
approximately 8 weight percent FeO as an impurity, and
the remainder impurities such as those listed above.
It is to be understood that the present
invention is not limited to sorelslag. Other titanium-
bearing materi~ls or ores as starting materials for thepresent invention are within the scope thereof. For
example, "chloride slag" may also be used in the
process of the present invention. A typical chloride
slag may consist of approximately 85 weight percent
TiO2, approximately 10 weight percent FeO as an
impurity and the remainder impurities such as those
listed above.
Another suitable raw material for the process
of ~he present invention can be an intermediate product
--- 30,371B-F _3_
formed during a beneficiation process of ilmenite such
as that described in U.S. Patent No. 3~825,41. A
typical raw material formed during the beneficiation
process above may consist of approximately 95 weight
percent TiO2, approximately 1 weight percent FeO as an
impurity and the remainder impurities such as those
listed above.
Examples of other titanium bearing materials
which can be used in the process according to the
present invention are any titanium-bearing materials
which are so treated that the titanium dioxide portion
thereof becomes reactive with an alkali metal compound
when heated at to a temperature of from 700C to 950C.
An alkali metal compound, as used herein includes, for
examplel an alkali metal hydroxide, an alkali metal
carbonate, or an alkali metal oxide or mixtures
thereof.
All of the equipment used in the process of the
present invention for grinding, mixing, roasting,
filtering and calcining and all other operations are
carried out by conventional equipment suitable for the
purpose of continuous or batch type operation. For
comminuting the titanium bearing starting material to
micron size it is preferred to use "sandmills" of the
type described and illustrated, for example, in U.S.
Patent No. 2,581,414. A "sandmilling" process will
refer herein to a process of grinding a material to
micron particle size using the type of equipment
~ described and illustrated, for example, in U.S. Patent
No. 2,581,414. However, the grinding media used in
such equipment is not limited to sand, but can be
35. glass, steel, ceramic or any other suitable grinding
30,371B-F 4-
p
~5--
media having a spherical or bead shape, generally, in
the range of from 0.5 to 3 millimeters in diameter.
The digesting step has to be carried out in
vessels with inner surface portions or linings
resistant, under normal operating conditions, to the
acid utilized in the process. Suitable materials for
such surface portions are, for example, glass, FRP
~glass fiber reinforced polymeric composites),
polyester, vinylester, epoxy and other suitable
plastics, Hasteloy (Ni/Mo alloy), rubber, refractory
metals (Ta, Zr, Cb) or acid resistant brick.
~ccording to a preferred embodiment of the
present invention, the particle size of the titanium
bearing material should be small enough for all or
substantially all of the material to react with an
alkali metal compound. With reference to Fiyure 1, the
titanium bearing starting material is first ground, for
example, by hammermilling to a size suitable for
sandmilling. Hereinafter, the process of the present
invention will be described with reference to sorelslag
as the titanium bearing material but, as afore-
mentioned, the material is not limited to sorelslag.
After the hammermilling step, the sorelslag ispreferably sandmilled to an average particle size of
about 15 microns , more preferably, a particle size of
about 10 microns. Even more preferably, the startin~
material may be sandmilled to a maximum particle size
of 10 mi¢ron or less.` Particles larger than about
15 microns may occlude impurities which may not be
` readily removed from the starting material in the
subsequent process steps of the present invention.
30,371B-F -5-
1 ~ ~3
--6--
After the sorelslag is ground to the preferred
particle size, it is intimately mixed with an alkali
metal compound such as, for example, an alkali metal
hydroxide, an alkali metal carbonate, an alkali metal
oxide or mixtures thereof. Alkali metals such as
sodium, potassium~ lithium, rubidium, cesium or
mixtures thereof may be used. The preferred compound
is an alkali metal hydroxide, and more preferably,
sodium hydroxide because it is readily reactive with
the finely ground sorelslag material. Hereinafter, the
process of the present invention will be described with
reference to sodium hydroxide as the preferred alkali
metal compound but it is understood that the present
invention is not limited thereto.
Sodium hydroxide may be mixed with the
sorelslag material prior to roasting and preferably
during the sandmilling step above or, alternatively,
prior to the sandmilling step. The mixture of
sorelslag and sodium hydroxide can contain about 30
parts by weight ~pbw) or above of sodium hydroxide to
100 pbw of sorelslag. Preferably, from 30 to 60 pbw of
sodium hydroxide to about 100 pbw of sorelslag is used. - 25 More preferably, the ratio by weight of sorelslag to
sodium hydroxide is from 100:35 to 100:45. Using a
ratio of sorelslag to sodium hydroxide above or below
the range of from 100:30 to 100:60 is operable,
however, it may result in an unsatisfactory TiO2
3 pigmentary product. When a material bearing a higher
than 70 weight percent Tio2 content is used, the
hydroxide portion of the mixture is increased
accordingly.
After the sandmilling step, the mixture of
sorelslag and sodium hydroxide is heated or roasted at
30 9 371B-F -6-
--7--
temperatures of from 700C to 950C for a length of time
of from l to 3 hours. At roasting temperatures above
950C a hard material or clinker may result, and below
700C the reaction between the sodium hydroxide and
sorelslag may not be complete. It is, therefore,
preferred to roast the mixture at a temperature of from
800C to 870C for 12 to 2 hours. Preferably, the
roasted mixture is subsequently ground to a particle
size of from 0.5 to 2 microns. As mentioned above,
fine particles will enhance the removal of impurities
during subsequent treatment of the roasted material.
During the roasting step, it is believed that
the Tio2 contained in the mixture reacts with the
sodium hydroxide forming a sodium titanate. Some of
the impurities in the material may also react with the
particular hydroxide used to form an alkali metal salt,
leaving them in an extractable form. For example, when
sodium hydroxide is used, the impurities in their
alkali metal salt form include sodium vanadate, sodium
chromate, sodium aluminate and sodium silicate, which
are readily soluble in water~ These impurities are
therefore preferably at least partially dissolved in
water by washing, after the roasting step, to reduce,
or more preferably, substantially entirely remove
deleterious amounts of the impuritiesO Other compounds
present in the starting material such as iron oxide,
magnesium oxide and calcium oxide are soluble in
3 mineral acids, such as hydrochloric acid (HCl), and are
removed during the digestion step as discussed below.
After the water soluble impurities are washed
off or dissolved from the roasted material, the
remaining insoluble alkali metal titanate with
additional insoluble oxides is digested in hydrochloric
30,371B-F -7-
::~L3~33~
--8--
acid. It is preferred to use about 6 normal tN) HC1
acid because a much weaker acid is not as effective as
about 6 N. A stronger acid may be effectively used in
-a pressurized container but tends to approach 6 N when
boiled in a vessel open to the atmosphere. It is
therefore entirely within the scope of the present
invention to use acid concentra-tions of from as low as
5 N to as high a concentration as 12 N. However, when
the concentration exceeds 6 N (which is the HCl
azeotrope boilin~ at 108C) a pressure vessel is
required. It is therefore mo,re practical to operate at
atmospheric pressure.
At standard atmospheric pressure, the digestion
step can be carried out at 80C or above and preferably
under reflux at a temperature of from 90C to 110C, and
more preferably at 108C, for a length of time ranging
from 10 to 120 minutes, preferably from 10 to 40
minutes. Higher temperatures can be used, however,
pressurized equipment may be necessary. The digestion
step above may be carried out one or more times,
however, it is preferred to carry out the digestion
step at least two times.
~A nodular or an acicular TiO2 particle shape is
` obtained depending on how long time the roasted
material (sodium titanate) dwells in 6 N ~Cl at a
temperature below about 90C. When the 6 N acidic
suspension in the digestion step is heated slowly (less
-than 5C/min) to its boiling point of 108C, the process
yields an essentially nodular Tio2. An acicular
particle shape results from raisin~ the temperature of
the digestion mixture very fast or by using an acid
concentration below 4 N in the heating step and
30,371B-F -8-
.
p~3V33~
g
increasing the acidity to 6 N when the temperature has
exceeded 90C.
In a preferred embodiment of the present
invention, the roasted material is mixed with HCl at
room temperature (i.e., from 20C to 30C)~ The roasted
material/acid mixture is then heated, slowly, up to the
boiling point of the acid at a rate of about 5C/minute
or less, preferably from 2C/min. to 3C/min., and more
preferably at 2C/min~ or less. It is believed that the
nodular sodium titanate material is formed during the
digestion step.
In either case, for obtaining a nodular or an
acicular particle shape, the suspension of the washed
roasted material in the about ~ N HCl is boiled with
reflux for a length of time to substantially complete
the digestion step. The boiling point, which varies
with prevailing pressure, is in the range of 90C to
~ 111C and preferably about 108C. The digestion is
substantially complete in from lO to 15 minutes.
Digestion times of up to about 120 minutes can be used
but preferably the digestion time is from lO to 40
minutes. Higher heating rates and time may be used,
- however, pressurized equipment may be necessary.
During the digestion step, the alkali metal
titanate formed is believed to hydrolyze into amorphous
hydrous TiO2-nH2O and the iron oxides solubilize as
ferrous and erric chloride. The iron chlorides and
other impurities in the acid suspension are removed by,
for example, centrifugation or filtration and,
optionally, disposed of or further treated to recover
unreacted acid. The insoluble amorphous titanium
dioxide residue is washed with a fluid such as water to
30,371B-F -9-
3~h..~l
-10-
further remove soluble impurities. Thereafter, the
Tio2 is recovered from the water by, for example,
filtering or centrifuging. A white residual cake
results after this step is carried out.
The iron impurities in the Tio2 pigment are
believed to be the cause of a non-white pigment.
Preferably the TiO2 pigment contains less than 520 ppm
Fe and more preferabIy less than 200 ppm.
The amorphous TiO2 is calcined, preferably, or
30 to 60 minutes at temperatures ranging from 800C to
1000C to convert the TiO2 to its crystalline rutile
form. More preferably, a temperature of from 875C to
925C for 30 minutes to one hour is employed, because
undesirable discoloration of the resulting pigment is
minimized and lower temperatures are not as effective
in converting the product into its desirable
crystalline form.
If the crystalline rutile product, which is
either acicular or nodular in shape, obtained after
calcination is severely agglomerated, it may be
pulverized or sandmilled. The final particle size for
the acicular version is from 0.05 to 0.3 micron in
thickness and from O.l to l.0 mic-ron in length,
preferably of about 0.2 micron in thickness and about
0.7 micron in length. Its value as an opacifier is
dlctated by a narrow optimum size range.
When the crystalline rutile product is nodular
in shape, the TiO2 particle has an average particle
size of less than about l micron and preferably about
0.3 micron.
30,371B-F -10-
`` ~3~33~
The TiO2 product can be used as a pigment in
any of the typical applications for which opacifying
pigments are used. As an illustration only and not to
limit the scope of the the present invention, the TiO2
pigment obtained from the process of the present
invention can be used as an opacifier in paint, paper
or plastics. The opacifying power and brightness of
the product is determined by measuring its light
scattering coefficient and reflectance. Pigment
obtained by the process of this invention desirably has
a light scattering coefficient of above 2000 cm2/g,
preferably above 4000 cm2/g, and more preferably above
9000 cm2/g, and a brightness of above 80 percent and
preferably from 85 percent to 93 percent when measured
in accordance to the method described in Examples l
and 12.
The examples which follow are illustrative of
the present invention but the present invention ought
not to be limited thereby.
Exa~ple l
A 600 gram (g) sample of hammermilled sorelslag
ore, about 75 micrometers (~um), with approximately 70
weight percent TiO2 and approximately 30 weight percent
impurities was dispersed in 450 milliliters (ml) of
water. The sorelslag in suspension was sandmilled
3~ with 800 ml of 1.5 millimeters ~mm) diameter steelshot
for 120 minutes (min.) at lQ00 revolutions per minute
(rpm)~ The sandmill used was a vertical water-cooled
laboratory sandmill constructed of stainless steel
having a cylindrical grinding vessel with an inside
diameter of ll.5 centimeters (cm) and a height of 19
cm; a shaft, driven by an air turbine, and two 8.5 cm
diameter
30,371B-F
3 ~ 3
-12-
polyurethane disc impellers 4 cm apart. The peripheral
velocity of the impellers was 4.4 meters per second
(m/s). Speed was controlled with an optical
tachometer. At completion of the sandmilling, the top
size of the sorelslag particles was about l0 ~m, the
average size being about 5 ~um. After the steelshot was
removed from the slag suspension by screening through a
screen having openings of 0.5 mm, 240 g of anhydrous
sodium hydroxide (NaOH) was added to the slag
suspension and then thoroughly mixedO The mixture,
having a sorelslag/NaOH ratio of l00/40, was then
evaporated to dryness in air in a shallow dish at 120C.
To break up any agglomerates formed after drying and
obtain a homogeneous mixture, the dried material was
hammermilled using a Weber Brothers Lab Mill S-500.
The resulting fine powder was roasted in air in
porcelain crucibles for 2 hours at a temperature of
820C. A 150 g sample of the roasted material was
pulverized in a mortar to remove any lumps formed after
roasting and dispersed in 350 ml water. The roasted
material and water was sandmilled with 700 ml of l.2 mm
diameter glass beads for 30 minutes at l000 rpm. Glass
beads were used in this sandmilling step instead of
steelshot to avoid discoloration of the material caused
by steelshot.
After removing the beads by screeniny, the
sandmilled roasted material and water dispersion was
3 centrifuged. The solid residue for~ed after centri-
fugation ~solids) was washed by redispersing in water
and recentrifuging. The washing was repeated twice.
The solids were then dispersed in l000 ml of
(about 100C) 6 N HCl acid and boiled at a temperature
of about 108C in an open beaker while agitating with a
30,371B-F ~12-
~3~332
- -13-
magnetic stirrer for 9o minutes. The acidic liquor
containing the solids was centrifuged. The solids were
washed with water twice and dried in air at 120C. The
dried solids were then calcined for 1 hour at 900C
The density of the resulting white calcined
product was 4.14 g/cm and X-ray diffraction analysis
showed the calcined product had a rutile structure.
Electron transmission microscopy revealed pigmentary
grade TiO2 particles with an acicular shape. The
particle size of the acicular pigment ranged from 0.05
to 0.3 ym thick and from 0.1 to 1.0 ~m long.
The bri~htness (R~) of the pigment was 88.4
percent based on the measurement of light reflectance
of a fumed magnesium oxide surface having a 100 percent
reflectance, and its scattering coefficient (S) was
6,835 square centimeters per gram (cm2/g). The iron
(Fe) content of the pigment was determined by X-ray
fluorescence and found to be 520 parts per million
(ppm).
Determininq Scatterinq Coefficient (S) and
Briqhtness (R~)
The primary function of a TiO2 pigment is to
provide opacity to a material, such as paint, paper,
plastics, etc., in which it is incorporated as a
uniform dispersion. Of the many modes used for
3 expressing a pigment's opacifying power, the term
scattering coefficient is particularly meaningful and.
can be easily determined accurately and reproducibly.
The principle of this test is to make a thin
film of the pigment over a black plate glass so that
the film is slightly translucent, i.e., has a
30,371B-F -13-
3~ ~ 3
-14-
reflectance of about 80 to 90 percent of that of the
reflectance of a thick, completely opaque film.
Another film of the same dispersion is applied on a
white glass plate and made so thick that a further
increase in thickness does not change its light
reflectance. When the reflectances of these two films
(R and R~), respectively, are measured and the weight
~W) of the film coating over the black plate is
determined (weight of dry film per unit area, g/cm2),
the scattering coefficient S (cm2/g) can be calculated
using the Kubelka-Munk Theory of light scattering
(Zeitschrift. fur Tech. Physik, 12, 593, 1931). The
reflectance measurement of the film over the white
~lass, R~, is often referred to as brightness. Tables,
based on Kubelka-Munk equations are found in an article
by Mitton-Jacobsen, "New Graphs for Computing
Scattering Coefficient and Hiding Power," Official
Digest, September, 1963, pages 871-913. Using the
tables of the above article, S can be easily computed
knowing R, R~r and W. Example A, below, further
illustrates a method of determining S.
Example A
A pigment is dispersed in water and a small
amount of binder is added which as a film former makes
a coherent film when the dispersion is cast on glass
plates for reflectance measurements. Since the opacity
of the film is very sensitive to the volume ratio of
pigment to binder, this ratio must be kept accurately
constant and at high enough level at which the
opacifying power of the pigment is not appreciably
depressed by the presence of binder. Tests are run at
pigment volume concentration (pvc) of 70.00 percent
(pigment's volume = 70.00 percent of total solids
30,371B-F -14-
~3(~3;~ f~
-15-
volume and total solids volume = pigment volume +
binder volume) and at low enough solids content so that
the film, when cast with a 37 ~m applicator on black
glass will be slightly translucent (having a
reflectance of about 80-90 percent of that of the
reflectance of a thick, completely opaque film of the
same dispersion). A solids volume of about 4 percent
is a suitable level for TiO2 dispersions (pigment
volume + binder volume = 4 percent of total volume of
dispersion). An example of a film composition with
TiO2 pigment for testing S and R~, is described in
Table A below:
TABLE A
ml ml
Grams gJml VolumeVolume
Weiqht Density of of
Solids Dispersicn
TiO2 pi~ent sample 15.00 4.20 ~.57 3.57
Nalco 2324 Di~persantl0.50 1.00 0.50
Dispersant Binder (50.1~
solids by weight)2 3.06 1.04 1.53 3.11
Water 120.32- 1.00 120.32
138.88 5.10 127.50
An anionic polyacrylate disper.sant, sold under the trade name
"Nalco~ 2324" by Nalco Chemical Company.
2A carboxylated styrene-butadiene latex sold under the trade
name "Dow Latex 620A" by The Dow Chemical Company.
In the above composition the pvc.= 70.OG percent and
solids volume of dispersion solids = 4.00 percent.
The pigment, water and dispersant is mixed for
5 minutes with a homogenizer sold under the trade name
"PT 45!80 Brinkmann Homogenizer" by Brinkmann
30,371B-F -15-
` ~ 3 ~ 3
-16-
Instruments Company, at speed setting 4. Then Dow
Latex 620 is added and the mixture is stirred at speed
setting 2 for s minutes.
A Bird Film Applicator, commercially available
from Gardner Laboratory, a Division of Pacific
Scientific Company, having a width of 15 cm and a gap
of 0.037 mm is placed on the top edge of an 50 x 50 cm
black glass plate (reflectance - 0) and about 3 ml of
the dispersion is put in front of the applicator. The
applicator is drawn down on a glass plate uniformly
with an even speed. The film is allowed to dry at room
temperature in a horizontal position. Using a Bird
applicator with a gap of 75 ~m, the same dispersion is
drawn down on a white glass plate (reflectance = 85.6).
After the films are allowed to dry For about 2
hours, a 5 x 6.25 cm rectangular template, having an
area of 32 cm2, is placed over the film on the black
~ plate and the coating outside the template is removed
with a razor blade leaving a 32 cm2 rectangular patch
on the black plate. Next, a Photovolt reflectometer
equipped with an external digital voltmeter and a
search unit with a blue filter, having a wavelength of
- 457 nanometers and sold under the trade name Wratten
filter by Eastman Kodak Company, was used to measure
the reflectances of the patch on the black plate and
the coating on the white plate. Next, the patch on the
black glass is removed with a razor blade and weighed
on an analytical balance.
The following values represent an example:
30,371B-F -16-
-17-
R - 79.5
R~ - 92.0
~ = 0.0186 gram/32 cm2 or 0.000577 g/cm2
From Table 8, on page 895 of the article by Mitton-
Jacobsen above, and given the above values for R and R~
the scattering power (SW) is found to equal 4.09. The
scattering coefficient (S) can then be calculated as
follows:
W 0.000577 g/cm2 = 7088 cm2/g
Example 2
A 600 g sample of sorelslag with approximately
70 weight percent TIO2 and approximately 30 weight
percent impurities was sandmilled as in Example l. The
steelshot-ground suspension contained 57 percent slag.
175 g of this suspension (=lO0 g solid slag) was mixed
with 67 g anhydrous Na2CO3, dried, hammermilled and
roasted for 2 hours at 1050C. The hard, roasted
material was broken into 3.6 mm bits in mortar and then
hammermilled into fine powder which was further
- sandmilled with glass beads as in Example l.
The roasted material was dispersed in water and
separated centrlfugally and washed as in Example l, and
3 digested in 1000 ml of 6 N HCl for 90 minutes, followed
by solids separation and washing as in Example 1.
After calcining for l hour at 900C, a white pigment
having a rutile structure and a density of 4.l0 g/cm3
3~ was obtained. The pigment had a brightness of 78~3
percent and an S of 4,128 cm2/g.
30,371B-F -17-
~ ~33~
- -18-
Example 3
A 600 g sample of hammermilled sorelslag with
approximately 70 weight percent Tio2 and approximately
30 weight percent impurities ~as sandmilled with
steelshot as in Example 1. The screened steelshot was
washed with water. The wash water was combined with
ground slag suspension. With the added water the
suspension had a solids content of 45.9 percent.
' 10
A 327 g sample of the slag suspension (=150 g
solid slag) was mixed with 52.5 g anhydrous NaOH
resulting in a slag/NaOH ratio of 100:35. The mixture
was evaporated to dryness at 120C, followed by
hammermilling and then roasting for 2 hours at 800C.
Without further sandmilling, the roasted material was
dispersed in water and centrifuged. The separated
solids were washed with water twice by redispersing in
water and recentrifuging. The washed roasted material
was then boiled fo~ one hour in 1000 ml 6 N HCl in an
open beaker. After digestion in the acid liquor the
solids were separated and washed centrifugally two
times. The solids were then redispersed in 300 ml
water and sandmilled with 700 ml glass beads for
60 minutes at 1000 rpm. After the glass beads were
screened out, the white, opaque suspension was mixed
with an equal volume of 12 N HCl and boiled for lG
minutes in an open beaker (the final boiling point
being about 108C). The solids were then separated and
washed twice with water and dried at 120C~ The solids
were then calcined for l hour at 900C. A pigmentary
TiO2 having a density of 4.0 g/cm and an iron content
of 150 ppm was obtained. The pigment had a brightness
of 8B.9 percent and a~n S of 3~760 cm2/g.
30,371B-F -18-
~ 3V ~
--19--
Example 4
A 327 9 sample of steelshot ground soxelslag
suspension prepared in Example 3 ~=150 g solid slag)
was mixed with 45 g anhydrous NaOH (the slag/NaOH ratio
= 100/30). The mixture was evaporated to dryness at
120C, followed by hammermilling and roasting as in
Example 3. The two digestion steps and other
processing steps were also performed as in Example 3.
This example was carried out to determine the affect of
reducing the amount of NaOH added to the sorelslag
material of Example 3 on final pigment properties.
After calcination, the pigmentary Tio2 obtained
was buff in color and had an iron content of 8300 ppm.
The pigment had a brightness of about 50 percent. The
value of S was not determined.
Example 5
A 600 g sample of hammermilled sorelslag with
approximately 70 weight percent TiO2 and approximately
30 weight percent impurities was dispersed in 480 g 50
percent NaOH solution (the slag/NaOH ratio = 100/40)~
~5 The suspension was sandmilled for 150 minutes at
900 rpm with 800 ml steelshot with the sandmill
described in Example 1. The sandmilled suspension had
solid particles up to about 13 ~m in size with the
average size being about 7 ~m.
After the steelshot was screened from the
suspension, the suspension was dried at 120C, hammer-
milled and roasted for 2 hours at 850C.
A 150 9 sample of the roasted material was
sandmilled with 700 ml glass beads and 350 ml water at
.
30,371B-F -19-
~3~3~
~20-
1000 rpm for 10 minutes. After the glass beads were
screened out, the solids were separated centrifugally
and washed twice. The solids residue formed after
centrifugation was then dispersed in 500 ml of 10.8 N
HCl and boiled with reflux at 107C for 25 minutes. The
solids were separated from the acid liquor centri-
fugally and washed once with water. The centrifuged
solids were redispersed in 500 ml 7 N HCl and boiled
with reflux at 108C for 30 minutes. The solids were
0 separated from the acid liquor centrifugally and washed
three times. The centrifuged solids were dried at 120C
and calcined for 45 minutes at 900C. A pigmentary
TiO2 was obtained with an iron content of 65 ppm. The
pigment had a brightness of 90.5 percent and an S of
4,472 cm2/g.
Example 6
A 400 g sample of hammermilled sorelslag with
approximately 70 weight percent TiO2 and approximately
30 weight percent impurities was dispersed in 2B0 ml
water and 180 g anhydrous NaOH (the slag/NaOH ratio =
100/45). The suspension was sandmilled for 150 minutes
at 1350 rpm with 600 ml of 1O5 mm steelshot with the
sandmill described in Example 1.
After screening out the steelshot, the sand-
milled suspension was dried at 120C, hammermilled and
roasted for 2 hours at-840C. A 150 g sample of roasted
material was dispersed in 350 ml water and sandmilled
with 70~ ml of 1.2 mm diameter glass beads for 10
minutes at 1000 rpm. After screening, the glass beads,
the suspension was centrifuged and washed twice. The
washed centrifuge cake weighed 234 g and contained 96 g
water, Thus, the yield of solid roasted material was
30,371B-F -20-
~3~
-21-
138 ~. This cake was dispersed in 300 ml water and
605 ml 12 N HCl. The liquid portion resulted in a
7.3 N HCl which after neutralization of solids became
6 N. Digestion with reflux at 108C lasted 20 minutes.
The solids were separated from the acid liquor centri-
fugally and washed twice, then redispersed in 600 ml
6 N HCl and digested another 20 minutes, followed by
centrifugal solids separation and 2 washings. The
solids were then dried at 120C and calcined for 1 hour
at 300C. A TiO2 pigment was produced with an iron
content of 30 ppm. The pigment had a brightness o
90.1 percent and an S of 6,170 cm2/g.
Example 7
In this example, the sorelslag/NaOH ratio was
changed to 100/50 and processed as follows:
A 600 9 sample of hammermilled sorelslag with
approximately 70 weight percent TiO2 and approximately
30 weight percent impurities was dispersed in 400 ml of
water and sandmilled with 800 ml of 1.5 mm steelshot
for 120 minutes at 1800 rpm with the sandmill described
in Example 1. The steelshot was removed by screening.
- 25
Into 200 ~rams of this slag suspension,
containing 120 g sorelslag, 60 g of anhydrous NaOH was
solubilized and dried in a shallow dish in an oven at
120C. The dried material was hammermilled and roasted
3 for 2 hours at 840C. The roasted material was
- dispersed in 350 ml of water and sandmilled with 700 ml
of 1.2 mm diameter glass beads for 10 minutes at
1000 rpm. After screening the ylass beads, the
sandmilled roasted material was vacuum filtered in a
15 cm diameter Buchner funnel with a filter paper
30,371B-F -21-
33~
-22-
having a pore size of about 7 microns made by W&R
Bolton, Ltd., England, and sold under the trade name
"Whatman Filter Paper No. 541" and washed with about
2 liters of water. The washed roasted material was
then digested with reflux in 1000 ml 6 N HCl at 108C
for 20 minutes. Then 7 g of a 1 percent solution of a
first flocculating agent was added. The first
flocculating agent added was a cationic homopolymer
manufactured by The Dow Chemical Company of the type
generally described in U.S. Patent No. 3,719,748. The
flocculated suspension was vacuum filtered and washed
on the filter with 2 liters of water. The filter cake
was dispersed in 1000 ml of 6 N HCl at about 30C and
the temperature was gradually raised (within about 20
minutes) to the boiling point of 108C which was
maintained for 20 minutes. Then 1000 ml of cold water
and 7 g of a 1 percent solution of a second
flocculating agent was added. The second flocculating
agent, also manufactured by The Dow Chemical Company,
was a slightly anionic homopolymex of acrylamide in
solid form with a degree of hydrolysis of from 1 to 5
percent. Viscosity of a 0.5 percent solids in water
solution at a pH of 3 and 25C of the second
flocculating agent was in the range of from 31 to~5a
- centipoise (cp). The flocculated suspension was vacuum
filtered and washed with 2 liters of water followed by
drying at 120C. The solids were calcined for 1 hour at
900C.
Electronmicrographs revealed a nodular or non-
acicular shaped pigment. An X-ray diffraction analysis
confirmed that the pigment had a rutile structure.
S-value was 2408 cm2/g and the brightness 83.9 percent.
30,371B-F -22-
-23-
Example 8
In this example, a titanium bearing material,
referred to as "chloride slag" with approximately 85
weight percent Tio2 and approximately 15 weight percènt
impurities was used to produce a pigmentary TiO2. This
material, in granular form, had a particle size of
about 840 um.
0 A 400 g sample of this material was mixed with
200 9 anhydrous NaOH and 300 ml water. The mixture
was sandmilled, using the sandmill described in
Example 1, with 700 ml of 1.5 mm steelshot at 1500 rpm
for 240 minutes to an average particle size below about
10 ~m. After the steelshot was screened from the
sandmilled suspension, the suspension was dried at
120C, hammermilled and roasted for 2 hours at 840C.
A 150 g sample of the roasted material was
dispersed in 350 ml of water and sandmilled at 1000 rpm
with 700 ml glass beads for 10 minutes. The glass
beads were screened out from the suspension and then
the suspension was centrifuged. The centrifuge cake
was washed 4 times. The centrifuged cake was digested
in 1000 ml of 6 N HCl for 20 minutes with reflux at
108C. At the end of the digestion, S g~of a 1 percent
solution of the first flocculating agent described in
Example 7, was added to the digested material and the
flocculated suspension was vacuum filtered. The filter
cake was then washed on the filter with 1000 ml water.
- The washed filter cake was digested a second time- in
1000 ml of 6 N HCl for 20 minutes with reflux at 108C.
After digestion, 5 g of a 1 percent solution of the
second flocculating agent described in Example 7, was
added to the digested material. The flocculated
30,37lB-F -23-
3 V 3
-24-
suspension was then vacuum filtered and washed with
2 liters of water on the filter. The filter cake was
then dried at 120C. The solids were calcined for
l hour at 900C. A pigmentary TiO~ was obtained having
an S of 5,670 cm2/g and a brightness oE 89.0 percent.
Example 9
A 500 g sample of hammermilled sorelslag with
approxlmately 78 weight percent TiO2 and approximately
22 weight percent impurities was dispersed in 350 ml
water and sandmilled for 180 minutes at 1800 rpm with
800 ml of 1.5 mm steelshot with the sandmill described
in Example 1. The peripheral velocity of impellers was
8 m/s.
The ground slag was screened through a 425 ~m
screen to remove the steelshot. The steelshot was
rinsed with water and the rinse water was combined into
the slag grind resulting in a slag suspension having a
solids content of 24.5 percent.
Into 612 g of this slag suspension (=150 g
solid slag), 67.5 g anhydrous NaOH was added giving a
sorelslag/NaOH ratio o 100/45. The mixture was
evaporated to dryness at 120C in a shallow dish. The
dried material was hammermilled to obtain a homogeneous
fine powder. The powder was then heated or roasted in
a porceIain crucible for 150 minutes at 840C. Upon
3 cooling, the roasted material was pulveri7ed in a
mortar. The roasted matexial was then dispersed in
350 ml of water and sandmilled for 10 minutes at
1800 rpm in the laboratory sandmill above using 700 ml
of 1.2 mm diameter glass beads as srinding media. The
sandmilled roasted material was screened free of
,
30 9 37lB-F ~24-
3~
-25-
beads, diluted to 1500 ml with water and vacuum
filtered through a 15 cm diameter Buchner funnel using
No. 541 Whatman paper. The filter cake, being abou-t
? cm thick was washed on the filter with about 2 liters
of water. Solids content of the filter cake was 62.6
percent.
The washed filter cake was dispersed in 300 ml
of ~ater plus 200 ml 12 N HCl. The mixture having a
normality of about 4.4 was put into a 2 liter, 4-necked
glass flask equipped with a reflux condenser, a
thermometer, an agitator and a funnel with a stopcock.
The dispersion was heated up to a temperature of 100C.
Thereafter, to raise the acidity to 6 N, 400 ml 12 N
HCl was added into the flask. The temperature dropped
momentarily to 85C, however, within 5 minutes the
temperature reached 108C, the boiling point of 6 N HCl.
The mixture was boiled for 20 minutes and then diluted
with cold water to 2000 ml. Under continued agitation,
7 g of a l percent solution of the first flocculating
agent, described in Example 7, was added to the
mixture.
The mixture now beiny about 3 N was vacuum
filtered. T~e filter cake was washed on the filter
with 2 liters of water. The solids content of the
ilter cake was 32.5 percent.
The filter cake, weiyhing 323 g (solids -
105 g), was dispersed in a solution containing 280 ml
of water plus 200 ml of 12 N HCl. This mixture was
placed in the 4-necked glass flask and heated to 100C.
Thereafter 300 ml of 12 N HCl was added to the mixture.
The temperature was raised to 108C boiling point of 5 N
HCl and kept at this temperature for 20 minutes. The
30,371B-F -25- -
~ 3~ 3
- -26-
mixture was diluted to 2000 ml with cold water. Under
continued agitation, 7 g of a 1 percent solution of the
second flocculating agent described in Example 7, was
added to the mixture. The mixture was filtered and
washed the same as after the first digestion.
The filter cake, about 3 cm thick, was dried at
120C, and thereafter ground in a mortar. The ground
cake was calcined for 1 hour at 900C. The calcined
material was immediately placed in a large shallow
porcelain dish and cooled at room temperature exposed
to air. A pigmentary TiO2 was obtained.
An X-ray diffraction analysis identified the
pigment as rutile which had a density of 4.1 g/cm3.
The pigment had an S of 6,900 cm2/g and a brightness of
92.2 percent. The crystal habit was acicular.
Example 10
A TiO2 pigment was produced as in Example 9,
except that the sorelslag/NaOH ratio was changed to
100/40 and the NaOH was added to the sorelslag prior to
sandmilling rather than after the slag was sandmilled.
The acicularly shaped pigment had an S of
8,900 cm2/g and a brightness of 31.0 percent.
Example ll
3 A titanium-bearing material made ~uring the
Benilite Cyclic Process from ilmenite by reduction
roasting and subsequent HCl acid leaching was used as
starting material in this example. This black material
had approximately 93 weight percent Tio2 and
30,371B-F -26-
~3~332'~
-27-
approximately 7 weight percent impurities and a
particle size of about 250 ~m.
A 400 g sample of the material was mixed with
200 g NaOH, and 350 ml H2O and sandmilled with the
sandmill described in Example 1 with 600 ml of 1.5 mm
` steelshot for 90 minutes at 1600 rpm. The sandmilled
material contained particles with an average size of
below about 10,~m. After the steelshot was screened
out, the ground material was dried at 120C, hammer~
milled and roasted for 2 hours at 840C. A 170 g sample
of roasted material was dispersed in 350 ml of water
and sandmilled with 700 ml of glass beads at 1600 rpm
for 10 minutes. After the beads were screened out, the
suspension was centrifuged and the centrifuged cake was
washed 2 times with water.
The centrifuged cake was digested with reflux
in 1000 ml 6 N HCl for 20 minutes. The solids were
separated from the acid liquor and washed centri-
fugally. A second digestion also in 1000 ml 6 N HCl
was carried out followed by centrifugal separation and
washing. The solids were dried at 120C and then
calcined for 1 hour at 900C. An a,cicularly shaped~
pigmentary TiO2 was o~tained. The pigment had an S'of
4,260 cm2/g and a brightness of 90.0 percent.
Table I, below, summarizes the examples above
excluding Examples 2, 8 and li.
30~371B-F ~27- ,, ,
~3V~
-` --28--
~ ~1 ~ ' o L~ ~ ~ N O
~ ~ 0 0 0 0 0 ~ ~ ~
S., C CO oo U~ 0 O~
m~
~ ~ o ~ o 0 o o
~ ~o ~ ~ o o o
E~ D N
o o L~ o
O ~
S . ~ ~ J N N N N N
~
H Z ~ ~
n ~ ~ z o = ~ O O n
~_
U~
n C ~ O O O O ~ O O O
o o U~ o o ~ o ~ o
~d ~ ~ t`~ J ~ ~ J
o z ~ ~ ~ ~ ~ ~ ~ ~
~ oooooooo
~ o o o o o o o o
~ V~ ~ ,_ ~ ,_ ~ ,_ ~ ,_
a~ O
30, 371B-F -28-
3~
-29-
Example 12
A sorelslag sample with a particle size of
about 74 microns was obtained from QIT-FER ET TITANEr
INC., a Canadian company. A 1200 9 sample of the
sorelslag and 480 g of sodium hydroxide (NaOH) were
placed in a laboratory sandmiil containing one liter of
1.2 mm in diameter steel shot and about 1,000 ml of
water. The mixture was then sandmilled until the
maximum particle size (top size) of the sorelslag was
10 microns. The slag/NaO~ ratio of the mixture was
100~40 by weight. After the steel shot was screened
out~ the mixture was dried in an oven at a temperature
of 100C. The dried material was hammermilled to break
up the agglomerates formed after drying.
The hammermilled material was then roasted at a
temperature of 875C or two hours. The roasted
material was run through a grinder to break up the
2Q agglomerates formed after roasting and then the roasted
material was sandmilled for five minutes in about 700
ml of water and one liter of 1.2 mm in diameter
zirconium oxide beadsO After the beads were scr~ened
out, the suspension was vacuum filtered and the filter
cake was washed with water two times, each wash using
about one liter of waterO
A 194 g sample of the roasted material on a dry
basis was digested in 1,0C0 ml of 6 N hydrochloric acid
(HCl). The digestion step was carried out as follows:
The temperature of the 6 N solution (i.e. roasted
material/acid mixture) was at room temperature (20C).
Then the temperature was increased slowly at a rate of
about 5C/minute up to the boiling point of the 6 N acid
solution and the solution was allowed to reflux for lS
30,371B-F 29-
3~ 3
-30-
minutes. The amount of water in the wet filter cake
was taken into account when the normality of the HCl
acid was calculated. A flocculant, 3 g of a 1 percent
solution of SEPARAN MG-205 ~a trademark of The Dow
Chemical Company) was added to the digestion
suspension. The suspension was filtered and washed two
times with wat~r, each wash using about one llter of
water. The filter cake was redispersed in water and
digested a second time with 800 ml of 6 N HCl in the
0 same manner as ~he first digestion. The same amount of
flocculant as in the first digestion was added to the
suspension. The suspension was then vacuum filtered
and washed as in the first digestion. A filter cake
was dried at a temperature of 110C for about three
hours. The dried material was then calcined for one
hour at a temperature of 900C. The obtained rutile
pigment had a distinctly nodular particle shape.
20The dry brightness of the calcined material was
measured by packing a portion of the material into a
sample vial cap which was about 1.3 cm deep by 2.5 cm
inch in diameter and measuring the reflectance of the
sample using a Photovolt equipped with a blue filter.
The instrument was first calibrated by using a white
- standard chip of known brightness of 86.9 with the blue
- filter. The dry brightness of the calcined material of
this example was 88.0 percent.
.
30The scattering coefficient oE the calcined
material was me~sured by the following procedureO
A 15 g sample of pigment and 1/2 g of sodium
tripolyphosphate in 70 g of water was dispersed in a
high speed disperser for 5 minutes. Then 3.1 g of a
latex sold under the trade name Rhoplex ~100 by Rohm
, 30,371B-F -30
-~ ~ 3
-31-
and Haas Co. and 1/2 g of a surfactant sold under -the
~- trade name Triton X-100 by Rohm and Haas Co. was added
to the dispersion and stirred gently by hand for about
3 minutes. A drawdown coating of the dispersion was
applied on a 50 um thick clear plastic sheet of Mylar
(a trademark of E.I. DuPont de Nemours & Co.) with a
37 ,um Bird film applicator and placed in an oven and
dried at a temperature of 100C for 3 minutes. A 5 cm
by 5 cm square sample of the coated Mylar sheet was
weighed and then placed on a 5 cm by 12.5 cm optically
flat black glass plate which was coated with propylene
glycol. The propylene glycol was used to ensure
optical contact. Using a Photovolt the reElectance
over the black glass, RBI was measured for the sample.
The coating on the 5 x 5 cm Mylar sheet was washed off
and the Mylar sheet was dried and then weighed to find
the coating weight per unit area, W. A practical
approximation to an infinitely opaque coating, R~, was
obtained as follows: A drawdown coating was applied on
a sheet of 50 micron Mylar and dried and this step was
repeated until a maximum reflectance was reached as
measured by the Photovolt. Using the RB, R~, and W
values, a scattering power value~ SW, was found by
using the Tables in the article Mitton Jacobsen, "New
Graphs for Computing Scattering Coefficient and Hiding
Power", Official Digest, September 1963, pages 871-913.
The scattering coefficient, Sj was then calculated
using the formula:
SW
30,371B-F -31-
:~3~3;~
In this example the scattering coefficient of
the calcined material was 3,336 cm2/g.
The iron content of the calcined material was
measured using ionic coupled plasma spectrometry. In
this example the iron content of the calcined material
was 200 ppm.
30,371B-F . -32-