Note: Descriptions are shown in the official language in which they were submitted.
17-21(~083)A
~3~)~B191
HEAT RECOVERY FROM CONCENTRATED SULFURIC ACID
Back~round of the Invention
This invention relates to a process for the recovery
of heat from a sulfuric acid plant. More particularly, this
invention relates to a process ~or the recoverY oE the exo-
thermic heat resulting from the absorption of sulfur trioxideinto concen~rated sulfuric acid. ~nis invention also relates
to a heat recovery tower which is used to recover the heat
energy from csncentrated sulfuric acid.
DESCRIPTION OF THE PRIOR ART
.
The process for the manufacture of sulfuric acid
starts with a gas stream which contains sulfur dioxide. The
sulfur dioxide is catalytically oxidized in a converter to
sulfur trioxide whi~h is removed from the gas stream in one or
more absorption stages to form sulfuric acid. The oxidation of
sulfur dioxide to sulfur trioxide is an exothermic reaction.
To prevent the loss of this heat, steam has been generated in
boiler~, and low level process heat has been recovered by
heating boil~r feed water in economizers.
~ After oxidation the gas stream containi~g ~he sulfur
trioxide passes through absorption towers in which the gaseous
suIfur trioxide is absorbed into concentrated sulfuric acid,
.
'
17-21 (5083~A
13~3~3~4
having a typical concentration of 98%. In a modern s~lfuric
acid plant there are typically two absorption towers, designat-
ed as the interpass absorp~ion tower and the f inal a~sorption
~ower, which are respectively located in the process upstream
from and downstream from the final catalyst stage in the con-
verter. In ~he sulfuric acid plants of today, the gas stream
is cooled prior to entry into the absorption towers to maximize
the recovery o~ energy f rom the gas stream. The absorption
to~er is operated at a temperature which is selected to facili-
tate the absorption of sulfur trioxide into ~he sulfuric acid,to minimize the corrosion of piplng and heat exchangers that
occurs at higher te~peratures, and to minimize the ~ormation of
acid`mist. The absorption of sul~ur trioxide into sulfuric
acid is a highly exoth~rmic reactionO and large amounts of heat
are lost to cooling water ~hile maintaining the low tempera-
tures in the typical absorption towers.
The absorption tower is typically constructed such
that the sulfuric acid flows downward through the tower and the
enclosed packing material while the gas stream which contains
the sulfur trioxide passes through the tower. The packing
promotes contact between the sulfuric acid and the gas stream
such that the sulfur trioxide is absorbed in the sulfuric
acid, The acid drains into a pump tank, where water is added
to dilute the acid to the desired strength. Both absorption
and dilution are exothermic reactions~ and the genera~ed heat
is removed in a heat exchanger which is typically located
between the pump tank and the absorption tower inlet. The
operation of the absorp~ion tower is characterized by the con-
centration of the recirculated sulEuric acidr typically 98~, a
low maximum acid exit temperature of approximately 120C, and a
typical acid inlet temperature of approximately ~0C. A lower
17-21 (5083 jA
~L3U3~14
acid inlet temperature would thermally shock the hot gas stream
and often create an undesirable acid mist. A higher acid inlet
temperature would increase the acid exit temperature and the
corrosion of related piping and heat exchangers It is thus
known that the operating temperature of the absorption tower is
set by and limited by considerations of the rate of corrosion
of the equipment, and the undesirable formation o~ acid mist.
The absorption tower has typically been constructed
a~ a brick lined carbon steel tower to limit corrosion. Typi-
cally, cast iron or ductile iron pipe has been used around theabsorption tower. Historically, a number of materials have
been used for acid cool~rs. These include cast iron pipe or
radia'tor sections, alloy C276 plate type heat exchangers,
polytetrafluorethylene (PTFE) tank coils and stainless steel
shell and tube heat exchangers.
The cast iron coolers are limited in operating
temperature ~o approximately 110C by corrosion. They have
poor heat transfer and occupy a large amount of space within
the sulfuric acid plant. In addition, they have many mechani-
cal joints which tend to leak and result in high maintenancerequirements.
Alloy C276 plate type heat exchangers can be cost
effec~ive relative to the cast iron coolers. However, this
expensive alloy is limited in use to a maximum acid temperature
of approximately 90C; thus, the liquid exiting from the
absorption tower at approximately 120C is mixed with cold
recycle acid before entering the heat exchanger This reduces
the thermal driving force and shows ~hat the use of expensive
alloys will not provide an easy solu~ion to the problem of heat
recovery rom sulfuric acid.
17-21 (5083) A
:13~3~3~4
PTFE tank coils have been used to minimize corro-
sion. Small thin wall tubes, which are easily plugged, are
required to obtain adequate heat transfer. The PTFE can
Withstand temperatures up to 200C; however, in heat recovery
applications its low mechanical strength limits the pressure of
steam that can be generated. Thus, an intermediate heat tran-
sfer ~luid is required for heat exchange with the hot sulfuric
acid. A second heat exchanger is then required or heat ex-
change between the hea~ transfer fluid and steam: thus, this
d~sign is too expensive for use in this hea~ recovery applica-
tion.
Stainless steel heat exchangers, typically type 316
stainless steel, haYe been used as acid coolers. These require
careful control of the acid temperature and the acid velocity
in order ta minimize corrosion. The more recent anodically
protected stainless steel acid coolers have proven to be a
reliable means of minimizing corrosion; however, practice has
been to limit acid operating temperatures to less than 115C.
The equipment to provide the anodic passivation is expensive.
Past practice with the aforementioned types of heat
exchangers generally has been limited to rejecting ~he heat
into cooling water or recovering heat in a low level form such
as hot water for boiler feed or district heating.
E~orts have been made in the past to recover the
heat generated ~hen the sulfur trioxide is absorbed into sul-
furic acid. ~.S. Patent 2,017,676 describes an apparatus for
condensing sulfuric acid. Sulfur trioxide and sulfuric acid
fumes are passed through a heat exchanger which has ceramic
tu~es to slowly and unifor~ly cool the gases from a temperature
of about 350C to about 140C. The ceramic tube material is
used in contact wi~h ~he sulfuric acid ~o prevent corrosion;
17-21 (5083)A
~l3¢~3~4
however, a metallic tube is used concentrically about each
ceramic tube to prevent mechanical stress and breakage of the
ceramic tubes. The cooling medium, a high boiling point oil or
boiling hot water, is allowed to become heated to high tempera-
tures such as would be present in a steam boiler. When oper-
ated in this manner, the patent s~ates that approximately 1.5
tons of steam may be generated per ton of sul~uric acid and thé
sulfuric acid manufacturing c~sts may be reduced.
British Patent 1,175~055 describes a method for the
manufacture of sulfuric acid in which the gases are alternately
pa~sed through a catalyst bed to convert sulfur dioxide to ~ul-
fur ~rioxide and a heat exchanger/condenser in which the gases
are cooled in ~he presence of water vapor to condense a part of
the sulfur ~rioxide as sulfuric acid. The heat exchangers are
lined with, or constructed of, materials which are resistant to
corrosion by the hot, concentrated sulfuric acid such as
ceramic materials and porcelain, metals such as steel coated
with polytetrafluoroethylene or other corrosion resistant
materials, or metals such as silicon-iron and nickel alloys.
The heat created by formation of sulfuric ~cid and the heat
released by condensation is utilized to create high pressure
steam which may be utilized as a source of power~ The 3ritish
Patent also discusses recovery of the sulfuric acid in a more
concentrated ~orm. By employing a stsichiometric deficiency of
steam during the intermediate csndensations, it is possible to
obtain sulfuric acid having a concentration of greater than
100~. Only in the final condensation after completion of the
conversion of sulfur dioxide to sulfur trioxide is the remain-
ing sulfur trioxide condensed in the prese~e of a sufficient
excess of steam to insure that substantially all of the sulfur
trioxide is removed from the gas stream.
17-21 (5083)A
~3~38~
Both U.S. Patent 2,017,676 and British Patent
1,175,055 teach methods of recovering energy from the sulfuric
acid process. However, both patents require the use of exotic
materials of ~onstruction and emphasize the use of ceramics,
porcelain materials, coated metals, brittle metals such as
silicon-iron, and expensive nickel alloys for con~truction to
preven~ rapid corrosion and ~ailure of the equipment.
Blumrich et al U~S. patent 4,330,364 describes a
process for strengthening dilute phosphoric acid using energy
derived from a contact sulfuric acid plant. In one e~lbodiment,
heat is transferred from an H2SO4 absorber acid ~tream to a
phosphoric acid stream wi~hout the use of any in~ermediate
1uid. In an alternative embodiment, a pressurized water
system is interposed between the sulfuric acid and phosphoric
acid systems. In the lat~er system the pressurized water is
said to be heated to 120C by transfer of heat from 98.5%
absorber acid which is cooled from 140C to 120CC in the
process. However, the reference contains no disclosure of the
materials of construction to be used in the heat exchanger for
~ransfer o~ heat from absorber acid to the pressurized water.
Sand~r and Beckmann, ~Concentration of Dilute Sul-
~uric Acid and Phosphoric Acid With Waste Heat~, paper 25 of
~Making the Most of Sulfuric Acid~, Proceedings of the British
Sulphur Corporations's Fifth International Conference, Part II:
Additional Papers and Discussions; London, ~ovember 16-18,
1981, includes a flow sheet for an integrated sulfurio acid
plant wi~h a venturi reconcentrator unit which uses waste hsat
from a double absorp~ion production unit. In this process, hot
acid from each of ~he intermediate and final absorbers is
passed through a heat exohanger which ~ransfers heat to spent
acid to be concentrated in the Venturi reconcentrator. In the
17-21 (5083) A
'3~
case of the intermediate absorber, absorber acid having a
strength of 98.5~ is passed through the heat exchanger where it
is cooled ~rom 130 to 110C by transfer of heat to the recon-
centratOr acid circulating streain. However, in the Sander
system, the heat exchanger utilized contains Teflon rather than
metal alloy tubes, and the recirculating acid i8 heated only to
a temperature of 70-80C.
In connection with the concentration of phosphoric
acid, the Sander et al reference refers to the possibility of
running the sulfur trioxide absorption system as high as 130
~4 140C to generate low pressure steam of 1.2 to 1.5 bar, and
the Use of such steam in a phosphoric acid vacuum concentra-
tor. Sander et al further report an actual installation of a
system apparen~ly patterned on the process of the Blumrich et
al patent. In the latter system, Sander et al state that the
sulfuric acid is cooled from 110C to 90C in the course of
heating demineralized water up to 90C.
SUMMARY OF THE INVENTION
It is an object of this invention to provide a method
for the recovery in high grade form of heat which is now lost
to cooling water in the sulfuric acid process.
It is a further object of this invention to provide a
method for recovery of the heat created in the sulfuric acid
25 process when sulfur trioxide is absorbed into sulfuric acid.
It is yet another object of this invention to provide
a me~hod ~or the ab~orption of sulfur trioxide into hot, con-
centrated sulfuric acid while greatly reducing the corrosive
effect of the sulfuric acid~
17-21 (5083) A
~3~3~
It is yet anothec object of this invention to provide
a method f~r recovering the heat oE absorption of sulf~r tri-
oxide in sul~uric acid at a higher temperature level, and thus
in higher grade form, than has heretofore been practicable. A
particular ~bject is to recover ab~orption energy in the form
of elec~ricity, steam or process heat.
~ n additional object of this invention is to provide
a heat recovery system consis~ing of a heat recovery tower,
heat exchanger and associated equipment such as pumps and
piping for use in a sulfuric acid plant which may be construct-
ed of cost effective alloy materials rather than the porcelain,
ceramic, and ooated materials heretofore proposed for high tem-
perature operation, all of which have mechanical, heat transfer
and economic limitations.
These and other objec~s are ob~ained through a novel
process in which the sulfur trioxide passiny from the converter
in a sulfuric acid plant is absorbed into hot concentrated
sulfuric acid and the heat is recovered for useful purposes
through heat exchange with a third fluid.
The process of the invention is implemented utilizing
a novel apparatus for recovery of the energy of absorption of
sulur trioxide in sulfuric acid. This apparatus comprises a
vessel containing an absorption zone in which sulfur trioxide
is absorbed in a sul~uric acid stream, the absorption zone
comprising m~ans for promoting contact and mass transfer
between a gas phase comprising sulfur trioxide and a liquid
phase comprising sulfuric acid having a concentration o~
greater than 98~ and less than 101% and a temperature of
grea~er than 120QC. The apparatus further includes means for
: 30 delivering sulfuric acid to the vessel for passage ~hrough the
- absorp~ion zone, means for delivering gas comprising sulfur
.
17-21 ~C83) A
~3~3~3~L4
trioxide to the vessel for passage through the absocption zone,
means for egress o sulfuric acid from the vessel after passage
through the absorption zone, means for egress of the gas s~ream
from the vessel after passage through the absorption zone, and
a heat exchanger wherein the heat of a~sorption is recovered
from the sulfuric acid in useful foxm ~y heat exchange with a
third flui~.[ The heat exchanger comprises ~eans for transfer
of heat from sul~uric acid to the third fluid, the hea~ tran-
sfer means being fabricated of an alloy having a low corrosion
rate when exposed to hot concentrated sulfuric acid. ~
More particularly, the invention is directed to such
apparatus including a heat recovery tower having top and bottom
inlets and top and bottom exit~. The sulfur trioxide con~ain-
ing yas stream from the converter~ a~ter being cooled, enters
lS the heat recovery tower through the bottom inlet and flows up-
ward through the tower and the hot sul~uric acid stream enters
the heat recovery tower through the top inlet and flows down-
ward through the ~ower. At all points in the heat recovery
tower and heat exchanger system ~he sulfuric acid has a con-
centration greater ~han 98% and less than 101~ and a tempera-
ture greater than 120~C. The acid concentration is defined as
being the weight percen~ of sulfuric acid. The counterflow of
the gas stream and sul~uric acid maximizes the driving force
for efficiently àbsorbing the sulur trioxide into the sulfuric
acid. Co-current flow of gas and acid can be utilized, but iç
less efficient. The absorption of sulfur trioxide into sul-
furic acid is a process which is known to ~hose having experi-
ence in the manufacture of sulfuric acid and will thus not be
further desc~ibed. This process will be referred ~o herein as
t~e absorption of sulfur trioxide into ~ulfuric acid and the
17-21 (5()83) A
:~3~3~4
heat generated by the process will be referred to as the heat
of absorption. The heat of absorption includes the heat liber-
ated when water is aaded to dilute the recycled sulfuric acid,
a process step whic~ may occur within or external to the heat
recovery tower. Af~er the absorption of sulfur trioxide, the
sulfuric acid stream passes through a hea~ exchanger wherein
the heat of absorption is recover~d ~hrough heat exchange with
other 1uids. It is desirable that the heat exchanger be ~ab-
rica~ed from a metal to facilita~e the transfer of heat from
the sulfuric acid stream to other ~luids. It has been dis-
covered that by operating the heat recovery tow~r in a very
narro,w acid concen~ration range between 9~% and 101%, and
preferably between 98.5~ and 100.0~, it is possible to absorb
sulfur trioxide efficiently and to dramatically reduce the
corrosion rate of certain alloys while operating at tempera-
tures heretofore considered impracticable. It has been
discovered that certain alloys exhibit excellent corrosion
resistance in the concentration range previously defined, and
at temperatures of 120C or hi~her. Stainless steel alloys are
generally superior to high nickel alloys. Excellent corrosion
resistance has been found or certain iron/chromium alloys,
iron/chromium/nickel alloys and nickel/chromium alloys having
austenitic, erritic or duplex structures. S~ainless steels of
such structure have been ~ound especially sui~able. Thirty
alloys were tested at service conditions typical of the heat
recovery systPm. It has b~en de~ermined that the corrosion
resistance of these alloys can be characterized in terms of the
percentages of major alloying constituents. ~ he alloys~best
suited for service in this heat recovery system~contained iron,
chromium, and nickel as the principal alloy constituents, and
had compositions which gave a corrosion index ~CI) greater than
3~
17-21(5083~A
~3~3B~L4
CI> 39
as deined by the following equation:
CI - 0.35(Fe+Mn) ~ 0.70(Cr~ + 0.30(Ni) - 0.12(Mo)
where:
~e z the weight percent of iron in the alloy,
Mn - the weight percent of manganese in the alloy,
: Cr - the weight percent of chromium in the alloy J
Ni 2 the weight percent o~ nickel in the alloy, and
Mo = the weight percent of molybdenum in the alloy.
,
In a conventional sulfuric acid plant, the heat of
absorption of sulfur trioxide into sulfuric acid is lost to
cooling towers. ~y use o the process and apparatus of this
invention, a hish percentage of this previously lost energy may
be recovered and profitably used, The heat may be used, for
example, to produce low to medium pressure steam for process
heating or to power a turbogenerator for the generation of
electricity. In a 2700 tonne per day sulfur burning sulfuric
acid plant approximately 6 megawatts of additional electrical
power can be produced from the heat recovered in the heat
recovery tower.
: : DESCRIPTIQN OF THE DRAWINGS
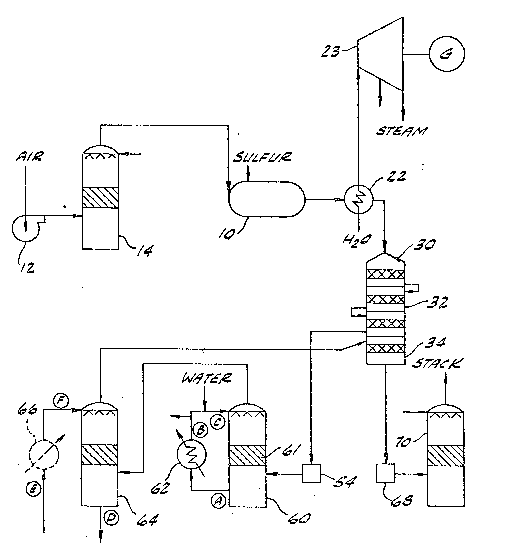
: Figure 1 is a process:flow diagram o~ a sulfuric acid
~la t Wh ch includes t~e ~pparacus f this invention.
; ' .
,
17-21 (5083)A
~3~1 3~3~4
Figure 2 is a schematic which illus~rates the
co~rosion rate of alloys and the degree of absorption of sulfur
trioxide into sulfuric acid at a given temperature as the con-
centration of the sulfuric acid varies.
Figure 3 is a diagram of the process and apparatus of
this invention.
Figure 4 is a graph showing the operating cycle of
the heat recovery tower in relation to the operating cycle of a
typical interpass absorption tower.
~igure 5 is a process flow diagram allustrating a
preferred scheme for implementing the improved process of the
invention,
Fig. 6 is a sulfuric acid temperatUre/conceQtration
diagram having plstted thereon corrosion data and estimated
~ 15 isocorrosion curves for type 304L stainless steel;
Fig. 7 is a plot comparable to Fig. 6 but for type
310S stainless steel;
Fig. 8 is a plot showing both the literature 5 mpy
isocorrosion curve for ~ncoloy 825 in sulfuric acid and ~he
approximate 5 mpy isocorrosion curve for Incoloy 825 in the
98%-100% acid concentration region as determined in the course
of the development of the improved process of the invention;
Fig! 9 is a plot showing both a litera~re 5 mpy
isocorrosion curve for type 316 stainIess steel in sulfuric
acid and the approximate 5 mpy isocorrosion curve for type 116
stainless steel in the 98% to 190~ concentration region as
determined in t~e course of the developmen~ of ~he improved
process o the invention;
~ .
12
~,. . .. .
17-21 ~5083)A
~L3U3~
Fig. 10 is a plot showing literature 1 mpy and 50 mpy
i~ocorrosion curves for Ferralium Alloy 255 in sulfuric acid
and the approximate 1 mpy isocorrosion curve for Ferralium in
the 98% to 100% concentration region as determined in the
course of the development of the improved process of ~he
invention;
~ ig. 11 is a plot showing seYeral isocorrosion curves
for Hastelloy C-275 in sulfuric acid, having plotted thereon a
point showing the corrosion rate ~or ~lloy C-276 in the 98~ to
100% region as determined in the course of the develop~ent of
the process of the invention;
, ~ig. 12 shows anodic potentiodynamic scans for type
304 stainless steel in 99.2% sulfuric acid at a scan ratP of
1.8 v/hr;
Fig. 13 shows anodic potentiodynamic scans for type
304 stainless steel in 99.2% sulfuric acid with a purge gas of
nitrogen, 5~ 2 and 0.5~ SO2 at a scan rate of 1.8v/hr.;
Fig. 14 shows anodic potentiodynamic -~cans ~or type
310 stainless steel in 99.2~ sulfuric acid at a scan rate of
1.8 v/hr. and
Fig. 15 shows anodic potentiodynamic scans for
E-Brite 26~1 in 100% sulfuric acid at a scan rate of 2 v/hr.
Corresponding re~erence oharacters indicate corres-
ponding process equipment elements throughout ~he several views
of the drawings.
DESCRIPTION OF THE PREFERRED EMBODIMENT
. .
Figure 1 illustra~es a proc@ss flow diagram for a
sulfuric acid plant which includes the apparatus of this
i,nvention. The sulfuric acid process is well known; thus,
17-21 (~083~A
313~4
portions of the sulfuric acid plant will not be described in
detail herein, The drawing shows a sulfuric acid plant which
burns sulfur to supply the gas stream containing sulfur dioxide
to the sulfuric acid plant.
Loo~ing now at Figure 1, blower 12 supplies air
through drying tower 14 to tne sulfur burner lO in which the
sulfur is burned, to provide a gas stream containing sulfur
dioxide. The sulfur dioxide laden feed gas stream exits from
the sulfur ~urner lO and passes through a ~irst heat exchanger
22, before entering the converter 30. The ~eed gas is cooled
in first heat exchange~ 22 to a temperature near the desired
inlet temperature to the converter. ~irst heat exchanger 22 is
used to generate steam for driving a turbogenerator 23 for the
generation of electrical power, but other uses are also practi-
lS cal.
Converter 30, a vessel for the catalytic conversionof sulfur dioxide into sulfur trioxide, typically has a plural-
ity of catalyst beds which are divided into a first oxidation
stage 32 and a second oxidation stage 34. ~etween any ~wo
catalyst beds there is heat exchange to remove the heat gener-
ated during the oxidation of the sulfur dioxide. These heat
exchangers are not shown in Figure l. In a typical sulfuric
acid plant, between the first oxidation stage 32 and the second
oxidation stage 34 the gas stream passes through an interpass
2S absorption tower to remove the sulfur trioxide from the gas
stream to provide a gas stream to the second oxidation stage 34
that i~ lean in sulfur trioxide. As the sulfur dioxide laden
~eed gas stream passes ~hrough the first oxidation stage 32,
greater than 90~ of the sulfur dioxide will be converted to
sulur trioxide. The oxidation reaction is a reversible
reaction which approaches an equilibrium: thus, some
14
17-21 (50833A
~3~3~4
of the sulfur trioxide must be removed from the gas stream to
enable the remaining sulfur dioxide to be oxidized easily.
Esonomizer 54 is used to cool the gas stream exiting from the
first oxidation stage 32 to a temperature which is above the
dew point of the gas stream.
The sulfur trioxide in the gas stream is then
absorbed into a sulfuric acid stream and heat is generated by
the process. According to typical conventional practice, the
absorption takes place in an absorption tower in which the acid
10 temperature is maintained at a low level, to minimize corrosion
of related piping and hea~ exchangers. However, the mainten-
ance of the low temperature in the absorption tower makes it
dif icult to recover energy in an economically viable manner,
that is, in a useful form.
~ 15 In accordance with the embodiment of Fig. 1, a heat
recovery tower 60 is provided downstream of the economizer 54.
The cooled sulfur trioxide laden gas stream enters the lower
portion of heat recovery tower 60 and flows upward through an
absorption zone in the tower, the absorption zone containing
means, such as a bed of packing 61, for promoting contact and
mass trans~er between the gas and liquid phases. While this
description is of a packed tower, it is contemplated that other
gas-liquid contacting devices such as tray towers or ve~turi
absorbers can be used~ Hot, liquid sulfuric acid is sprayed
~rom the top of the heat recovery ~ower 60 onto the bed of
packing 61 and, as the sulfuric acid and sulfur trioxide con-
tact one another in the absorption zone, the sulfur trioxide is
absorbed in~o the sulfuric acid. As delivered to the eounter-
current absorption zone in the hea~ recovery tower 60, ~he
s~lfuric acid has a concen~ration greater than 98~ and less
than 101%, preferably between about 98~5~ and about 99.3%, and
17-21 ~083)A
~3~3~i~
a temperature greater than 120C. As discussed above, the heat
of absorption of the sulfur trioxide into the sulfuric acid i5
released in this process. Throughout ~he absorption zone, and
throughout the course of SO3 absorption therein, the strength
of the sulfuric acid s~ream is maintained between 98%-and 101~,
pre~erably between 98.5~ and 10~.0%, and the acid temperature
is maintai~ed a~ greater than 120C. After a~sorbing the
~ulfur trioxide and being heated by the exothermic reaction,
the sulfuric a~id èxits the absorption zone and passe3 through
~he bottom outlet of the heat recovery tower. At the exit of
the absorption zone and outlet at ~he tower, the acid has a
conc~ntration of greater than 98~, preferably greater than 99%,
optimally between about 9g.~3 and about 100.0~ and a tempera-
ture o~ greater than 1~0C. After leaving the absorption zone,
the hot concentrated sulfuric acid stream passes through heat
exchanger 62 to remove the heat of absorption of he sulfur
trioxide, prior to again baing circulated through the heat
recovery tower. Preferably heat exchanger 6~ comprises a means
for indirect transfer of heat between two fluids, ordinarily a
solid partition such as, for example, the tube wall of a shell
and tube heat exchanger, or the plates of a plate type exchang-
er. ~he absorption of the sulfur trioxide increases the
concentration of the sulfuric acid; therefore, the sulfuric
acid must be diluted at some point. The required water may be
added within the heat recovery tower 60 or into the piping
between the heat recovery tower 60 and the heat exchanger 62;
however, it is preferred that the dilution water be added
following cooling of the sulfuric acid in heat exchanger 62 at
.
16
17-21 (~0~3)A
~3~ 4
a point in which sufficient mixing may occur before the
sulfuric acid enters the heat recovery towee 60. The dilution
water can also be added as a vapor. This increases the amount
of heat recovered and provides a means of upgrading atmospheric
pressure steam to a useable higher pressure Alternately, the
dilution water can be added as dilute sulfuric acid.
In Figure 1 the heat exchanger 62 is shown external
rom the heat recovery tower 60. While this is the preferred
arrangement, it is recognized that the heat exchanger 62 may be
located within the hea~ recovery tower 60~ In heat exchanger
62 the heat of absorption released in the process is removed by
heat transfer to a ~hird fluid. Preferably, th~ cooling fluid
is water and the heat of absorption is recovered ~y the
generation of low to medium pressure steam, for example, steam
having an absolute pressure between apprsximately 150 and 2000
kPa and normally between approximately 300 and 1200 kPa. Where
liquid wat~r is fed as the cooling fluid to the acid cooler,
all or part of the water may be converted to steam. The steam
produced by the heat recovery system is approximately 0.5
tonnes per tonne of acid produced when li~uid water is used for
acid dilution. This steam may be used within the manufacturing
complex surrounding the sulfuric acid plant or to generate
electrici~y. It is a common practice to remove low and/or
medium pressure steam from a turbogenerator for process use.
The removal of this steam reduces the electrical output of the
turbogenerator. The steam generated in t~e heat exchanger 62
may be used to reduce the amount of steam normally removed from
the turbogenerator or to eliminate this removal of steam alto-
gether. If additional steam ~s avai~able, it may be injected
17-21(,083)A
~3~J 3B14
into the turbogenerator 23. The cessation of removal of steam
from the turbogenerator increases i~s electrical output and the
injection of additional steam will also increase the turbo-
generator's electrical output. In a 2700 tonne per day sulfur
burning sulfuric acid plant, approximately 6 megawatts of addi-
tional elec~rical power can be produced as a result of the use
of the steam generated in the heat exchanger 62. Alterna-
tively, electrical power can be generated by using heat
exchanger 62 as ~he boiler for vaporizing an organic liquid for
use in driving a generator or otherwise producing work in an
organic Rankine cycle. The higher temperatures available
through the use of the heat recovery tower have now made such
usage economically feasible.
In this way the heat of absorption of sulfur trioxide
into sulfuric acid may be removed from the sulfuric acid
process in a useful form; that is, in a form which may be
utilized to produce a benefit either through use in a process
or through the generation of electricity. This is in contrast
to the current typical loss of this heat through removal by
cooling water and release of the heat to the atmosphere in
cooling towers.
Implementation of the improvements illustrated in
Figure l provldes a major increase in the overall recovery of
energy fro~ a contact sulfuric acid manufacturing plant. Prior
to the present invention, developments such as increased
converter reaotion gas strength, low tempera~ure economizers,
reduced gas stream pressure drop, heating of boiler feed water
with low temperature effluent heat, and u~e of suction drying
tower had improved to about 70% the recovery of energy in high
grade form, as electrici~y, steam or otherwise as a source of
process heat. Introduction of the improved process of the
18
17-21 ~5083~A
13~';3~
invention increases that high grade energy recovery efficiency
to 90-95%.
Following passage of the gas stream through the heat
recovery tower, the gas stream exits from the top of the hea~
recovery tower 60 and passes to the absorption zone of an
interpass absorption tower 64 where absorption of any sulfur
trioxide remaining in the gas stream takes place. Although
shown as comprising a separate-tower in Fig. 1, the interpass
sbsorber may comprise a separate stage positioned within the
same housing as ~he hea~ recovery z~ne. In a typical sulfuric
acid pla~t in wbich all of the sulfur trioxide is absorbed into
a su~furic acid stream in the interpass ~bsorption tower 64, it
is necessary to remove ~he heat of absorption. Thus, an acid
cooler 66 is provided for the sul~uric acid recirculating
~ 15 through the in~erpass absorption ~ower 64~ ~owever, in a sul-
furic acid plant utilizing the current invention, most of the
sulfur trioxide is absorbed into sulfuric acid in the heat
recovery tower 60: thus, only a small portion of the sulfur
trioxide remains to be absorbed in the interpass absorption
tower 64. Only a small temperature rise occurs within the
interpass a~sorption tower 64. In the circumstances this small
heat load may be removed elsewhere in the system; thu~, the
acid cooler 66 is usually unnecessary and may be eliminated.
The dotted lines used to show the acid cooler 66 in Figure 1
indicate that the a~id cooler 66 has been removed from the
process.
As a result of the high temperature operation of the
heat recovery tower, the gas stream exiting ~he heat recoverY
tower is relatively hot, and is in contact with hot acid. This
.
in turn results in stripping of sulfuric acid from the acid
19
17-21 (5083) A
~L3V3131~
stream into the gas stream. On passage through interpass
absorption tower 64, the gas stream is cooled, typically to a
temperature in the range of between about 7~C and about 120C,
preferably between about 75C and about 100C, by contact with
the acid stream circulating through the interpass tower. At
the gas exit ~rom the interpass tower absorp~ion zone, it is
preferred that the acid temperature be below about 120C,
preferably below about 100C, and a~ leas~ about 10C lower
than the temperature of the acid at the inlet of the heat
recovery tower. In a countercurrent interpass tower, th~ acid
preferably enters at a temperature of abou~ 75 to about
100C. Where an interpass tower is used, it is preferred that
the acid temperature throughout the interpass t~wer be lower
than the acid temperature at the inlet of the heat recovery
tower.
As the gas stream cool~ during its passage through
the interpass tower, sulfuric acid vapor condenses and is
either absorbed by the interpass tower acid stream or collected
by a mist eliminator positioned in or at the exit of the inter-
pass tower. Such lowering of the dew point of the ga~ streamhelps minimize condensation of acid and corrosion of downstream
ducts and equipment.
Where there i~ no separate interpass tower, the
interstage absorption may be accomplished essentially in a
single absorption zone with n heat recovery tower 60. In this
instance, the gas leaving the tower is preferably cooled before
return to the converter, for example, by passing it through an
economizer ~or heating boiler feed water. In this case, She
dew point of the gas stream is also preferably reduced ~o 75C
to 120DC by cooling i~ to a temperature in that ran~e. How-
ever, in the latter instance, condensation of sulfuric acid in
17-21 ~5083) A
the economizer creates conditions which are corrosive to many
metals. Thus, an economizer used to lower the dew psint should
be constructed of a metal of the type discussed above for use
in the heat recovery tower acid cool~r. It is also important
to control the addition of dilution water to the heat recovery
tower recirculating stream so that the acid condensed in ~he
economizer has a strength of at least 98.~.
Alternatively, the dew point may be lowered by pas-
sage of the gas stream through a direct contact cooler, such as
a packed tower, countercurren~ to sulfuric acid flowing at a
rela~ively low flow rate, i.e., so that th~ temperature of the
acid leaving ~he contact cooler approaches ~he temperature of
the acid at the inlet of the heat recovery tower. Thus, con
tact with the acid is effective in reducin~ the dew point of
~ 15 the gas (and absorbing residual sulfur trioxide), but the acid
is heated to a temperature high enough so ~hat i~ may be
blended into the acid entering the heat recovery absorption
zone withou~ significantly reducing the temperature at which
energy is recovered from the heat recovery system. The ~ela-
tively small temperature difference between gas and liquid
throughout the cooling zone minimizes acid mist formation.
Such a packed tower cooler may be constructed either as a
separate vessel or as a separate stage within the same housing
that contains the heat recovery absorp~ion zoneO In either
instance, it is pre~erred that the temperature of the cooling
acid that i9 in contact with the gas leaving the cooling zone
be at least 10C lower than the acid temperature at the inlet
of the heat recovery absorption zone. Whatever approach is
taken, lowering of the gas dew point is important for protect-
ing carbon s eel heat exchangers conventionally locateddownstream of ~he converter to which ~he gas stream is
returned.
17-21(~083)~
~3¢~3~
The remainder of the sulfuric acid process shown in
~igure 1 is well known The sulfu~ trioxide depleted gas
stream is returned to the second oxidation s~age 34 of the
converter 30 to complete oxidation of the remaining sulfur
dioxide. This final passage through an oxidation stage will
complete the conversion of sulfur dioxlde ~o sùlfur trioxide.
The gas stream exits from the converter 30, passes ~hrough an
economizer ~8 for cooling, and passes through ~he final absorp-
tion tower 70 in which the sulfur trioxide in the gas str~am is
absorbed into sulfuric acid. The amount of sul~ur trioxide
present to be absorbed is much smaller than that which is
absor~ed in the hea~ recovery tower and interpass absorption
tower; thus, only a small amoun~ of heat is created by the
absorption of the sulur trioxide into sulfuric acid in the
- 15 final absorption tower 70. Following absorption of the sulfur
trioxide, the gas stream is released to the atmosphere.
While the above descrip~ion is for an interpass
plant, i~ is contemplated that the heat recovery tower can be
installed upstream of the absorption tower in a non-interpass
plant. For some operating conditions, as noted above, it is
also contemplated that the heat recovery tower can replace ~he
interpass absorption tower in an interpass plant, or the
absorbing tower in a non-interpass plant. As further noted
above, this is not the preferred mode of opera~ion for a heat
r~covery tower located between converter stages, unless the
heat recovery tower includes a cooling zone to reduce the high
sulfuric acid vapor content of the gas exiting a hea~ recovery
tower, which would otherwise lead to corrosion of downstream
equipmen~. Without a cooling zone, it is not preferred in the
case of final absorption either, since residual sul~ur trioxide
17-21(~083)A
~ 3~33~L4
and sulfuric acid vapor contained in the exit gas from the heat
recovery tower constitute undesirable atmospheric emissions if
not recovered in a lower temperature final absorption tower or
in other emission control apparatus. Moreover, in the case of
either an interpass or final absorption system, problems of
corrosion and/or emissions would be aggravated by any upsets in
the operation of the heat recovery tower.
~ eferring to Figure 2/ it can be seen that at
constant temperature, both the corrosion rate of certain alloys
and the degree of sul~ur trioxide absorption decrease rapitly
as the sulfuric acid concentration increases. It has been
deter~ined for this inven~ion ~hat there is a narrow window of
operation in which the corrosiveness of the sulfuric acid to
certain alloys at high ~emperature is greatly reduced while the
absorption of sulfur trioxide into sulfuric acid is maintained
at a sufficient level to remove the sulfur trioxide from the
gas stream passing through ~he heat recovery tower.
Referring now to ~igure 3, the heat recovery tower S0
of this invention and its associated pipi~g, including the heat
exchanger 62 and pump 63 are shown. The sulur ~rioxide laden
gas stream, leaving the first oxidation stage 32 of converter
30, enters the heat recovery tower 60 ~hrough bottom inlet 82.
The gas stream passes upward through the absorption zone com-
p~ising the bed of packing 61 in which ~he gas contacts the
sulfuric acid stream and the sulfur trioxide is absorb~d in~o
the ~ulfuric acid~ The sulfur trioxide depleted gas stream
exits ~rom the hea~ recovery tower 60 through mist eliminator
89 and top outlet 88. The sulfuric acid enters the heat re-
covery tower 60 through top inlet 84 and is sprayed through a
plurality of acid distri~utors 85 onto the upper surface of the
bed of paoking 61. The sulfuric acid flows downward through
.
17-21 (5083) A
~3~3~ ~
the bed oE packing 61 in which it comes in contact with the
rising sulfur trioxide laden ~as stream and the sulfur trioxid~
is absorbed into the sulfuric acid. The absorption of the sul-
fur trioxide into the sulfuric acid is an exothermic process.
The hot sulfuric acid enters the heat recovery tower 60 at a
temperature greater than 120C and, after absorbing the sulfur
trioxide and being heated by the exothermic reaction, the sul-
furic acid exits from the heat r~covery tower 60 at a temper-
ature as high as about 250C. The sulfuric acid exits from the
heat recovery tower through bottom outle~ 86 and is pumped by
pump 63 ~hrough heat exchanger 62 to remove, by transfer to a
third, fluid, the heat generated by the absorption of the sulfur
trioxide prior to again being circulated through tower 60.
Preferably, the acid Qtream has a concen~ration of greater than
~ 15 98% and less than 1013 and a temperature of greater than 120C,
more preferably greater than 125C ~hroughout the course of
heat transfee to the another fluid. It is also preferred that
the third fluid be heated to a temperature of at least 120C,
more preferably greater than 125C, in its passage through
exchanger 62. Corrosion of the heat exchangec is minimized if
the acid concentration is at least 99~ throughout the course of
heat transfer. Following passage through heat exchanger 62, a
portion of the sulfuric acid is removed as p oduct through ~he
acid pipe 95. In addition to the rise in the temperature Qf
the sulfuric acid, the absorption of the sulfur trioxide
increases the concentration of the su}uric acid; therefore,
the sulfuric acid must be diluted~ The sulfuric acid may be
dilu~ed by ~he addition of dilute sulfuric acid or water in the
liquid or Yapor state; and the terms water or dilution water
will be used ~o refer to the diluent. The required wa~er for
'
24
17-21 ~50~3)A
~3~38~
dilution of tne acid is shown being added in line through pipe
90 upstream of ~he absorption zone. It is preferred that the
dilution water be added, as shown, in the piping 91 between the
heat exchanger 62 and the entry of the sulfur~c acid into the
S heat recovery tower 60 th~ough top inlet 84. However, the
addition of the dilution water at this point is not required
for this inventlon. ~he dilution water may be ~dded prior to
passage of th~ sulfuric acid through ~he heat exchanger 62 or
may be added to the sul~uric acid within the heat recovery
~ower 60, ei~her upstream, downst~eam or within the absorption
æone omprising packing 61. The pref~rred location for the
addi~ion o~ dilution water, represented by pipe gO, allows the
alloy pump and heat exchanyer to operate at the highest sul-
furic acid concentration, which gives the lowest rate of cor-
rosion, at any given temperature within the operating range.It is particularly preferred that the acid strength be main-
~ained above 9g~ throughout the alloy heat exchanger. Acid
strength downstream of the dilution point is monitored by a
conductivity probe. Conveniently, ~he rate o~ dilution water
addition is controlled by throttling a feed control Yalve in
response to a feedback con~roller that in turn operates in
response to the conductivity probe downstrea~ of the addition
point.
For the purposes of this invention it has been
determined that ~he preferred operating conditions for th~ heat
recovery tower compri~e sulfuric acid temperatures greater than
120C and concentrations greater than 98% and less than 101~,
more preferably 98.5% to 100. n~ . operation of ~he hea~
reco~ery tower under these conditions provides the reduced
s~lfuric acid corrosiveness to cer~ain alloys necessary for
.
17-21 (5083) A
~3~ 4
operation of the equipment for long periods of time, and a high
degree of absorption of sul~ur ~rioxide into the sulfuric
acid. A reduced level of sulfur trioxide absorption would
reduce the amount of energy recovered in useful form. To pro-
vide yood absorp~ion efficiency, the temperature of the acid atthe outlet of the tower, and at the inlet of the acid cooler,
is preferably not greater than about 250C. Within that limit,
however, the acid temperature may be varied rather widely,
depending on the manner in which the absorption heat is
ultimately to be utilized. Thus, for examplei the e~ergy may
be used ~o generate low pressure steam for concen~ration of
phospporic acid in an integrated sulfuric ac~d/phosphoric acid
plant, in which case the temperature of the acid should be
above 120C through the hea~ exchanger, and consequently some-
what higher than tha~ at both the inlet and exit of the heatrecovery ~ower. To provide a working margin to compensate for
line pressure drop, phosphoric acid evaporator steam chest
fouling, and the like, it is preferred that the acid tempera-
ture throughout the heat exchanger ~e above 130C, more
preferably above 140C, and most preferably above 150C.
Temperature~ significantly above 150C are generally
unnecessary in an integrated sulfuric acid/phosphoric acid
plant.
~owever, it has been found that the process of the
invention is capable of operating with substantially higher
acid temperature~, both at ~he inle~ and exit of the tower and
throughout thP heat exchanger. Operation at such higher ~em-
pera~ures makes pos~ible the generation of medium pressure
steam for such applications as the operation of a tur~ogenera-
tor for the production of eleotricity. In such instance, it is
26
17-21 (5083)A
~3~
preerred that the acid temperature throughout the heat
exchanser be above 150C, more preferably above 1759C, and even
more preferably above 200C. To provide such temperatures
throughout the heat exchanger, it is, of course, necessary to
maintain even higher temperatures at both the exit and inlet of
the heat recovery tower. For certain alloys, it has been found
that at some temperature above 120C the rat~ of corrosion in
98 101% acid actually begins to decrease with temperature,
par~icularly at concentrations above 99~. Fsr such alloys, an
especially attractive mode of operation may be to maintain an
acid temperature entering the heat exchanger at somewhat above
the ~oint where the negative relationship between temperature
and corrosion ra~e begins to obtain.
The upper acid concentration limit of 101% is estab-
lished on the basis of achieving a high degree of absorption in
~he heat recovery tower while opera~ing at conventional acid
plant pressures which are near atmospheric. The upper acid
concentration limit can be extended to approximately 105% if
the heat recovery tower is operated at a pressure up to 1000
kPa. Where the tower is operated under pressures up to 1000
kPa, the preferred acid temperature range in the tower is
between about 150C and about 270C.
It has been de~ermined that certain materials are
particularly suitable for construc~ion of the heat recovery
tower 60, the heat exchanger 67, the pump 63 and the other
equipment associated with them. In partioular certain alloys
have baen found to be preferable for their corrosion resistance
at the conditions under which the heat recov~ry tower, heat
exchanger and pump will be operated. Similarly; such alloys
can be utilized as materials of construction for other equip-
men~ or components exposed to sulfuric acid at ~he above-
described concentrations and temperatures, for example, in
27
17-21 ( ~083) A
~3'~ 4
piping, ductwork, heat exchangers or other equipment loca~ed
downstream of the heat recovery tower. These alloys exhibit
minimal, industrially acceptable, corrosion at acid concentra-
tions of 98-101% and temperatures above 120C. It has been
determined that the corrosion resistance of these alloys can be
characterized in terms of the percentages o the major alloying
constituents. Provided that the acid concentration is main-
tained within the ranges specified hereinaboVe, it has been
determined that the rates of corrosivn are sutprisingly low for
a su~stantial range of iron/chromium, nickel/chromiUm, and
iron/chromium/nickel alloys, even at highly elevated tempera-
tures substantially above 12~C. As indicated above, the
alloys used for hea~ exchanger ~u~es, and other equipment
elements exposed to the hot concentrated heat recovery tower
- 15 absorber acid, should be consti~uted of either a ferrous alloy
containing chromium, an iron-nickel alloy containing chromium,
or a nickel alloy containing chromium. In each instance, the
alloy should have an austenitic, ferritic, or duplex
structure.
Generally suitable stainless steel (ferrous) and
iron-nickel alloys may contain between about 16% and about 30
by weight chromium, up to about 33~ by weight nickel, up to
about 6~, pre~erably up to about 4%r by weight molybdenum, and
between about 35~ and about 83~ t pre~erably about 46~ to about
83%, by weight iron. Other alloying elements such as copper,
manganese, silicon, cobalt, tungsten, niobium, titaniuml
nitrogen, sulfur, phosphorus, and carbon may also be present in
minor proportions.
28
17-2i!~G83iA
~3~`3~
Nickel base alloys useful in the method of the
invention may generally contain between about 32% and about 76%
preferaDly about 37% to about 43~, by weight nickel, be~ween
about 1% and ~bout 31%, preferably about 23% to a~out 31%, by
weight chromium, between about 2~ and about 463, preferably
between about 16% and about 28%, by wPight iron, and up to
about 28%, preferably up to about 3~, by weight molybdenum. As
in the case of stainless steel type and iron/chromium/nickel
type alloys, the nickel base alloys may also contain minor
proportions of other allsying elements.
The alloys best suited $or service in this heat
reco~ery system have compositions which give a corrosion index
(CI) greater than 39,
CI ~ 39
l~ as defined by the following equation:
.
CI = 0.35(Fe+Mn) + 0.70(Cr) + 0.30(Ni) - 0.12(Mo)
where:
Fe 5 the weight percent of iron in the alloy,
Mn = the weight percent of manganese in the alloy,
Cr = the weight perc~nt of chromium in ~he alloy,
Ni - the weight percent of nickel in the alloy, and
Mo = the weight percent of molybdenum in the alloy.
For alloys of the above-described type, industrially
acceptable corrosion ra~es are a matter oE economics. In
addition to varying with general economic factors bearing on
the profitability of a given plant, ~he acceptable rate of
29
17-21(5083)A
13~13t3~L4
corrosion varies with the function of the particular piece of
equipment for Which an alloy is considered as a candidate. Por
heat exchanger tubes, however, it may be said that an
acceptable rate is generally in the range of 0~05 to 0.08
mm/yr. For piping, pump tanks, distributor~, or the shell of
an absorption tower, slightly to moderately higher rates of
corrosion may be tolerated. Based on corrosion data obtained
in connection with the development of the process of the inven-
tion, it has been found hat stainless steel type alloys are
generally preerred to nic~el allvys for use in equipment
exposed to the heat ~ecovery towe~ abso~ber acid at ~empera-
turesq above 120C.
A preferr~d material of cons~ruc~ion is 304~ stain-
less steel which typically contains about 18-20% by weight
chromium, about 8-12~ by weight nickelJ up to about 2~ by
weight manganese, up to about 1.0~ by weight silicon, up to
about 0.030% by weight carbon, up to about 0.045~ phosphorus,
and up to about 0.030~ sulfur and the balance essentially
iron. Another preferred materi~l is 309 stainless steel which
comprises approximately 20-24% by weight chromium, about 12-15
by weight nickel, up to about 1.0~ by weight silicon, up to
about 0.045% phosphorus, up to about 0.03~ sulfur and the
balance essentially iron. Especially preferred is 310
stainless steel`which contains between about 24~ and about 26%
by weight chromium, between about 19% and about 22% by weight
nickel, up to about 2.0% manganese, up to about 1.5~ by weight
silicon, up to about 0~08% by weight carbon, up ~o about 0.045%
phosphorus, up to about 0.030% sulfur, and the ~alance
essen~ially iron. Other particularly useful alloys include
`
17-21 (5C83)A
~3~ 4
E-Brite Alloy 26-1, which comprises between about 25.0% and
about 27.5% by weight chromium, between about 0.75% and about
1~50% by weight molybdenum, between about 0.05% and about 0.20
by weight niobium, up to about 0.50~ by weight nickel, up to
about 0.20% by weight copper, up to about 0.40% by~ weight
manganese, up ~o about 0.02% by weight phosphorus, up to about
0.02% by weight sulfur, up to about 0.40% by weight ~ilicon, up
to about 0.01~ by weight carbon, up to about 0.01S% by weight
nitrogen, and the balance essentcially iron; and ~erralium Alloy
10 255, which comprises between about 24.0% and about 27.0% by
weight chromium, between about 2O0% and about 4.0% by weight
molybdenum, between about 4.5~ and aboutc 6.5~ by weight nickel,
between about l.S~ and about 2.5% by weight capper, between
about 0.10% and about 0.25% by weight nitrogen, up to about
- lS 1.0~ by weight silicon, up to abou~ 1.5% by weight manganese,
up to about 0.04% by weight carbon, up to about 0.04% by weight
phosphorus; up to abou~ 0.03~ by weight sulfur, and the balance
essentially iron. As generally exemplified by Alloy 26-1,
Alloy 255, 304L stainless steel, 309 stainless steel and 310
stainless steel, a preferred range of alloys compris~s between
about 16% and about 30% by weight chromium, up to about 23~ by
weight nickel, up to about 4% by weight molybdenum, and the
balance essentially iron~ Other conventional alloying elements
may be present in minor proportions.
While the~e alloys of the above-described type are
pref2rred for construction, sometimes conventional materials of
construction for the hea~ recovery ~ower will be more cost
effec~ive. A~ these times the heat recovery tower can be
construc~ed of carbon steel and lined with a ceramic ma~erial
17-21 t5083)A
13E~
to protect the carbon steel shell from the corrosive attack of
the sulfuric cid. Such construction is ve~y similar to tha~
typically utilized for the interpass absorption tower. In the
case of heat exchanger 62, either ~he entire exchanger or only
5 the means for ~ransfer of heat ~rom the acid to the third
fluid, for example, the ~ubes, tube sheet, and channel3 of a
shell and tube exchanger, are fabricated of the corrosion re-
sistant alloy. In the latter case, the acid is passed through
the tubes of the exchanger and the shell is constructed of a
- 10 rela~ively low cost material such as mild steel for containment
of a relatively non-corrosive cooling fluid.
The function of the heat recovery tower is to contain
the sulfuric acid and the sulfur trioxide laden gas stream and
to provide for the contacting o~ these two streams such that
~ 15 the sulfur trioxide is absorbed into the sulfuric acid. As the
abs~rption of the sulfur trioxide into the sulfuric acid is an
exothermic reaction, the acid is heated. It is preferred that
the sulfuric acid entering ~he heat recovery tower have a
temperature greater than 120C. The heat of absorption will
raise this temperature, generally within the range of 10-60~C,
more ~ypically by 20-40C. The exit temperature ~rom the tower
may be as high as about 250C. While this is the preferred
operating range or the acid temperature, it is possible to
operate at higher temperatures by increasing the pressure or
reducing the degree of sulfur trioxide absorption. In the
preferred temperature range low to medium pressure ~team, for
example, steam having a pressure between approximately 150 and
2000 kPa, pre~erably 300-1200 kP , may be generated. Increas-
ing ~he steam pressure will require a cor~esponding increase in
the temperature of ~he sul~uric acid entering the heat recovery
tower.
32
17-21 (5083)A
13~:3~
Steam generated by transfer of the absorption heat
may be used in a variety of applications. Relatively low
pressure steam at a temp~rature greater than 120C, more
preferably at a temperature greater than about 125C, is
advantageously used as a source of energy for concentrating
phosphoric acid in an integrated sulfuric/phosphoric acid
plant. As noted above, it may be advantageous to generate
steam at somewhat higher temperatures to compensate for such
problems as line pressure drop between the heat exchanger for
cooling sulfuric acid and the phosphoric acid evaporator, and
fouling of the tubes of the phosphoric acid evaporator steam
chest. Thus, for such applications, it is preferred that the
third fluid be heated to a temperature of at least about 130C,
more preferably at least about 140C, and most preferably at
- 15 least about 150C.
Alternatively, steam may be generated at higher
temperature and pressure for use in operating a turbogenerator
or for delivering process heat at temperatures higher than that
re~uired, for example, in concentrating phosphoric acid. For
such higher temperature applications, it is preferred that the
other fluid to which the absorption heat is transferred,
whether steam or some other fluid, be heated in the sulfuric
acid heat exchanger to a temperature o~ at leas~ about 150C,
more prefsrably at least about 175C, most preferably to at
least about 200C. It will be understood that the inlet
tempera~ure to the sulfuric acid heat exchanger will exceed the
temperature ~o which the third fluid is heated by an increment
o~ roughly 20-70C, mo-e typicallg 30-50C.
17-21(5083)A
13'J3~
In a particularly preferred mode of operation, the
tower is operated at essentially atmospheric pressure, the acid
leaving the tower 60 is maintained at a temperature greater
than 150C, and steam is generated in heat exchanger 62 at a
pressure o~ 440 kPa absolute or greater.
Again, it must be stated tha~ this invention is not
limited to the configuration shown in Figure 3. ~he héat
exchanger 62 is shown located externally of the heat recovery
tower 60~ This is Eor numerous reasons the p~eferred embodi-
ment; however, the heat exchanger may also be located withi~the heat recovery tower 60. Similarly, the dilution uater is
shown being added through pipe 90 into pipe 91 betwee~ the heat
exchanger 62 and the top inlet 84 to the heat recovery tower
60. With this preferred configuration the heat exchanger 62
and pump 63 always contact acid of the highest concentration
which provides the lowest corrosion rates and the highest
degree of protection to the heat exchanger and pump. It is
also contemplated, and is a part of this invention, that the
dilu~ion water may be added into pipe 92 which is located
between the bottom outlet 36 of heat recovery tower 60 and the
heat exchanger 62. It is also contemplated that the dilution
water could be added directly to the heat recovery tower 60 or
to the gas stream entering the tower. It is noted that the
addition of dilu~ion water downstream of ~he absorption 20ne,
or in the zone near the acid exit point, will reduce the
sulfuric acid concentration beore it passes through the pump
63 and heat exchanger 62 and that this lower sul~uric acid
concentration will result in a higher corrosion rate for the
heat exchanger 62 and pump 63.
34
17-21(5083)A
:~3~
Illustrated in Figure 5 is a particularly preferred
embodiment of the process of the invention. In this system,
boiler feed water is preheated in a heat exchanger 96 by
transfer of heat rom final absorption tower circulating acid,
and in a heat exchanger 100 by transfer of heat from acid
circulating through drying tower 14 and interpass a~sorption
tower 64. Preheated boiler feed water is sent to a de-aerator
~no~ snown), and a portion of ~he water exiting the de-aerator
i returned to another heat exchan~er 98 where it is further
heated by transfer of heat from the produc~ acid stream ~hat is
withdrawn from the heat recovery ~ower circulating loop via
disc~arge line 95. ~ m~jor portion of the boiler feed water
stream leaving exchanger 98 is delivered to h~at exchanger 62
- where it is vaporized to produce steam ~hat may be used for
power generation or process heat. However, a side stream of
the water leaving exchanger 98 is diverted to line 90 through
which it is injected as makeup water into the heat recoverY
tower circulating loop. Depending on the volume of feed wa~er
and the particular conditions prevailing in the final
absorption tower, the boiler feed water m~y be partially
vaporized by the time it reaches pipe 90.
A circulating acid pump tank 99 is provided for
drying tower 14 and interpass absorption tower 64. Product
acid from exchanger 98 is delivered to tank 99. Some or all of
~he acid returned to ~ower 64 may bypass exchanger 100 through
line 101, thereby increasing ~he temperature of the ~cid in ~he
pump tank and allowing the boiler feed wa~er passing through
the exchanger ~o be heated to a higher temperature than would
otherwise be feasible. A product acid stream is removed ~rom
tank 99 through boiler ~e~d water preheater 109.
.
17-21 (5083)A
3~
Figure 4 is a graph which shows the operating cycle
of the heat recovery tower 60 in terms of the temperature and
concentration of the sulfuric acid. Shown on the graph are
isocorrosion lines for type 304L stainless steel. Also shown
is a line representing the sulfur trioxide equilibrium between
typical inlet gas and outlet acid. Thi~ equilibrium line
defines the limiting conditions for the absorption of sulfur
trioxide in the acid at atmospheric pressure. The operating
cycle of the heat recovery ~ower is shown as a triangle A3C.
The loca~ion of points A, ~ and C in ~he process may be found
in Figure 3 and Figure 1. Point A of the operating cycle
repr~sents the conditions, the temperature and concentration
of the sulfuric acid, at the bottom outlet 86 ~rom the heat
- recovery tower ~0. Point B represents the Qulfuric acid
conditions after pas~age through the heat exchanger 62. Point
C represents ~he sulfuric acid conditions as the sulfuric acid
is entering the heat recovery tower through top inlet 84,
after the addition o~ the dilution water.
Looking at Figures 3 and 4, it is of interest to
discuss a complete operating cycle by considering the flow of
the sulfuric acid starting at point C. The sulfuric acid
enters the heat recovery tower 60 at a temperature of
approximately 165C and a concentration of approximately 99~.
As the sulfuric acid passes downward through the heat recovery
~ower and absorbs ~he sulfur trioxide from the gas stream
passing upward through the hea~ recovery tower an exothermic
reaction takes plaoe. The temperature o~ ~he sulfuric acid
rises and the concentration increases. At the outlet of the
heat recovery tower, represented by point A, the sulfuric acid
has a temp~ratur~ of approximately 200UC and a concentr~tion
of approximately ~00%. Following the heat recovery tower, the
17-21 (5083)A
~3~3B14
sulfuric acid flows theough heat exchanger 62 and is cooled.
Re~erence point B is at ~he exit of the heat exchanger 62. At
this point the acid has been cooled from approximately 200C
to approximately 157C and the acid concentration has remained
constant. Before the sulfuric acid en~ers the heat recovery
tower again, dilution water is added. The addition of water
to the concentrated sulfuric acid reduces the concentration
and causes a rise in temperature. ThereforP, the ~riangle of
the operating cycle shows that the concentration of the sul-
furic acid is reduced from approximately 100% to approximately99% and that, during this reduction, the temperature of the
sulf~ric acid rises from approximately 157C to 165C. At
~his point the sulfuric acid again enters the heat recovery
tower and ~he opera~ing cycle is repeated.
lS It is easy to see in Figure 4 the relationship
between the operating cycle of the heat recovery tower 60,
which includes the heat exchanger 62, the corrosion rates for
type 304L stainless steel at di~ferent temperatures and sul-
furic acid strengths, the equilibrium line for the absorption
of sulfur trioxide into the sulfuric acid, and the ~emperature
of the steam that may be generated. This operating cycle is
compared to the operating cycle of a typical in~erpass absorp-
tion tower represented by the triangle DEF. The lo~ation of
points D, E, and F in the process may be found in Figure 1.
Point D represents the conditions o~ temperature and acid
concentration leaving the interpass tower. Point E represents
typical condition where the acid in the pump tank has been
diluted with water and cooled by mixing wi~h colder acid
d~aining from the drying tower circuit. Point F represents
the temperature and acid concentration lPaving ~he acid cooler
and recirculating to ~he interpass absorbing ~ower. It can be
17-21(5083)A
3~
seen in Figure 4 that the heat recovery tower of this inven-
tion permits absorbing sulfur ~rioxide at significantly higher
temperature than previously practiced, while reducing cor-
rosion rates for type 304L stainless steel by a ~actor of ten
or more, compared to that obtained at acid t0mperatures and
concentrations characteristic of past practices. Significant
reductions in corrosion rates were ~ound ~sr the other alloys
Whlch have a corrosion index (CI) greater than 39, with the
extent of the reduction dependent upon the specific alloy.
It may be noted that the discoveries of the present
invention with respect to materials of construction have
gene~al application to the storage, transpor~ation or handling
of hot concentrated sulfuric acid streams irrespective of the
- particular process or other operation that may be involved.
~hus, a basic method has been discovered for storing, trans-
porting or handling sulfuric acid having a concentration
greater than 98.5~ and less than 101~ at temperatures greater
than 120C. This method comprises containing the açid in a
conduit or vessel constituted of an Fe/Cr, Ni/Cr or Fe/Cr/Ni
alloy having an austenitic, ferritic, or duplex structure and
having a corrosion index corresponding to the algorithm set
orth hereinabove. Particularly preferred alloys have com-
posiitio~s falling within the ranges particularly specified
hereinabove.
Further in accordance with the invention, a method
has been di-~covered for condensing sulfuric acid vapor from a
gas stream satur~ed with sulfuric acid vapo~ at a emperature
above 120C. This me~thod is of important value in reducing
the dew point of the gas stream so as to reduce its corrosive-
; 30 ness to materials or equipment with which it may later come
into contac~. It is particularly useful in oooling and reduc-
: . ing the dew point of gas exiting the heat recovery zone in the
38
17-21 (~083)A
13~3B14
heat recovery process of the present invention. In accordance
with the novel method for condensing sulfuric acid vapor, the
saturated gas stream is brought into contact with a heat
transfer surface having a tempera~ure below the saturation
temperature of the ~as with ~espect to sulfuric acid vapor,
but above 120~C and hiyh enough so that the sulfuric acid
which condenses on the heat transfer surface has a concentra-
tion greater than 98.5~. The heat transfer surface is com-
prised of a errous alloy containing chromium, an iron-nickel
alloy containing chromium, or a nickel alloy containing
chromium, and has either an austenitic, ferritic, or duplex
struc~ure. The composition of the alloy further corresponds
to the corrosion index set forth hereinabove.
The novel method for condensing sulfuric acid vapor
from a saturated gas stream may be implemented, for example,
by passing the gas stream exiting the hea~ recovery tower of
the invention through an economizer having tubes constituted
o~ the above described alloy, and controlling the temperature
of the cooling fluid inside the economizer tubes so that the
outside of the tube wall in contact with the gas stream and
condensate has a temperature greater than 120C and high
enough so that the condensate has a concentration greater than
98.5~.
EXAMPLE 1
Sta~ic corrosion immersion ~ests were carried out in
hot concentrated ~ulfuric acid over a range o~ temperatures to
determine ~he corrosion resis~ance of carbon steel and various
alloy me~als whose compositions are set forth in Table 1.
39
17-21 (5083)A
~3
W
U~ ., o
.~ ..
, o o
~ C c
,, .~ ~
~, , 1 o o
~ ~ o . ,
.c ~ V C~ o o
` U~ `
~ a~
Q. ~ ~ O 0 ~
~ ~ ~ o ~ o
J
U~ s ~
~J
Ut ~ o
;
o ~, o
E~
~1 ~a
. t,
~; D ~ Z I I o
o
U~
O ~
O~1
V O I ~
o~ a~
1:; V
U~
.
OV ~1 ~ C~
~a ~ t~ o~
U~
s u~ : ~n
C
c ~
O ~ U~
aJ
o o
In E sn ~ c~ ~r ~r
' ,,., ~ o
.
.
qO
'
17-21(;083)~
~3~3~
~, to
o~ ~C
.r~ V
,c o o
U
~ o C~ o o
0 C~
3 Q~
o o ~: o
U G~ 13
_ ~n ~ v o ~ ~
3 ~ X O O OO
~ C: Ql ~
O ~
ca ~ ~:
~ ~ Ul ~
r-1 r-l N
o 1~
,~ ~ C h¦ Wl C
E ;~ ~ æ
O
.
:: :
41
:
i
17-21 (5083~ A
~3L3~1;3~
o
~ CO
q~ ~ o
V~ s C o Z
3 ~ o
~, ,
.. ..
Q~ ~ u~
s , V
, U7
o In In
~ 3 ~ 4 ~ ~) o ~ ~
c E~ ~ o
a~ ' o~
~3 ~. o a)
o _~ ~
o
ov t) a~l ~ n ~ ^ ~ '
~ 3
~
s ~q ~
` .C ' 5E
~ ~ ~ s
E: ~ n~ 3 o s o
~n O aJ ~ 4 3 ,t
o ~ ~
.r~ ~ ~ ~ Z
U~ o
42
17-21 (~083)A
. æ z
~ Z
.~ ,.
s o o C~ o
C V C
~ ~ I~
a~ ~ o ~ . ~ _~
~Q ~ -- o ~ -- o O
~ ~ ~ .
o~
tn
~ ~ ~
E~ c tn o
-- o o -- o o
~ ~ ~ .
~.U~ o
.~ E~
Ul U.
C ,~
~ u~ _, ~
_l U~ C Z ~ CO ~ U'l
~ ~ U~ ~
~U~ ~
a~ o ~ _
~3 ~ h ¦ _~ N ~ 1
O ~ U~
~ E~ . -- N
o ~ a)
~:: U
t) ~ ~ U~
O ~ rl a~ u~ . O
~a
J.1 Q~ C 4~ ~
r~ _ _
O ~ 3 : ~
~ .
U: O .c U~ U~ .~
aJ
1 ~ . "~ J~
O U~ ta
t) ~ ~ ~ :
~a ~ O
~ ~ ~ O O .~ V ~
h
.J -~1 h
er er S: ~ ~ Ll
C~ ~ t.~
O
43
17-21 (5083) A
~3ll 3
u~ s~
.t; O o o
V ~ .c
o ~ _~ o ~
~ U~ ~
3 ,_, ~, .
O O O O
V 0 C
~U~ S O
cE~
,
C
O.
--~ ~ C u~ O N 1
~ o~ ~, O
~ o = ~
o 1~ a
o ~ q . .: ~ ~ ~r N
s 3 1 ~ `D ~ ~ ~ eP
8::~ d~c=~ :~ : ~o=
:: ~ ; ~ C ~ ~ : ~: Z
1~ ~ 4 ~ ~ ~ ~ s:~
W ~ 0 nJ ~ ~ E
: I ~ CI. O 9 $
~ ~ ~ ~ ~ C C 3
q4
17-21 (5083) A
~3~ 14
~ ~ r~
o o o
U~ ,c ~ U V
.,.~ J~
J- .C C~ ~0
C ~ ~
~, C s
.. ..
v ~ s Vl
s 9 ~ _ _ _
~u
~o ,.
cr r~O O ~ ~ ~ ~ C~
C U~ . . . ~1 ~ Il
~o ~ ~, o o o
C
U~ ~ o~ ... _
g .,,~ ~o ~ r~
o ~ ~ ,,
. C
~
Q~ 0
_~ ~ ~ Cl~
_
U~ ~
r~l ~ ~ 1~ r~~D U7 r~ 0 0 M
O
U~ L~
a~ ~ o ~
i~ V~ 'O CO r~
U o U
O
C
0
C o r- U~
o ~ , . ~ ~ _
~ la ~ C4 Ou~ N CO ~ ~
V Q~ ~: ~ P~i ~1
.
O ~ V~
~0
~ O
_/ ~ C ~ r~ U~
~ 4 (~ O a~ t~ ~ o o
.,., ~ "~ ~ ~ ~ C~
E ~ ~ ~ " ~ ~D ~D ~1 ~ ~ u~
a~ ~ ~ .~c o o ,~c ~ ~ ~o
_~ ~1 ,1
U ~ .. ~ ~ ~ L~ V
a~ at ~ a~
3 ~ e ~ V o ~ ~ 6~ E E
_~ g ~ ~ u~ In U ~ ~ ~ 2 3
v aJ ~ . n~ C
Z ~ 4
o U~
--I
.
17-21(50833A
~3ql3~
For purposes of these tests, samples were cut to a size of
approximately 10 mm x 6 mm x 2 mm and the surfaces of the
samples ground smoo~h with a 129-grit belt surface grinder.
These samples were immersed in various sulf~ric acid solutions
in 80 ml capacity Teflon cups fabricated by Savillex Corpora-
tion, The cups were provided with screw caps de~igned to
afford a seal. A glass rod grid was inserted in the bottom of
each cup to support the sample with minimum contact, so that
there would be little difference be~ween the areas of exposure
to the acid on the top and bottom of the sample. ~f~er the
sample was placed on ~he glass qrid, a portion of sulfuric acid
~50 ml) of the desired stren~th was poured into ~he cup, thus
providing a Eatio o acid voluma to sample surface area of 29
ml/cm2, a ratio wi~hin the range of 20-40 ml/cm2, as recom-
mended in ASTM Method G-310 The acid solutions used in the
corrosion tests were prepared by mixing 98% by weight sulfuric
acid (Axton-Cross) with either water, to provide solutions more
dilute than 98%, or with 20~ oleum to provide solutions of a
strength greater than 9R4, Acid concentrations were determined
by measuring the conductivity of the solu~ion at a prescribed
temperature. After the acid solu~ion was poured into a cup
containing a corrosion sample, the cup was sealed and inserted
into an oven at the test temperature and the sample exposed to
the acid solution at that temperature for seven days. After
exposure, the cup was removed from the oven and allowed to
cool, and the sample then removed and rinsed. Most samples
required no extensive clean-up after rinsing, but some were
cleaned with a rubber era er to remove an adherent film.
Thereafter, ~he samples were weighed ~o detarmine loss of m~ss
on corrosion, and from such loss of mass the linear corrosion
rates were determined. Set forth in Table 2 is a compilation
of the data obtained in Shese static immersion tests. This
data provided ~he basis for the co~rosion index ~et forth
- hereinabove.
46
17-~1 (5083) A
~3~3~
o o o o o
o ~ o . ..... . ..
o o o o o o
_,
Cr~ ,, ~ ~ ", ~ U~ U~ U~ ._
~ _, ~ o o o C~ o o o o o
O cn o o o ~ o o o o o
~ a~
Z
_. U U~
~n ~ . Q O O I O I I ~ I O
~ C:~ _~
o aJ ~ r~
_~
.
~ _l ~ ~ ~ ~O
O '' ~1 O O Q O O
O o o o i I
~a ~ r
El ~;
~1 ~ ' Ln , , ,, ~ ~ ~
_1 C!~ 0 . o ~
~ æ o r~ ~
E~ ~ ~ a~ o o c~ o o o
IY o
~ ~ .
n: --
Fn ~ N ~ N
. O O O O O
X C~ . . I I I .
O ~1 Ch O la o o
H
U~ ~
~ O~
U~ ~ 0 t~
~ r~ C~ O O O O O
: ~ ~n o sa o o o o
C~
~A Q Il
: , ~ ~ O
Q O O
E cl~ e o~o co
4J
E~ 3: . ~ .,.,
~P E~ v o
~ l
Z e o
~ l o ~
; w ~ ~ v ~
~ u~
.
47
17-21 (~083)A
P3~4
~ U~ ~ t~ er ~ o~ ~
o o o o o o," o o
o o Q o ~ o o
.o~
0~ ~1 ~ oo ~ ooQo ~
Z
-- ~1 o o e~
1 O ~ C: O O ~ O O O O
~:
- _ o ~ I ~ o CO ~ o_/
c: E~ o~ t~ ~ o c:~ o o o o o o o o
V ~ _l Ln
O Z
o~ o ooo_lo o ooo
C~ u~ ~ 0 ~
O CO -~ ~ I t -~
~ _1 ol 6 ~ 0 0 0 0 0 0 C~ O O
DS O~ .
u~ o ~ o ~
~ O ~ O O ~ ~ ~ O O O
V : r~O O O O O O O O O O
~I
r~NN D ~ U'~ ~ N
O U JJ ~ ~-- O tD U ~'
~p C Z ~ Z
.~
ut ~ ~n .
~8
17-21 (5083)A
~3~3~
. ~ ~ , ~ C~
s~ o ~ o o ~ U Ul
o ... , .... , _,
o o C~ oo o o ~
~ ~ ~ o o
Q a~ o o Io~ ~
æ ~ ~ ' O N t~ 0
~r) CD r~ a- o c~
_ ~ O O O I O _I O _I N O
~ _i ~
E
E u ) a~ o Cl3 ~ o~
O Q~ , cn o ~ ~ I o o o o ~ I
~:: E-l C:; r`
o E~ O ,1 u~ u~ ~ ~" o~
--1 h ~ O O O O _I O
5~
W O ¦ ~r
H _l C~ O O O O O O
P~ O~
. IA~
` ~ .
C~ ~ O O O O _l
U~
E~ t~ ~ ~ oa V ~
n, O C~
u~ o C~ O O O ~ t~q
E~ ~ ~ ~ C
~. ~ ~ u~ V a~
~ ~ ~ V ~ Ut U~
~ o o C ~ ~
~ ~ .
o U~
49
17-21 (~083)A
~3~38~
Collated in Table 3 is data at a variety of acid con-
centrations for several alloys, including certain alloys which
exhibited favorable corrosion resistance based on the data of
Table 2. Among the specific alloys for which corrosion data is
presented in Table 3 are E-Brite Alloy 26-1, a ierritic stain-
less steel, Alloy 255 duplex s~ainless steel, Alloy 304L, an
austenitic stainless steel, and Alloy C276, a high nickel
ailoy. Other alloys for which data is presented in Table 3 are
304L stainless, 310 stainless and Alloy 29-4-2. Thi data
illustrates thP very significant e~fect o~ small differences in
acid concentration on the corrosion ra~es of ~hese alloys.
Thus,. the stainless st el alloys experience as much as a
35-~old increase in the corrosion rate when the sul~uric acid
~ concentration is decreased from 100 weight percent acid to
approximately 98%. Alloy C276 shows a reduction in corrosion
in the 98-99% concentration range, but the corrosion rate in-
creases between 99~ and 100%. If the Alloy C276 data is com-
pared with any of the stainless steel data, it can easily be
seen that there is a considerable advantage ~or stainless steel
alloys when used in accordance with the teachings of this
invention.
At the elevated temperatures normally encountered in
the heat recovery tower and heat exchanger many alloys become
more passive, or resistant to corrosion. Thi~ effect can be
seen to prevail for a number of alloys as illustrated by the
data given in Table 3.
Set forth in Fiq. 6 are estimated isocorrosion curves
based on the data of Table 3 ~or type 304L stainless steel. It
may be noted that the isoco~rosion curves of Fig~ 6 are no~
~traight. The data of Table 3 shows ~he corrosion rate
51~
17-21 (~083) A
~3~3~
increasing rapidly with ~emperature in the range of 250C and
exposure to 97-9~% acid concentration. However, for acid
concen~rations between 99-~00%, the temperature effect was
found not to ~e substantial. It may be noted that the
isocorrosion curves o~ ~ig. 6 exhibit an ~S~ shape, resulting
from an actual decrease in corrosion as a function of
temperature in elevated temperature regions typically above
130C.
17-21(5083)A
~3~3~1
C~l
~ o o ~ o o o
: 'U~ o o o o o c~
_~ ~n
~ U~ ~o
C~ ~ ,` o _, _l U ,` CO C~
~ Z 0~ O O O O G O _I
O ~ ~ rG
CO ~010~0_1
6~ E ~C ~ r I o o c o
H O 0~ ~ Cl~ CO cn O
~ O O O O ~ _~
c~ o o o o o la o
:
O ~O
C OD ~ i
~; ~r ~J
a~ I ~ o er
I ~ 1 o _~
O W N
:
~1
52
17-21 (~083)A
~L3~73E~
O
_~ c~ o I I o ~ r~
o
~n
o,, o o _, ~
~ o ooo
U~ C~
E~
o o o
- ~o~ ~ ooo o
~ ~ ~ U~ ~ ~ ~
~ E~ ~ o ~ o o ~ ~ c~
O g~ = _~ r O O O o O O O
_ o; ~ ~ ~
0~ ~ ~ r o o o o o _l
t l l l l ~ I
u~
:~ co ~ ~ ~ O
~q I I ~
_l .
~ 1 ~ V
V
:C
~P E~
~:
I ~ O ~'
. Icr~ ~ ~ O _I
O W t~ h ~ ~ ~ 5:
o
53
17-21(;08~)A
~3~3
o o ~ o
~, o oo ~,
U~ .
~ o o o o o
:~ . cr~ o o
g~ Uo~ ~
~ Ul ~ .` ~
Z ~ ooooo ~
3 Oo~ ~ In
O O O O
., o ~ '~ o o o ........ o
0 ~D' I~I~ II
: ~9
U~ U~ ~
0~ ~ ~ ~
t~ ~N
0~ ~ N
O ~ ~ L~ ~.2 ~ ~
1 Ll O ~ ~D
I O~ O i~
~: ~ ~:
'
.
O
In . ~
54
17-21 (5083)A
~3C~3~
_, ~ ~ ~ o
o o o o ~, ~
o o o o o ~,
_, ~ U~
. o o c: o r~ I ~
~ ~ ~ o o o
a c~
o ~ a~ o
o _, ~ ~ I
~n
er O O O O ~ O
a~
r~ ~r ~ In ~ I ~r
U~ . . .
U~ oo~oo o
U~
E~
o o o C:~ o ~, ~
, . . o . ~ o
~r c~ o o o o o
o _l
o O
~:5 o ~n ~ = o o ~ o
v ~ :~ E ~ ~ ~ r 7 a~
' O ~g ~ E o o o o o _I N
t) a:; ~ QJ ~ O O O O O O O
~ ~ _( I I I N N
U~ I I I ~I U') O~ I
~O O O ~_~
~ r~
C ~ u7 I I I I I 1~
0 . C~
0
0~ : ~1, Y ~
oP E~
: o
~ ~ cJ~
o
17-21 (~0~3)A
~3U.3~
Set forth in Fig. 7 is a plot of corrosion data of
Table 3 on a temperature vs. acid concentration diagram for
Type 310S stainless. Also set forth in Fig. 7 are estimated
isocorrosion curves. Chemical analysis of the Type 310S
stainless steel used in the static immersion tests of this
example is set forth in Table 4.
TA~LE 4
Chemical Analysis o~ Type 310S Stainless Steel
Used in Static Immersion Tests
- ~eight
Element Percent
.;
C 0.055
Fe 52.5
Cr 24.04
Ni 21.33
; 15 S~ 0.46
Figures 8 to 10 illustrate the unexpected results
observed in the corrosion tests o Example 1. Thus, in the
temperature concentration diagram of Fig. 8, curve 1 is the
isocorrosion curve at 5 mpy ~or Incoloy 825 as reported in Fig.
38 of International Nickel Company Corrosion Engineering,
~Bulletin CEB-lU (January 1983)~ while curve 2 is ~he
approximate 5 mpy (0.13 mm/yr) isocorrosion curve in the
98-100% acid region for Incoloy 825 as derived from data ob-
tained in the above-described static immersion tests. In Fig.
9, ourve 1 is based on 5 mpy (0.}3 mm/yr) corrosion data pub-
lLshed by Fontana ~or 316 stainless steel, while ~urve 2 is the
56
.
17-21(5083)A
13~3B~4
approximate 5 mpy (0.13 mm/yr) isocorrosion cu~ve as derived
from the above-described test. In Fig. 10, curves 1 and 2
are the 1 mpy and 50 mpy isocorrosion curves ~or Ferralium
Alloy 255 as published ~y Cabot Corporation, whil~ curve 3
is the approximate 1 mpy (0.03 mm/yr) isocorrosion cu~ve in
the 98-100~ sulfuric acid region as deriYed from the above-
described corrosion test. In Fi.g. 11, curves 1-4 are
isocorrosion curves for Hastelloy C-276 as published at page 75
o Cabot Corporation's ~Corrosion Resistance of Hastelloys~
(198~). Inscribed as point S on Fig. 11 is the da~a obtained
for this alloy in the above-described test.
EXAMPLE 2
Despite the g2nerally favorable resul~s obtained in
the static immersion tests described in Example 1, tests of
such nature cannot necessarily be relied upon as a basis for
specifying materials of construction for the tubes of a heat
exchanger cooling hot abso~ber acid. Static tests do not pro-
vide a satisfactory basis for determining whether the alloys
involved are or may be subject to active-passive type oor-
rosion. In order to provide a further assess~ent of thesuitability of various alloys in absorber acid cooler service
at elevated temperatures, ~lec~rochemical tests we~e carried
out to establish the stability of the passivation process under
specific conditions. In these tests, the voltage was monitored
during approach to the freely corroding potential (FCP~. Once
the ~CP was reached, potentio-dynamic scans were used to assess
stabi}ity. Tests were run under th~ following conditions:
17-21(;083)A
13~?3Bl~
(~) type 304 stainless steel and 99.2% sulfuric acid
at 143C, 171C and 199C;
~ B) same conditions as (A) but the acid was purged
with a ~as mixture to simulate the absorber atmosphere, i.e.,
nitrogen containing 5% by volume oxygen and 0~5% by volume
sulfur dioxide;
(C) type 310 stainless steel under the same
conditions as (A);
(D) E-~rite 26-1 in 100% sulfuric acid at 143C and
171C.
-
In test series (A) for 304 stainless steel, the acid was takendirectly from a carboy and no attempt was made to aerate or
purge the acid before or during the electrochemical tests.
After an electrochemical cell was set up and the desired
temperature achieved, the circuit was left open and the cell
was allowed to stabilize at the FCP. Table 5 shows the reely
corrGding potential at the various temperatures involved in
this test. After the FCP was established at each test
temperature, an anodic scan was run at 1.8 volts per hour.
The scans were then reversed and the voltage was allowed to
decrease until ~he current reached 103 microa~ps/cm2 in the
cathodic direction. The voltage was then turned off and the
cell allowed to again reach the ~CP. These last FCPs are also
shown:in Table 5. The anodic scans are shown in Fi~. 12.
58
17-21 (5083~ A
13~3~
TA~LE 5
Freely Corroding Potentials (~CPs~ or
Type 304 Stainless Steel in 99.2~ Sulfuric Acid
Freely Corroding Pote_tlals, ~olts_~S.C.E.)
~efore ~oan ter scan
143~ (290F) ~.343 ~.309
171C (340F) +.330
199C (390F) ~.334 +.329
The FCPs shown in Table 5 were found to be very stable since
all settled out in less than one hour, except for the FCP at
143C after the cathodic scan, in which case two hours were
required. Such behavior indicates a system with a strong
tendency to passivity. Moreover, ~he shape of the anodic scans
indicates that the natural corrosion potential is in the pas-
sive region of the potentiodynamic curve. ~ased on the cor-
rosion current, and assuming that the corrosion consists of
oxida~ion of metallic iron to ferrous ion, the curves indicate
corrosion rate~ of less than 0.013 mm/yr at 143C and about
. 0.02S mm/yr at both 171C and lg9C. These results are in good
agreement with the immersion tests of Example 1.
In the ~lectrochemi~cal tests on 304 stainless steel
in 99.2% sulfuric acid purged~with nitrogen, 5~ oxygen and 0.5
sul~ur dioxide (Seri~s (B) as described above) the ~est
sequence was as follows: (l) establish ~CP; (2) conduct a
: 25 cathodic scan; (3)~ re-establ~sh FCPs; (4) run an anodic s~an;
: and ~4) re-establish F~P. Tnis seguence increased the severity
::
. Sg
,
17-21 (5083) A
13~3~
of the test, since the cathodic scan was expected to strip the
prot~ctive passive layer, and thus provided an evaluation of
the ability of the alloy to repassivate under adverse
conditions The FCPs observed in the tests of series ~B) are
set forth in Table 6 with their anodic scans ~eing shown in
Fig. 13. The results under the conditions of series (B) reveal
that at 143C the FCP is beyond the passive range and entering
tne ac~ive zone, bu~ the scans at the two higher temperatures
show that the type 304 stainless steel specimen is in a passive
10 s~ateO
TABLE 6
~ ~reely Corroding Potentials (FCPs) for Type 304
Stainless Steel in 99.2~ Sulfuric Acid wi~h a
Nitro~en, 5% 2~ and 0.5~ SO2 Gas Purge
Freel~_Corroding Potentials, Volts ~S.C.~.)
3efore Before After anodic
scans cathodic scan scan
r ........... _ _ _
143C (290F) -~093 +.11~ ~.115
171C (340F) +.343 ~.344 ~.335
199C (390F) +.350 +.360 ~.350
Table 7 shows the freely corroding potentials and
Fig. 14 shows ~he three anodic seans for the tests of s~ries
(C) or type 310 stainless steel. The voltage traces for 143C
(290~F) and 171C (340F) showed initial instability and
active-passive behavior fcr less than five minutes, but then
17-21 (~083)A
~3~1 3B14
gradually approached their stable FCP. In at least three of
the four FCPs measured, one hour was re~uired to achieve a
stable voltage. The scans in Fig. 14 indicate higher corrosion
ra~es for the passive regions at the two lower temperatures,
S approximately 0.13 mm/yr and up ~o approximat~ly 0.23 mm/yr,
but only approximately 0.012 mm/yr at 199C (390F).
TA3~E 7
Freely Corroding Potentials ~FCPs) for
Type 310 Stainless Steel in 99.~ Sulfuric Acid
, (Parentheses Indicate That Value Might be
Higher Than Shown.)
-
Freely Corroding Potentials, Volts (S.~.E.)
Before scan After scan
143C ~290~ ~.245
171C (340F)(+.271) ~~~~
199C (390F) +~342 +o317
In series (D), for E-Brite 26-1l only two temperatures were
evaluated, i.e., 143C and 171C. The freely corroding
potentials are shown in Table 8, and the potentiodynamic scan
29 curves are shown in Fig. 15. These results ~oth reflect a very
stabIe passivation situation. Calculated passive corrosion
rates Are less than about 0.012 mm~yr and thus in excellen~
agreement wi~h immersion test results shown in Table 3.
17-21(5583)A
13~3~314
TABLE 8
Freely Corroding Potentials (FCPs) for E-Brite 26-1
Ailoy in 100~ Sulfuric Acid
-
Freel~ Corrodin~ Potentials, Yolts (S.C.E.)
Before Af~er Ater anodic
~cans cathodlc scan scan
143G t290F) 0.227
171C (340F) 0.408 0.408 0.390
-.: Based on the static corrosion tests of Example 1, as
generally corroborated by the electrochemical tests of Example
2, the alloys tested were ranked according to their relative
suitability for use as the material of construotion of ~he
tubes of a heat exchanger for r~covery o~ the heat of absorp-
tion from 98% to lOQ% absorber acid at temperatures of greater
than 120C, in accordance with the process of the invention.
In making such ranking, ~he corrosion data was compared for
the various alloys at each of the three corners of ~he heat
recovery tower operating diagram as shown in Fig~ 4O Given the
vicissitudes of ~tartup and process upset conditions, the rank-
ing also took into account the corrosion performànce at temper-
atures and concentrations adjacent but somewhat outside the
operating ranges~ The ranking is set forth in Table 9, to-
gether with the corrosion index fo~ each sf the listed alloys
~ as:calculated from the corr~sion index equa~ion se~ forth
: ~ 2S :hereinabove. ~ ;
' ~ ~
S2
17-21 (~083)A
~3~31~4
The dotted line on Table 9 divides the alloys con-
sidered to be most suitable ~or implementation of the process
o~ the invention from those which would not currently be con-
sidered for commercial application, at least not for use in the
tubes of the heat ~xchanger in which the heat of absorption is
recovered by ~ransfer from the absorption acid to another
fluid. It should be understood tha~ the corrosion rates for
most of the alloys below the dotted line are reasonably
satisfactory, and in fact lower ~han would have been expected
prior to the corrosion testing program described in Examples l
and 2. However, the alloys below the line are not currently
consi~ered commercial candidates because o the even more
favorable corrosion performance of the alloys listed above the
dotted line. I~ should further be noted that ~he ranking set
forth in Table 9 is based entirely on corrosion data and does
not necessarily reflect the exact current ranking when detailed
economic or fabrication considerations are taken into account.
However, regardless of whether the ranking incorporates design
optimization considerations or is done strictly on the basis of
corrosion results as in Table 9~ the general correlation
between a corrosion index greater than 39 and suitability for
use in the heat recovery process of the invention remains
consistently valid.
63
17-21 (5083)A
~3~J3
C~
X X X X X
, '
C~
- 'X X X X
H X X X X X ~ X X X X X X X X
~ .
............. ,............ O . O
~ ~ ~ ~ ~ ~ ~ cn ~ o o a:l ~ o t~ n o c~ o 1`
a:
rn u~ rn rn ~n ~n z rn z rn z r~ rn . u~ ~n cn æ z rn z tn rJ~
O
I ` ~ r~ o u~ o ~ O O
~,o o ~ u~ t~
c~ E ~~D co ~ ~D u7 ~r
3 ~ v c
~ ~r ~ ~ ~ o O ~ ~ c E h E~
a~ ~ ~ o ~3 ~ ~ o ~ o ~ c~
t~ O ' ~ t~ ~ U ~ ~
~ia ~ h ~ t~ ) ~ r,3 ~ ~ ~ z ~ tr~ e~
* ~ :
~: ~ :
Il~ _I N t~ ~ 6~ ~ O ~ O _~
~ o
.
64
17-21 ( 5033~ A
~3~3~
v
.,~
E
~o
o
U V
a~ , c:
~
v X x x x x x æ C
~ o
U
~ o
~ ~ U~
ry ~ ~
~: ~ I r~ ~c
. o Vl ~ ~ ~ ~ ~ ~
C J
C~ ~ C ~
~ U~ Z V~ Z Z Z o ~
_I ~ ~ .Q ~ ~
1~1 ~ A
~ 1 t~
C ~q In
O O O
O ~ 1 C
1~1 Ql ~ C ~ O
:~E ~ ~ C ~ ~
~ 8 ~ Q
C h~
cn ~ ~ ~ ~ 0 ~ O
,. " ~ ~
~: ~ g
c~ N ~
O
:
'
~: 65
17-21 (5083)A
13~ 3~
EXAMPLE 3
A pilot heat recovery tower is insta}led ahead of tne
final absorption tower in a non-interpass sulfur ~urning sul-
furic acid plant. A process gas slipstream of 5.0 Nm3/min at
a temperature of 260C and containing 7.5 volume percent suliur
trioxide is ~ed to the heat recovery towerO Sulfuric acid with
a concentration of 99 . O weight percent and a temperature of
L6~C is ~ed ~o the ~9p of the heat recovery tower at a rate of
35 kg~min. The acid leaves the tower at a concentration of
99.9 weight percent and a temperature of 201C. Overall ab-
sorption of sulfur trioxida from the feed gas stream is
appr~ximately 96~ Acid flows by gravity from the ~ower to a
pump tank, from whence it is pumped to a boiler where 0.8
- kg/min of steam is generated at a pressure of 450 kPa. Acid
leaves the boiler at a temperature of 15SC and i5 diluted to a
99.0 weight percent by in-line addition of liquid water. The
heat of dilution increases the acid temperature to 162C. This
stream is then circulated to the top of the ~ower, completing
the cycle.
EXAMPL~ 4
As described in Example 3, a pilot plant heat
recovery tower was installed ahead of ~he final absorption
tower in a non-interpass sulfu~-burning sulfuric acid plant.
The pilot plant tower was 0.~4 m I.D. and contained an
:25 absorption zone comprising No. 2 ceramic intalox saddles with a
~acked height of 1.1 m. A process gas ~lipstream con~aining
7.8 volume percen~ sulfur trioxide at a temperature of 2~C
was fed to the bottom inlet of the heat recovery tower at a
volumetric ra~e of 5.8 Nm3 per minute. Sulfuric acid having
an average concentration of 9~.9~: and temperature of 161C was
.
66
17-21 (~083)A
~3-~3~
fed to the top inlet of the tower at a rate of 50 kg/min. At
steady sta~e, a sulfuric acid stream having an average concen-
tration of 99.6% by weight and a temperature of 188C flowed
outward through ~he bottom outlet of the tower. During passage
through th~ heat rec~very tower; approximately 95% of the sul-
fur trioxide con~ent of the feed gas stream was absorbed into
the sulfuric acid stream. Suifuric acid leaving the tower
~lowed by gravity to a pump tank from whence it was pumped to a
boiler where 1.1 kg/min of s~eam was generated at a pressure of
445 kPa. Acid le~t the boiler at a temperature of 154C, 1.9
kg/min product acid was removed, and the remaining acid ~tream
was tpereafter diluted to 98.9 welght percent by inline addi-
tion of liquid water prior to recycle to the tower. The heat
of dilution increased the acid temperature to 161C. Corrosion
probes of type 304~ stainless steel were mounted in the acid
line before the boiler. The corrosion rate of type 304L
stainless steel was less ~han 0.03 mm per year.
EXAMPLE 5
Operation of the heat recovery process of the inven-
tion was continued in the pilot plant described in Example 4,the conditions being varied to demonstrate varying temperatures
in the heat recovery tower and boiler.
~ he boiler used for cooling the absorber acid in the
operation of the pilot plant was a vertical shell and tube heat
exchanger having a shell constructed of 310 stainless steel.
Various ~aterials were used for the tubes of the exchanger.
One tube had an O.D~ of 25 mm and was oonstructed o' ~ype 310
stainless steel, while the remainin~ 22 tubes, all 19 mm O.D.,
~ere constructed of type 304L stainless steel (9 tubes),
E-Bri~e alloy XM-27 t7 tubes), and Yerralium alloy 255 ~6
tubes) During pilot plant operation, absorber acid having a
11-21(5083)A
~ `3 ~ ~ ~
strength of 99-100% was passed through the shell side and
boiler fee~ water through the tube side of the exchanger.
Steam was generated on the tube side and, during the two-month
period of pilot plant operation, the acid temperature was
varied as required to generate steam at various pressures.
Thus, the acid temperature varied from 144C to 217C at ths
~_ d inlet on the bottom of the ~oiler and between 132~C and
194C at ~he acid outlet at the top of ~he boiler. The highest
st~am pressure produced was 1140 kPa corresponding to 181C at
saturation. Over the course 9~ the pilot plant operation,
measurements were made to determine the effect of the high
temperature concentrated acid on the hea~ exchanger tubes.
Additionally, corrosion coupons constituted of various metals
were installed at various points throughout the acid
recirculation system. Nlne different metals were corrosion
tested by means of coupons located under ~he acid distributor
within the heat recovety ~ower, and in the pump tank. Pipe
spools of type 304L and type 310 stainless steel were located
in the acid line at points before and after the boiler and
immediately downstream of the dilution sparger, i.e., the
Teflon sparger through which water was introduced into a
Teflon-lined pipe section in the recirculating system to adjust
the acid s~rength in compensation for the sulfur trioxide
absorbed Additionally, various distributor parts of type 304L
and type 310 stainless steel were tested, and a type 304L mesh
pad was installed at the top of ~he tower.
After the pilot plant run was compl~ted, the heat
exchanger was removed and cut up for visual inspection and
observation. An attempt was made to directly measure 1QSS in
6~
17-21(,083)A
:~L3V3~
tube diameter, but these measurements proved erratic and
unreliable, However, visual observations at up to 40
magnifications of the outside of the surfaces of ~he tubes
revealed:
310 - uniform light etch
Ferralium 255 - etch, few craters, probably
at weld
E-Brite 26-1 - uniformly dispersed craters
304L - uniform etch, indications of
some in~ergranular attack
Water-side observations also indicated that the various tube
materials tested were satisfactory under the conditions
prevailing. Tube sheet welds were found to be generally in
satisfactory condition. There were numerous white deposits on
the sufaces within the acid side o~ the exchanger. These were
most often found on the tubes at the baffle areas, on the tops
of the ba~fles and on the top of weld beads conneoting the
baffles to the positioning rods. A tube with such deposits on
it was analy2ed by X-ray 1uorescence. Areas with a~d without
deposits were compared. The only difference ~ound was in
sulfur content, thus indicating that the deposits were
primarily sulfates.
Using ~he standard NACE test, the corrosion rate on
the Yarious corrosion coupons was determined. Comparable
techniques were utilize~ to determl~e ~he corrosion rates on
the pipe spools, distributors, and type 304L stainle~s steel
mesh pad. The results of th~se corrosion measurements are set
orth in~Table 10.
69
17-21 ( ~0~3) A
~3~3~4
~q
O O
U~
tn
~ ~ ~ ~: ~ ~C -'
~ : ~^
~ ~ z ~
E~ O u~u~ OOO~ 0
: g ~ ~ C~ ~ O ~ _l
~ ~; u ~ m ~
o
:~E X~:
~ ~ r~ o ~
E~ ~ . . . . . ~ . u~
:: D o o o _I o
8 co ~ ~
Z ~ N N
;: ~ :
: :::: : ~n
E3: ~ ~: : : ~
_I V V ~O ~ ,
~ O er a~ ~S ~.1
~ _I o o ~ '-P o
::
: . - O U~
7 0
17-21(~083)A
~3~38~L4
~ CC
8 -- ~ ~ ~ _, ~
U~ ~ ,¢ ,, ~ _, o
,, I ¦ 2
~:
c ~ E~ ~ . - - -
O kl E~
"~ 2 ~N`
~ ~3
~ ~ ~ ~ : O
E~ ~ ~ o o ~r
O o ~ _I O O
r ~ ~ ~ ~
~ O
'
71
~ ~ ~, ~ , r;
13U3~
Additional data on the corrosion rate of type 304L stainless
steel was taken during the pilot plant run using an electro-
chemical device (~CorraterR*sold by Rohrback Instruments,Division of Rohrback Cocporation, 11861 E. Telegraph Rd., Santa
Fe Springs, CA 90670) for measuring corrosion rate. -This de-
vice was installed in the acid stream on the inlet end of the
boiler. Conditions at the inlet side varied as the pilot plant
run progressed, and there was also some variation in the cor-
rosion rates measured with the Corrater~* Overall, however,
the results were favorable. For operation at temperatures
varying from 144F to 217F and acid concentrations varying
from 99.2% to 100.9~ the Corrater indicated a mean corrosion
rate of 0.03 mm/yr, with a standard deviation of 0.03 mm/yr.
-
EXAMPLE 6
A heat recovery tower is installed ahead of the
interpass absorption tower in a sulfur burning sulfuric acid
plant.
A process gas stream of 2914 Nm3 per min. at atemperature of 166C containing 11.8 volume percent sulfur
trioxide is fed to the bottom of the heat recovery tower.
Sulfuric acid having a concentration of 98.6~ by weight and a
temperature of 168C is fed to the top of the tower at a rate
of 22670 kg per min.
The acid leaves the tower at a concentration of 99.8
weight percent and a temperature of 198C. Overall absorption
of sulfur trioxide from the feed gas stream is approximately
97~.
Acid leaving the tower flo~s by gravity to a pump
tank, from whence it is pumped to a boiler where approximately
830 kg~min. of steam is generated at a pressure of 377 kPa.
* ~rade Mark
72
17-21~5083)A
13~3~
Acid leaves the boiler at a temperature of 152C and,
after product removal, is diluted to gB.6 weight percent by
in-line addition of liquid water. The heat of dilution
increases the acid temperature to 16BC. This ~tream is then
recirculated to the top of the tower, completing the cycle,
EXAMPLE 7
A heat recove~y towe~ is intalled ahead of the
interpass absorp~ion tower in an existing sulfur burning
sulfuric acid plant.
A process gas stream of 2067 Nm3/min at ~
temp~rature of 232C containing 10.~ volume percent sulfur
trioxide is fed to the bot~om o the heat recovery tower.
Sulfuric acid with a concentration of 99.1 weight percent and a
temperature of 206C is ~ed to the top of the tower at a rate
of 43157 kg/min.
The acid leaves the tower at a concentration of 99.5
weight percent and a temperature of 215C. Overall absorption
of sulur trioxide from the feed gas stream is approximately
89%.
Acid leaving the ~ower flows by gravity to a pump
tank; from whence it is pumped to a boiler where approximately
507 kg/min of s~eam is generated at a pressure of 1342 kPa.
Acid leaves the boiler at a temperature of 201C and,
after product removal, is dilu~ed to 99.1 weight percent by
in-}ine addition of liquid water. The heat of dilution
increases the acid temperature to 206C. This stream is then
recirculated to the ~op o the tower, completing the cycle.
- 1 7 - 2 1 ( 5 0 8 3 ) A
~3~3B14
EXAMI:'LE 8
A heat recovery ~ower is installed ahead of the final
absorption tower in a non-interpass metallurglcal sulfuric asid
plant.
A process gas stream of 2470 Nm3/min a~ a tempera-
ture of 232C containing 9.8 volume percent sulfur trioxide is
fed ~o ~he bottom of the hea~ recovery tower. Sulfuric acid
with a concentration o~ 98.6 weigh~ percent and a ~emperature
of 162C is fed ~o the top o~ the towPr at a rate of 16242
kg/min.
The acid leaves ~he tower at a concentration of 99.8
weig~t percent and a temperature of 206C. Overall absorption
of sulur trioxide from the feed gas stream is approximately
97%,
Acid leaving the tower flows by gravity to a pump
tank where it is diluted to 99.3 weight percent. Dilution is
accomplished by addition o~ 1375 kg/min. of 66C, 93.0 weight
percent acid.
Ater dilution in the pump tank the resulting acid
enters a boiler at 196C where approximately 641 kg/min. of
steam is generated at a pressure o~ 377 kPa.
Acid leaves the boiler at a temperature of 153C and,
after product removal, is diluted to 98.6 weight percent by
in-line additisn of 112 kg/mm of 141C water containing 1.2%
steam. The heat of dilution inceases the acid tempera~ure to
162~C. This stream is ~hen cecirculated to the top of the
tower, completing the cycle.
A heat recove~y ~ower is installed ahead of the
interpass absorption tower in an exis~ing Culfur burning
sul~uric acid plant.
17-21 (~083)A
:~ 3~
A process gas stream of 2542 Nm3/min. at a tempera-
ture of 154C contaiing 11.8 volume percent sulfur trioxide is
fed ~o the bottom of the heat recovery tower. Sulfuric acid
with a concentration of 98.6 weight percent and a temperature
of 168C is fed to the top of the tower at a rate of 19871
kg/min.
The acid leaves the tower at a concentration of 99.8
weight percent and a temperature of 197C. Overall absorption
of sulfur trioxide from ~he feed gas str~am is approximately
97~.
Acid leaving the tower ~lows by gravity to a pump
tank~ from whence it is pumped o a boiler where approximately
691 kg/min. of steam is genera~ed at a pressure o 377 kPa.
- Acid leaves the ~oiler at a temperature of 152C and,
after product removal is diluted to g8.6 w~ight percent by
in-line additon of liquid water. The heat of dilution
increases the acid temperature to 168C. This stream is then
recirculated to the top of the tower, completing the cycle.
Example 10
A heat recovery tower is installed ahead of the
interpass absorption tower in a sulfur burning sulfuric acid
plant.
A process gas stream of 2969 Nm3/min. at a
temperature of 162C containing 11~6 volume percent sulfur
trioxide is fed to the bottom of the heat recovery tower.
Sulfuric acid with a concentration of 98.8 weight percent and a
temperatur~ o~ 206C is fed to the top of the tower at a rate
of 29293 kg/min.
17-21 (~083) A
3~3~3~
The acid leaves the tower at a concentration of 99.7
weigh~ percent and a temperature of 222C. Overall absorption
of sulfur trioxide from the feed gas stream is approximately
90~ .
Acid leaving the tower flows by g~avity to a pump
~ank, from whence it is pumped to a boiler where approximately
710 kg/min. of steam is generated at a pressure o~ 1136 kPa.
Acid leaves the boiler at a temperature of 194C and,
after product removal, is diluted to S8.8 weight percent by
in-line addition of liquid water. The hea~ of dilution
incr~ases the acid temperature to 206C. Thi~ s~ream is then
recir'culated to the op of the tower, comple~ing the cycle.
-
76