Note: Descriptions are shown in the official language in which they were submitted.
~3~3~
- 1 - C3146
DETERGENT GRANULES AND A PROCESS
FOR THEIR PREPARATION
TECHNICAL FIELD OF INVENTION
The present invention is concerned with a process for
the production of detergent granules built with alkali
metal carbonate and containin~ a water-insoluble seed
crystal material, such as calcite, for the alkali metal
carbonate. Such products are useful especially for the
domestic laundering of fabrics,
BACKGROUND AND PRIOR ART
Detergent compositions usually contain, in addition
to a detergent active material, a detergency builder whose
role, inter alia, is to remove hardness ions from the wash
liquor which would otherwise reduce the efficiency of the
detergent active material. Water-soluble phosphate
materials have been extensively used as detergency
builders. ~owever for a number of reasons, including
eutrophication allegedly caused by phosphates and cost,
~a~r~g
- 2 - C3146
there has been a desire to use alkali metal carbonates,
especially sodium carbonate, instead. Alkali ~etal
carbonate detergency builders suffer however from a number
of disadvantages. Firstly, the reaction between the
alkali metal carbonate and calcium ions which are present
in hard water results in the formation of water-insoluble
calcium carbonate which, depending on the conditions, may
be in such a form as to become deposited on the washed
fabrics. Secondly, the reaction between the alkali metal
carbonate and the calcium ions of the water is slow,
especially at low temperatures, and is readily inhibited
by materials which act as calcium carbonate precipitate
growth inhibitors, referred to herein as poisons. The
result of this is that the concentration of calcium ions
in the wash liquor is not reduced as far or as fast as
desired, so that some free calcium ions are still
available to reduce the efficiency of the detergent active
material.
As a possible solution to this problem it has been
proposed to include in the detergent composition a
water-insoluble material which can act as a seed crystal
for the precipitating calcium carbonate and can absorb the
poisons from the wash liquor. Among other materials,
finely divided high surface area calcite has been proposed
as such a material: see GB 1 437 950 tUnilever) and the
corresponding US 4076653 (Davies et al).
However, the inclusion of calcite in detergent
compositions is hampered by its physical formO One might
consider putting small particle size calcite in a slurry
together with other ingredients for spray-drying, but we
have found that where alkali metal silicates are included
this process leads to a loss of calcite seed activity.
Calcite having a large surface area is required for
maximum seed activity, but generally such material has a
~3i`~
- 3 - C3146
relatively small particle size, is dusty and is therefore
difficult to handle. One alternative is to handle the
calcite in a slurry, without drying to a powder, but this
could also involve high storage and transport costs. It
is therefore necessary to granulate the calcite, for
example by conventional techni~ues of pan granulation or
spray-drying, and to keep any silicate away from the
calcite. The term "granulation" is used herein to mean
any process of agglomerating fine particles into granules
of a suitable size for incorporation into, or use directly
as, detergent compositions.
Granulation of the calcite with a suitable binding
agent has been proposed, for example, in GB 1 515 273
(Unilever). However, in order to be efective in its
intended role in the wash liquor, it is necessary for the
calcite to disperse rapidly when the product is added to
water. Binding agents have generally been found sexiously
to reduce the dispersibility of the calcite. Attempts to
granulate calcite with materials known to be good
dispersing agents, for example some nonionic detergent
active materials, have also been unsuccessful. The
resulting granules may not have the necessary mechanical
strength to solve the handling problems of the calcite.
The problem is further complicated by the fact that some
binding agents and dispersing agents proposed in the prior
art are themselves poisons and will therefore reduce the
seed activity of the calcite, thereby further adding to
the problems which the calcite is intended to solve.
GB 2 174 712A (Unilever) discloses silicate-free
detergent granules comprising a seed crystal such as
calcite, a non-soap detergent-active material (preferably
anionic) and a sugar. The preferred sugar is sucrose.
~3~3~
- 4 - C3146
The granules are typically prepared by spraying a
mixture of detergent-active material, water and sucrose
onto calcite particles in a granulator. Alternatively
they may be prepared by spray-drying. The granules are
then dry-mixed with a detergent base powder containing
other desired ingredients, notably sodium silicate; this
might, for example, be a spray-dried base powder. If
desired the calcite granules may contain other
ingredients, notably sodium sulphate or sodium carbonate,
but not sodium silicate.
The detergent powders prepared by this method contain
separate calcite and silicate granules and, unless
measures are taken to control their relative densities and
particle sizes, segregration of the powder during storage
and transport may occur. Such powders may also be more
expensive to prepare by this method~
It has now been found that it is possible to prepare
detergent products containing a detergent active, sodium
carbonate, sodium silicate and calcite by a process which
avoids the risk of such segregation.
DEFINITION OF THE INVENTION
According to the present invention there is provided
detergent granules comprising at least:
(i) a detergent active material;
(ii) an alkalimetal carbonate
(iii) an alkali metal silicate, and
35 ~iv) a water-insoluble particulate carbonate
~L3~3~
_ 5
which iy a s~ed cryst~l for calGium ~Qrbona~e
and whi~h ha~ a sur~ace area of at least 10
maJ~
wherein the granules are ln the forln o~ hase powder
partlcles compriqlng the deter~ent active material, ~he
alkali metal carbona~e and the alkali metal s$1icate, the
seed crystal materlal being located at the surface ~f ~ald
ba~e powder paxticles and adh~red thereto.
The detergent granule~ o~ ~he invention may further
comprise a wat~r-soluble o~ water-disper~ible blnder
material whlch serve~ to adhere the seed crysta~ ~o the
base p~wder part~cles. The binder ~ater~al may compri~e
further detergen~ active material.
The invention further pro~ides a pr~cess for the
preparation of such detergent granules compr$sing the
steps of:
(a) making ba~e powder p~rti~les compri~ing the detergent
actlv~ materl~l, alkali m~tal carbonate, an~ alkali
metal silicate, and
(b) ~dh~ring ~he seed crystal material to the surfaoe o~
th~ Yaid base powder partlcles.
We ar~ aware of Jap~nese Patent publication
60~2628~5 l~ion Co., ~td.) whlch is ~irected to lmprovlng
the flo~ p~operties of ~ran~lar composlttons oon~aining
det~r~ent
~L
:~3~33~
~ 6 - C3146
active materials, alkai metal silicate and alkali metal
carbonate by adhering on the surface thereof small amounts
of cubic calcium carbonate particles having a primary
praticle diameter of 0.1 to 1.5 microns. Such calcium
carbonate material has an insufficient surface area for
use as an effective seed crystal in the context of the-
present invention.
We are also aware of GB 1583081 ~Unilver) which describec
a process comprising contacting an alkali metal carbonate
in particulate form with a liquid or pasty detergent
active compound and admixing calcium carbonate powder so
that the calcium carbonate adheres to the alkalimetal
carbonate particles. Such a process was said to prevent
interaction between the alkalimetal carbonate and the
calcium carbonate, which interaction was believed to have
a negative effect upon detergency. If conventional or
high levels of detergent active are used in such a method,
the resulting product may suffer from unacceptable
physical properties.
In contrast, the present invention requires that the
detergent active, the alkali metal carbonate and the
alkalimetal silicate constitute a common granulated base
powder to which the seed crystals are adhered~
DESCRIPTION OF THE INVENTION
The detergent granules built with alkali metal
(preferably sodium) carbonate, alkali metal silicate and a
seed crystal material, preferably calcite, are prepared by
a process comprising ~he two steps of granulation. A
granulated base powder containing the components other
than the calcite is first prepared. The base powder is
~L3~3~
- 7 - C3146
then granulated with calcite, preferably in the presence
of a liquid binder, as will be discussed in more detail
below.
Detergent products prepared in accordance with the
invention consist essentially of agglomerate particles
composed of base powder and seed crystal material, held
together, preferably by means of a binder. Of course
other solid materials added by postdosing may also be
present as discrete particles.
The base powder may be prepared by spray-drying an
aqueous slurry containing all desired ingredients
sufficiently heat-insensitive to be processed in this
manner, other than the seed crystal material. These
ingredients include not only the alkali metal carbonate
material, the alkali metal silicate, and the detergent
active material but may also include other detergency
builders, fluorescers, antiredeposition agents sush as
sodium carboxymethyl cellulose, and salts such as sodium
sulphate.
Alternatively the base powder may be made by
marumerising or by non~slurry granulation, such as by pan
granulation. These techniques are well known in the art
and need no further description.
THE ALKALI METAL CARBONATE
An essential ingredient of the base powder used in
the process of the invention is an alkali metal carbonate
builder salt, preferably sodium carbonate.
The sodium carbonate typically amounts to from 20 to
80% by weight of the base powder, and the base powder often
constitutes from 30 to 70% by weight of the final product,
~3~3~
- 8 - C3146
so the amount of sodium carbonate in the final composition
will be correspondingly less, i.e. from 5~ to 56% of the
final product.
If desired, other builders may also be present to
supplement the sodium carbonate, provided that they do not
inhibit calcium carbonate crystal growth. Examples of
suitable supplementary builders include citrates,
nitrilotriacetates and soaps.
THE ALKALI METAL SILICATE
-
A further essential ingredient of the base powder is
an alkali metal silicate. Sodium silicate is an important
ingredient of spray-dried detergent compositions. It
helps to give structure to the spray-dried powder and in
the wash liquor it prevents the corrosion of metal
surfaces of the washing machine. It is an advantage of
the process of the invention that sodium silicate can be
included in the base powder without the problem of calcite
deactivation.
The alkali metal silicate is particularly sodium
neutral, alkaline, meta- or orthosilicate. A low level of
silicate, for example 5-10% by weight of the final
composition is usually advantageous in decreasing the
corrosion of metal parts in fabric washing machines.
Lower levels eg. 2% to 5% may provide beneficial
structuring of the powder. If higher levels of silicate
are used up to a practical maximum of 30%, for example
from 10~ to 20% by weight, there can be a more noticeable
improvement in detergency, which may permit some decrease
in the water-soluble carbonate material content. This
effect appears to be particularly beneficial when the
products are used in water with appreciable levels of
magnesium hardness. The amount of silicate can also be
:3L3r.33~
- 9 - C31~6
used to some extent to control the equilibrium pH of the
wash liquor, which is generally within the range of 9-11,
preferably 10-11 for an aqueous solution of the
composition at the recommended concentration. It should
be noted that higher pH (ie over pH10.5) tends to be more
efficient as regards detergency, but it may be less
desirable for domestic safety. Sodium silicate is
commonly supplied in concentrated aqueous solution, but
the amounts are calculated on an anhydrous basis.
THE DETERGENT ACTIVE MATERIAL
,
The granulated base powder also includes one or more
detergent active materials, such as anionic and/or
nonionic surfactants.
Anionic surfactants are well-known to those skilled
in the detergents art. Examples include alkylbenzene
sulphonates, particularly sodium linear C8 C15
alkylbenzene sulphonates; primary and secondary alkyl
sulphates, particularly sodium C12-Cl5 primary alkyl
sulphates; olefin sulphonates; alkane sulphonates; and
fatty acid ester sulphonates.
Nonionic surfactants that may be used include primary
and secondary alcohols ethoxylated with an average of from
3 to 20 moles of ethylene oxide per mole of alcohol.
The base powder may also contain one or more soaps of
fatty acids. The preferred soaps are sodium soaps derived
from naturally occurring fatty acids, for example, the
fatty acids from coconut oil, beef tallow or sunflower
oil.
The total amount of detergent-active material
(surfactant), excluding soap, in ~he base powder may
~L3~3~
- 10 - C31~6
suitably ranye from 10 to 60% by weight: in a fully
formulated product containing perhaps 30 to 70% by weight
of base powder the amount will be correspondingly less
i.e. from 3% to 42~ of the final product. For low-sudsing
powders intended for use in European drum-type automatic
washing machines the weight ratio of anionic surfactant to
nonionic surfactant in the final product preferably does
not exceed 10:1, and more preferably does not exceed 6:1,
but it should be remembered that nonionic surfactant may
be sprayed on or postdosed on a carrier rather than
included in the base powder, so that the ratio in the
base powder may be higher, or indeed infinite.
Medium-sudsing or high-sudsing products tend to have a
higher ratio, and nonionic surfactant may be omitted
altogether from such products.
THE SEED CRYSTAL
In the process of the invention, the base powder is
granulated with a particulate water-insoluble carbonate
capable of acting as a seed crystal for the precipitate
which results from the rPaction in the wash liquor between
calcium water-hardness ions and the water-soluble
carbonate builder salt present in the base powder. Thus
this water-insoluble particulate material is a seed
crystal for calcium carbonate, and is preferably itself a
crystal form of calcium carbonate.
The water-insoluble particulate carbonate material
should be finely divided, and should have a surface area
of at least lO m2/g, and preferahly at least 15 m~/g.
The particularly preferred material has surface area from
30-100 m2/g. Insoluble carbonate material with surface
areas in excess of 100 m2tg may be used, if such materials
are economically available.
~l3~;1 3~
~ C3146
Surface area is measured by nitrogen adsorption using
the standard Bruauer, Emmet & Teller ~BET) method. A
suitable machine for carrying out this method is a Carlo
Erba Sorpty (Trade Mark) 1750 instrument operated
according to the manufacturer's instructlons.
It is most preferred that the high surface area
material be prepared in the absence of pOiSOllS, SO as to
retain its seed activity.
The insoluble carbonate material will usually have an
average particle size of less than 10 microns, as measured
by conventional techniques.
When the insoluble carbonate material is calcium
carbonate, any crystalline form thereof may be used or a
mixture thereof, but calcite is preferred as aragonite and
vaterite axe less readily available commercially, and
calcite is a little less soluble than aragonite or
vaterite at most usual wash temperaturesO When any
aragonite or vaterite is used it is generally in admixture
with calcite. In the following general description, the
term "calcite" is used to mean either calcite itself or
any other sui~able water-insoluble calcium carbonate seed
material.
The amount of calcite in a final powder prepared in
accordance with the invention is preferably at least 5~,
such as up to 40% by weight, more preferably from 10 to
30% by weight.
OPTIONAL INGREDIENTS
Many detergent compositions also contain a bleach, to
bleach stains and assist in the removal thereof from
fabrics. Peroxybleaches which generate hydrogen peroxide
~3n3~s?~
- 12 - C3146
in solution, such as sodium perborate, have been used for
this purpose but are not especially effective at low
temperatures. Products capable of bleaching at lower
temperatures contain peracid yenerating systems which may
comprise a peracid itself or, more commonly, a mixture of
peroxybleach and an activator therefor, such as sodium
perborate together with tetraacetylethylene diamine
(TAED). The performance of such systems is especially
sensitive to the presence of low levels of transition
metal ions, which are often present in small amounts in
raw materials used for preparing the compositions. It has
been proposed therefore to include in such compositions
materials such as the salt of a polymethylene phosphonic
acid, generally available under the Trade Mark DEQUEST,
and described in British patent specification GB 2 048 930
(UNILEVER) and the corresponding US 4259200 (Heslam et
al). These materials stabilise the peracid bleach system
against the effect of transition metals, but since Dequest
is itself a phosphorus containing material it has been
thought desirable to exclude Dequest from detergent
compositions not containing phosphate builders.
The use of a sodium c~rbonate/calcite builder mixture
in place of sodium tripolyphosphate leads to a number of
differences, including higher alkalinity in the wash
liquor. It is known that the bleaching performance of
peracetic acid (generated from a mixture of sodium
perborate and TAED3 is reduced at a higher pH and this,
together with the absence of Dequest to stabilise the
peracetic acid from the effects of transition metals would
lead one to expect that the bleach performance of a
phosphorus free composition based on a sodium
carbonate/calcite mixture would be significantly reduced
in comparison with its phosphate containing equivalent.
~3~3~
- 13 - C3146
We have now discovered however that the performance
of such compositions is substantially better than might
have been predicted and in some cases a benefit occurs
relative to an equivalent phosphate containing
composition.
The peracid generating bleach system may be selected
from peracids themselves, or a mixture OL a peroxybleach
such as an inorganic persalt and a peracid bleach
activator. The activator makes the bleaching more
effective at lower temperatures, ie. in the range from
ambient temperature to about 60C. The inorganic persalt
such as sodium perborate, both the monohydrate and the
tetrahydrate, acts to release active oxygen in solution,
and the activator therefor is usually an organic compound
having one or more reactive acyl residues, which cause the
formation of peracids, the latter providing for a more
effective bleaching action at lower temperatures than the
peroxybleach compound. Whilst the amount of the bleach
system, ie. peroxybleach compound and activator may be
varied between about 5% and about 35% by weight of the
detergent compositions, it is preferred to use about 6% to
about 30% of the ingredients forming ~he bleach system.
Typical examples of suitable peroxybleach compounds
are alkali metal perborates, both tetrahydrates and
monohydrates, alkali metal percarbonates t persulphates and
persilicates of which sodium perborate is preferred. The
peroxybleach compound is normally added in separately to
the detergent base powder.
We have found that the present invention is
especially applicable when the peroxybleach compound is
sodium perborate monohydrate, especially such material
which has a surface area in excess 5m2/g and a caking
index, as described in European Patent Specification No,
:~l3~3~
- 14 - C3146
164778 (UNILEVER - and the correspondiny US 4650599
(Farnworth et al) above zero.
Activators for peroxybleach compounds have been amply
described in the literature, including British patents 836
988, 855 735, 907 356, 9Q7 358, 970 950, 1 003 310 and 1
246 339; US patents 3 332 882 and 4 128 494; Canadian
patent 844 481 and South African patent 68/6 344.
The N-diacylated and N, N'-polyacylated amines are of
special interest, particularly N, N, N', N'-tetraacetyl
ethylene diamine (TAED)~
It is preferred to use the activator in granular
form, preferably wherein the activator is finely divided
as described in British Patent Specification No. 2 053 998
(UNILEVER) - and the corresponding US 4283302 (Foret et
al1.
It is a feature of the invention that the products
are preferably free of phosphorus. In particular, the
products should contain less than about 0.01% poly-
methylene phosphonic acids and their salts, calculated as
phosphonic acid.
Examples of other optional ingredients include the
lather boosters such as alkanolamides, particularly th~
monoethanolamides derived from palm kernel fatty acids and
coconut fatty acids, lather depressants, fabric softening
agents, such as quaternary ammonium salts and smectite
clays, inorganic salts such as sodium sulphate, and,
usually present in very minor amounts, fluorescent agents,
perfumes, enzymes such as proteases and amylases,
germicides and colourants. Particularly when the
composition does not contain an anionic detergent active
material, it can be beneficial to include an anti-ashing
13~3~
- 15 - C3146
material to reduce the deposition of calcium carbonate
onto fabrics.
THE BINDER MATERIAL
The granulation of the base powder with the calcite
may be carried out in the presence of a liquid binder,
although if the base powder is in a tacky state when
contacted with the calcite, the addition of a binder at
this stage is not essential.
According to a preferred embodiment of the invention,
the liquid binder is an aqueous solution of a sugar. By
the term "sugar" is meant a mono-, di- or polysaccharide
or a derivative thereof, or a degraded starch or
chemically modified degraded starch which is water
soluble. The saccharide repeating unit can have as few
as five carbon atoms or as many as fifty carbon atoms
consistent with water solubility. The saccharide
derivative can be an alcohol or acid of the saccharide as
described in Lehninger's Biochemistry (Worth, 1970). By
"water-soluble" in the present context it is meant that
the sugar is capable of forming a clear solution or a
stable colloidal dispersion in distilled water at room
temperature at a concentration of 0.01 g/l.
Amongst th~ sugars which are useful in this inv~ntion
are sucrose, which is most preferred for reasons of
availability and cheapness, glucose, fructose, maltose
(malt su~ar) and cellobiose and lactose which are
disaccharides. A useful saccharide derivative is
sorbitol.
If sucrose is the chosen sugar, it is preferably used
in an amount corresponding to from 1 to 5~ by weight of
the final product, and the amount of water that enters the
product by way of the sucrose solution is preferably from
1303~?~
- 16 - C3146
2 to 10~ by weight: these percentages are based on the
ultimate, fully formulated product including any postdosed
ingredients. Thus a relatively concentrated sucrose
solution (1 to 3 parts of water per part of sucrose) is
preferably employed. It may be necessary to evaporate of~
some water after spraying on the sucrose solution, rather
than allowing all of it to remain as free moisture in the
final product.
The use of sucrose as the binder has the advantage
that no loss of calcite seed activity occurs.
Alternatively, the binder may comprise an aqueous
solution containing a low level of an anionic polymer, for
example, sodium carboxymethyl cellulose, which is not a
calcite poison. Advantageously an aqueous solution
containing both sugar and a low level of a suitable
anionic polymer may be used: the final product is less
dusty, albeit at the cost of a small loss of calcite seed
activity. The amount of sodium carboxymethyl cellulose
incorporated in the binder solution suitably corresponds
to a level in the final product of from 0.01 to 0.1% by
weight.
Another binder material that may be used in the
process of the invention is a nonionic surfactant, for
example, a C12-C15 primary alcohol ethoxylated with 3-10
moles of ethylene oxide. Nonionic surfactants may be used
alone, in admixture or conjunction with water, or in
admixture or conjunction with sugar solution. Nonionic
surfactants used alone may if necessary be warmad to a
temperature at which they are mobile liquids. When
nonionic surfactants and sugar solutio~s are both used, it
may be advantageous to apply them separately to avoid
gelling problems.
~3~3~2~
-- 17 --
APHERING S~ED CRYSTAL ~AT~RIAL
The adherin~ of` s~ed cry~tal mat*rial to ba,sc
powd~r parl:icles may be carried ~f t l~y er~nulLItion ln
t;he presence of llquld binder 9 u31ng any
~uita~le mixiny appara~us, and n~ay be carried out
batchwi~e or continuously. The solid ~onstitu~nts (bas~
powder and calcite~ Inay be agitated tos~ether while the
liquid binder or b~nders is or ar~ sprayed on. A dxylng
~tep may be required depending on the amount of water
present in the liquid binder.
We have also now discover~d th~t calcltR may successful1y
be inc:orporated by the use of a ~mple ~nodi~iica~ion ~ a
conv~3ntional single level ~pray-drying l~ower.
The proc~ss comprise~:
i) spray-drying an aqueous slu~ry ~mprisin~ the
deteryent active material ~ the alkali metal
carbor,ate, the alkali metal silicate and option,~lly
other con~ent~.onal detergent ingredien~s, in a
spray-drying t~we~; and
( ii) simu1t~neou~1y injec~cing ~tle seed c~y~al ~naterial
ir~to the tower, whereby pa~ticle~ of the seed crystal
materi~l encounter wi~hi~ the tower par~icl~ formed
by the dry~ng ol~ the aqueous ~lurry. .'he ~oed cry~tal
materl al adheres to ttle ~urface of thesf3 par t ~ cle~ .
The calcit:e may ~e int:rodu~ed in part~cula~e o~n
in~co the to~er by any suitable method. Two ~uitable
nlethod~ are known as "~low-in" and "screw-in". "Blow-ira",
as i~ts name suggest~, in~olve~; ~eedlng tl~e calc~te to a
hopper of a~ air pump which blo~s cal~i te throu~h a pip6!
in~o th~ tower. "Screw-in" involves thë u~e of a ss:~rew
f~eder. A less pre~err~3d meth~d i~ t~ spray-in a calc:lte
31ur~y,~ op~'cionally co~tainlng a- s~rfact~n~, such as an
B anionic surf actan~ ~o reduce vi~co~ity .
~L3~3~
- 18 - C3146
slowing in offers the advantage of flexibility with
respect to the direction in which the calcite enters the
tower.
The calcite may be blown in upwardly and vertically,
downwardly and vertically, radially and horizontally,
tanqentially, or in any intermediate direction. In most
spray-drying towers the detergent slurry is sprayed
downwardly from nozzles situated in a upper region of the
tower, and the calcite should be introduced at a level
below that of the spray nozzles so that calcite particles
will encounter base powder granules formed by drying of
the slurry droplets. Advantageously, the calcite enters
the tower at a level below that of the hot air inlet. Of
course, in a multilevel tower slurry may be sprayed in at
various levels and it is then possible for the calcite too
to be injected at several different levels and/or in
several different directions.
In a preferred embodiment of this process, the
calcite is injected tangentially at a level below that of
the hot air inlet, preferably in the bottom cone of the
tower.
Because the base powder slurry contains alkali metal
silicate, any direct contact between the silicate in
solution and the calcite will lead to loss of seed crystal
activity of the latter, and it is therefore desirable that
the slurry droplets should be sufficiently dry when they
encounter the calcite particles. On the other hand, it is
necessary that the base powder granules should not be too
dry when they encounter the calcite particles, ie. they
must be tacky enough that the calcite particles adhere to
the surface thereof. It follows therefore that the
positioning of the calcite injection is critical. In a
small (1.8 m diameter~ tower, i~ has been found that the
~3~3~9
- 19 - C3146
calcite should preferably be injected at a level and/or in
such a direction that the calcite particles will come into
contact with slurry droplets or granules at least about 4
metres below the spray nozzles; but different limits may
be applicable to larger towers.
Finely divided high surface area calcite is a fine
and dusty material and metering to the air pump or screw
feeder may be difficult. It has been found that a
variable speed volumetxic screw feeder - the ACCU-RATE
(Trade Mark) ~eeder, ex March Systems Ltd., Newbury,
Berkshire - linked to a mechanically flexed mass flow
hopper, wilL perform this task successfully.
If desired, other solid materials that are not to be
incorporated via the slurry may be injected together with
the calcite, as an alternative to postdosing. This only
applies, of course, to materials that are stable to the
relatively high temperatures in the tower, and is not a
suitable method for introducing such components as
enzymes, bleaches or bleach precursors. One example of a
material that may be introduced together with calcite is
sodium bicarbonate.
To the agglomerated powder obtained in the
granulation step there may be postdosed any required
additives that cannot be incorporated in the base powder
because of heat-sensitivity or adverse interactions with
other slurry ingredients. Examples of such materials are
bleaches, bleach activators, bleach stabilisers, enzymes,
lather suppressors and perfumes.
Detergent compositions according to the invention
combine maximum calcite seed activity with good powdPr
properties.
~3~13~3~
- 20 - C3146
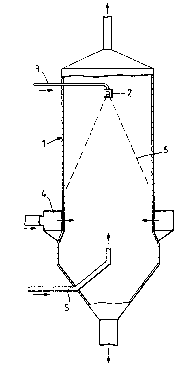
DESCRIPTION OF DRAWINGS
~ . ~ ~
Embodiments of the invention will now be described in
greater detail, by way of example only, with reference to
the accompanying drawings, in which:
Figure 1 represents a first spray-drying tower adapted
for preparing the granules of the in~ention;
0 Figure 2 represents a second spray-drying tower adapted
for preparing granules of the invention.
Referring now to Figure 1 of the accompanying
drawings, a spray-drying tower 1 is provided in its upper
region with a downwardly directed spray nozzle 2 fed by a
supply line 3. A ring main 4 through which drying air may
be introduced is located in a lower region of the tower 1.
A flexible pipe 5 connected to a solids feeder (not shown)
via an air pump (not shown) enters the tower at a level
below the ring main 4.
In preparing the granules of the invention, a
detergent slurry is pumped along the line 3 to the nozzle
2 where it is atomised into droplets forming the shape of
a cone indicated by the dotted lines 6. Hot air is forced
into the tower and upwards through the ring main 4 and the
falling droplets of slurry dry to form granules, which are
initially tacky, as they fall through the tower, Solid
finely-divided calcite is blown upwardly through the
flexible pipe 5, the calcite particles encouter tacky
granules of dried slurry and adhere thereto, and the
composite particles fall to the base of the towar.
The tower shown is Figure 2 is used in a similar
manner, differing only in that the solid calcite blown in
through a flexible pipe 7 enters the tower at a higher
.
;;~3S~3~13~
- 21 - C3146
level, above the ring main 4, so that the calcite gxanules
have the opportunity to collide with wetter slurry
granules.
In an alternative arrangement, differing from those
of Figures 1 and 2, the solid calcite may be blown in
through a flexible pipe which enters the tower
tangentially, at a level substantially below the ring main
4, in the bottom cone of the tower.
EXAMPLES
The invention is further illustrated by the following
non-limiting Examples, in which parts and percentages are
by weight unless otherwise stated.
Example 1 to 3
A spray-dried base powder was prepared to the
following composition:
~L3~3~3~
- 22 - C3146
Parts
Sodium linear alkylbenzene 11.0 18.9
sulphonate 5Dobane (Trade Mark)
113 ex Shell)
Nonionic surfactant 7EO 4,0 6.9
~Synperonic (Trade Mark) A7
ex ICI)
Sodium soap (Pristerine (Trade 2.5 4.3
Mark) 4910 ex Unichema)
Sodium carbonate 30.0 51.5
lS
Sodium carboxymethyl cellulose 0.55 0,9
Sodium silicate 6.0 10.3
20 Minor ingredients 0.3 0.5
Wa~er 4.0 _6.7
58.35 100.0
This was a crisp, free-flowing powder.
Four powders were prepared by mixing 58.35 parts of
this base powder with 20 parts of calcite having a nominal
surface area of 100 m2/g (Socal (Trade Markj U3 ex Solvay)
in a batch mixer.
One (Comparative Example A~ was used as a control,
and onto the other three were sprayed various liquid
binders as specified below (in parts by weight):
~3~3~3~
- 23 - C3146
A 1 2 3
Sucrose - 3 3
Sodium carboxymethyl - - 0.05 0.05
cellulose
Water - 6 2.45 6
The properties of the resulting powders were as
follows:
~3~3Q~
- 24 - C3146
A l 2 3
Calcite seed activity (%) 100 100 100 68
Bulk density (g/litre) 385 443 382 432
Dynamic flow rate (ml/s) 52 86 67 92
Compressibility (~ v/v) 19 20 22 21
Respirable dust ~mg/lOOg) 3.2 0.4 0.27 0.07
It will be seen that the powder A consisting simply
of calcite postdosed to base powder had a poor dynamic
flow rate and was very dusty. The spray-on of sucrose
solution (Example 1) improved both properties
substantially. Use of sodium carboxymethyl cellulose
solution (Example 2) improved the dustiness but the
dynamic flow rate was not ideal. Use of sucrose/sodium
carboxymethyl cellulose solution gave good powder
properties and very low dustiness at the cost of a
slightly reduced calcite seed activity.
Examples 4 to 7
A spray-dried base powder was prepared to the
following composition:
~L3~3~
- 25 - C3146
Parts %
Sodium linear alkylbenzene 11.0 19.9
sulphonate (Dobane (Trade Mark)
113 ex Shell)
Nonionic surfactant 7EO 1.0 1.8
(Synperonic (Trade Mark) A7
ex ICI)
Sodium soap (Pristerine (Trade 2.5 4.5
Mark) 4910 ex Unichema~
Sodium carbonate 30.0 54.2
Sodium carboxymethyl cellulose 0.55 1.0
Sodium silicate 6.0 10.9
20 Minor ingredients 0~3 0.5
Water 4.0 7.2
55,35 100.0
This was a crisp, free-flowing powder.
Eive powders were prepared by mixin~ 55.35 parts of
this base powder with 20 parts of the calcite used in
Examples l to 3, in a batch mixer. One powder
(Comparative Example B) was used as a control, and onto
the others were sprayed various liquid binders as shown in
the Table, which also shows the properties of the
resulting powders.
~3~3~
- 26 - C3146
The nonionic surfactant 7EO used in Examples 4 and 5
was heated to about 50C before spraying. The powders of
Examples 5 and 7 were prepared as follows: first the
nonionic surfactant (100% active matter) was sprayed on,
followed by an aqueous sucrose solution (2 parts sucrose
and 4 parts water).
The powders of Examples 5 and 7, containing nonionic
surfactant, sucrose and water as binders, combined
excellent powder properties, low dustiness and high
calcite seed activity.
13~39a.~
I~ ~ o
I~
~9
~r
o u~
~o I I ~ I I o
c,~ ~ ~
o o ~ ~ o
u~ I ~ I ~ e~- o ~ co ~1
ul a~
o ~ o ~9
~r I ~ I I I o
r~ ~1 ~
t~ ~r
u~ o~
,~ ml I I ~ ~ o
. O U~
OP _
o o ~ o
~ ~ _ ~ O
1--~r :~ a~
s~
.,~
~ .,, OP ~3
0
0
W rl
h 5~ ~ ~1 :3 r l
:~ ~ a~ ~ o rl
~o tq a) ~1 ~1 .4 a
U~
,)
.
O O O
C~
o o ~ ~ ~ ~ ~ o a)
z z~n ~ c~ a~ a ~ ~;
U o U~ O
' ' ~ : ''
- . :
~3~
- 28 - C3146
Example 8
A slurry having a moisture content of 38-40% weight
was prepared using the following ingredients:
Wei~ht ~ (of powder)
Branched alkylbenzene sulphonate, 28.0
sodium salt
Sodium carbonate 40.3
Anhydrous neutral sodium silicate 8.0
15 Sodium carboxymethyl celLulose 0.5
Fluorescer 0.2
Salts ~from the alkylbenzene l.0
20 sulphonate) 78.0_
The slurry was spray-dried using the tower shown in
Figure 1. Finely divided calcite of surface area 63 m2/g
(Socal (Trade Mark) U3 ex Solvay et Cie) was blown in at a
rate equivalent to a nominal level of 10~ by weight in the
formulation. The powder was spray-dried to a moisture
content of about 8~ by weight. 5% of sodium bicarbonate
was then postdosed.
The final powder had the following properties:
Actual calcite content ~wt %) 7.5
Actual moisture content (wt ~) - 8.3
Bulk density (g/litre) 385
35 Dynamic flow rate ~ml/s) 87
Compressibility (~ v/v) 13
~30~3~3~
- 29 ~ C3146
The powder properties were thus satisfactory.
The actual calcite content was measured by dissolving
t}le powder in dilute hydrochloric acid, adjusting the pH
to 10 with ammonia, and titrating with
ethylenediaminetetraacetic acid.
The calcite seed activity of the powders was checked
by means of a water softening test. 3.5g of powder were
dissoled in 1 litre of 24FH ~all Ca~ water containing 10
ppm of sodium tripolyphosphate to simulate the
calcite-poisoning effect of the soil on a dirty laundry
load. The solution was stirred for 2Q minutes at ambient
temperature; precipitated calcium salts were removed using
a very fine millipore filter (0.1 ~m); and the total
soluble calcium level in the resulting filtrate was
determined by atomic absorption spectroscopy. Powders
giving values of 2FH and below for the tota~ soluble
calcium concentration are regarded as acceptable; values
of 1FH and below indicate excellent powders.
The powder of Example 1 gave a total soluble calcium
concentration of 0.95FH, showing that its calcite had
retained its seed crystal activity.
Exam~le 9
A slurry having the same composition as that of
Example 8 was spray-dried using the tower shown in Figure
2, calcite being blown in ~t a somewhat higher position in
the tower. The powder had the ~ollowing properties:
.
~3C~3~
- 30 ~ C3146
Actual calcite content Iwt %) 7.2
Actual moisture content (wt %) 11.9
Bulk density (g/litre) 352
Dynamic flow rate (ml/s) 80
5 Compressibility (~ vtv) 34
Total soluble Ca concentration (FH) 2.38
The compressibility was inferior to that of the
powder of Example 8. The water-softening properties were
also inferior, showing some loss of calcite seed activity.
The lower blow-in position used in Example 8 is thus
to be preferred on both counts.
Examples 10 and 11
The procedure of previous Examples 8 and 9 was
repeated, using the modification of the tower shown in
Figures 1 and 2 in which the calcite is blown tangentially
into the bottom cone of the tower. The powders had the
following properties:
11
25 Actual calcite content Iwt %) 6.7 8.6
Actual moisture content (wt ~)11.6 9.0
Bulk density (g/litre) 430 410
Dynamic flow rate (ml/s) 96 80
Compressibility (% v/v) ~4 18
Total soluble Ca concentration (FH) 0.9 1.05
The powders thus showed good physical properties and
undiminished calcite seed crystal activity.
13~3~3~
- 31 ~ C3146
The powders prepared as described in Examples 1 to 11
may be converted into fully formulated products by the
subsequent addition of conventional ingredients, up to a
total of 100 parts.
: .
'