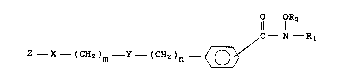Note: Descriptions are shown in the official language in which they were submitted.
3$,~ f:3q~
QA198
PIIIII~ AMI C AC IDS INCLUD ING A
HETERO-CONTAINIIIC
The present invention relates to phenyl
hydroxamic acid derivatives which include at least
one hetero-containing substituent and more
particularly concerns such derivatives which are
inhibitors of arachidonic acid 5-lipoxygenase and
as such are useful, for example, as antiallergy
and antipsoriatic agents.
In accordance with the present invention new
phenyl hydroxamic acid derivatives useful as
~5-lipoxygenase inhibitors are provided. These new
compounds have the general formula
I O OR2
Z - X - (CH2)m - Y - (CH2)n ~ C - N - R
or a phaxmaceutically acceptable salt thereof,
wherein X is NR, oxygen, sulfur,5
or a single bond, and Y is NR, oxygen, sulfur,
S
(l)g
--
9~
QA198
-2-
or a single bond, where R can be hydrogen or lower
alkyl, g can be 1 or 2, and with the proviso that
at least one of X and Y is other than a single
bond; Z is aryl, aralkyl or cycloalkyl; Rl is
hydrogen, lower alkyl, cycloalkyl, lower alkenyl or
aryl; R2 is hydrogen, lower alkyl, aroyl or acyl; m
is 0 to 4; and, n is 0 to 4 carbon atoms. Further
in accordance with the present invention, a method
for using the above compounds is provided.
The hydroxamic acid derivatives of the
present invention where R2 is hydrogen may form
salts with alkali metals, such as lithium, sodium
or potassium. In addition, the compounds of
formula I will form weak salts with
dicyclohexylamine or other amines as well as with
tris(hydroxymethyl)aminomethane, glucamine and
other amines as set out in United States patent
4,294,759. The compounds of the invention wherein
X or Y are NR and wherein Z is 2, 3 or 4-pyridyl
will form salts with acids, e.g. hydrochloric acid
and the like.
The term "lower alkyl" or "alkyl" as employed
herein by itself or as part of another group
includes both straight and branched chain radicals
of up to 12 carbons, preferably 1 to 8 carbons,
such as methyl, ethyl, propyl, isopropyl, butyl,
t-butyl, isobutyl, pentyl, hexyl, isohexyl, heptyl,
4,4-dimethylpentyl, octyl, 2,2,4-trimethylpentyl,
nonyl, decyl, undecyl, dodecyl, the various
branched chain isomers thereof, and the like as
well as such groups including a halo-substituent,
such as F, Br, Cl or I or CF3, an alkoxy
substituent, an aryl substituent, an alkyl-aryl
substituent, a haloaryl substituent, a cycloalkyl
~--f,~
QA198
-3-
substituent, an alkylcycloalkyl substituent,
hydroxy, an alkylamino substituent, an
alkanoylamino substituent, an arylcarbonylamino
substituent, a nitro substituent, a cyano
substituent, a thiol substituent or an alkylthio
substituent.
The term "cycloalkyl" employed herein by
itself or as part of another group includes
saturated cyclic hydrocarbon groups containing 3 to
12 carbons, preferably 3 to 8 carbons, which
include cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, cyclodecyl and
cyclododecyl, which groups are substituted with the
same, or a different cycloalkyl.
The term "aryl" or "Ar" as employed herein by
itself or as part of another group refers to
monocyclic or bicyclic aromatic groups containing
from 6 to ln carbons in the ring portion, such as
phenyl, 2, 3 or 4-pyridyl, naphthyl, substituted
phenyl or substituted naphthyl wherein the
substitutent on either the phenyl, pyridyl or
naphthyl may be 1 or 2 lower alkyl groups, 1 or 2
halogens (C1, Br or F), 1 or 2 lower alkoxy groups,
1 or 2 hydroxyl groups, 1 or 2 alkylamino groups, 1
or 2 alkanoylamino groups, 1 or 2 arylcarbonylamino
groups, 1 or 2 amino groups, 1 or 2 nitro groups, 1
or 2 cyano groups, 1 or 2 thiol groups and/or 1 or
2 alkylthio groups.
The term "aralkyl", "aryl-alkyl" or
"aryl-lower alkyl" as used herein refers to lower
alkyl groups as discussed above having an aryl
substituent, such as benzyl.
The term "lower alkenyl" or "alkenyl" as
employed herein by itself or as part of another
group includes an unsaturated hydrocarbon group
QA198
--4--
having from 2 to 8 carbons and a single
carbon-carbon double bond, such as ethenyl,
l-propenyl, 2-propenyl, 1-butenyl, 2-butenyl,
3-butenyl and the like.
The term "acyl" as used herein by itself or
as part of another group refers to an alkyl
carbonyl or alkenyl carbonyl group.
The term "aroyl" as used herein by itself or
as part of another group refers to an aryl carbonyl
group.
The term "halogen" or "halo" as used herein
refers to chlorine, bromine, fluorine or iodine
with chlorine being preferred.
Preferred are those compounds of the
invention wherein X is O, Y is O or NH, Z is
phenyl, R1 is CH3, R2 is H, m = 2, and n = 0.
The various compounds of the invention may be
prepared as described below.
To make compounds of formula I wherein X is
NR, R is hydrogen, Y is a single bond and Z is
phenyl, a carboxylic acid of the formula
II o
HO~CH2)m ~ C - OH
in an organic solvent, e.g. dichloromethane and
methanol, is added to a solution of ethereal
diazomethane to afford a compound of the formula
III o
HO(CH2)m ~ C - OCH3.
f~9~
QA198
--5--
A solution of the compound of formula III and
p-toluenesulfonyl chloride in pyridine can be
reacted at a temperature of within the range of
from about 0C to about 25C to obtain a compound
S of the formula
IV o o
CH3 ~ S -O -(CH2)m ~ C- OCH3
Compound IV can be reacted with aniline and
anhydrous sodium bicarbonate in the presence of
hexamethylphosphoric triamide (HMPA) at a
temperature between about 25C and 70C to provide
a compound ha~ing the formula
V O
~ N - ( CH2 )m ~ C - OCH3.
Compound V can thereafter be reacted with benzyl
bromide, and anhydrous sodium bicarbonate in the
presence of HMPA to afford a compound having the
formula
VI o
~ N (CH2)m ~ C - OCH3
CH2 ~
A mixture of compound VI with lithium hydroxide in
a solution of dioxane and water produces a
carboxylic acid of the formula
13043~0
-6- QA198
VII O
~ I - (C~2)m - ~ - C -- OH
CH2 ~
A chlorinating agent, e.g. oxalyl chloride or
thionyl chloride, is added to a mixture of compound
VII in a solution of a solvent, e.g. benzene, and a
catalytic amount of dimethylformamide at a
temperature between about 0C and 25C to produce
compound VIII having the formula
VIII O
~ N - (CH2)m ~ C - Cl
CH2 ~
A solution of compound VIII in a solvent, e.g.
tetrahydrofuran, can be reacted with a compound of
the formula
IX OR2
HCl HN - R
in the presence of an organic base, e.g.
triethylamine, to obtain a compound of the formula
x OR2
~ N (CH2)m ~ C - N - R
CH2 ~
QA198
-7-
A mixture of compound X and 5% palladium on carbon
in a solvent, e.g. methanol, can thereafter be
hydrogenated at about room temperature and
atmospheric pressure to afford the compounds of the
invention having the formula
XI l OR2
~ NH-- (CH2)m ~ C--N--Rl
that is, compounds of formula I wherein X is NR, R
is hydrogen, Y is a single bond, Z is phenyl and n
= O.
To make compounds of the invention as in formula XI
but where the phenyl is substituted with hydroxy,
the compound of formula II can be reacted with
N-bromosuccinimide in the presence of triphenyl
phosphine and an organic solvent, e.g. benzene, at
a temperature in the range between 0C and 25C.
This produces a compound of the formula
XII
~r(CH2)m ~ C - OH
which when reacted with a chlorinating agent, e.g.
oxalyl chloride, in the presence of an organic
solvent, such as benzene, preferably in the
presence of a small amount of dimethylformamide at
a temperature in the range between 0C and 25C
affords a compound of the formula
1304390
-8- QA198
XIII o
Br(CH2)m ~ C - Cl.
A compound of the formula
XIV HCl NH2 - O - CH2 ~
can be reacted with a compound of the formula
XV R1 - Br
in the presence of dry EMPA and dry NaHCO3 to
obtain a compound of the formula
XVI R
N - o - CH
H
Compounds XVI and XIII, each in solution in an
organic solvent, e.g. tetrahydrofuran, and in the
presence of triethylamine can be reacted in a molar
ratio of between about 1:1 and 2:1 and at a
temperature within the range from about 0C to 25C
to afford a compound of the formula
XVII 1 IOCH
Br(CH2)m - ~ C - N - R1.
The compound of formula XVII is thereafter reacted
with p-aminophenol in the presence of HMPA and dry
NaHCO3 under nitrogen at a temperature of between
:~-3~4~
QA198
_g_
about 0C and 25C to produce a compound of the
formula
XVIII O OCH
OH ~ NH (CH2)m ~ C N - Rl-
Compound XVIII can then be reacted in an organic
solvent, e.g. methanol, in the presence of 5%
palladium on carbon under hydrogen atmosphere to
yield compounds of the invention having the formula
XIX l OR2
HO ~ NH - (CH2)m ~ C N - R1
that is, compound of formula I wherein X is NR, R
is hydrogen, Y is a single bond, Z is phenyl
substituted with hydroxy and n = 0.
To obtain compounds of the present invention
wherein Z is aralkyl and X and Y are oxygen,
alpha-bromophenetole can be reacted with
p-hydroxybenzoic acid in the presence of a base
such as sodium hydride in an organic solvent such
as dimethylformamide in a molar ratio from between
about 1:1 to about 1:2, and at a temperature within
the range from about 0C to 60C to form a mixture
of the compound
XX
~ o - CH2 - CH2 - ~ C - OH
and the p-hydroxybenzoic acid. This mixture, when
reacted in a solvent, e.g. methanol, and chloroform
with diazomethane in ether yields an ester compound
of the formula
13C~439~)
QAl98
--10--
XXI O
~ O CH2 CH2 - O ~ C - OCH3.
S A solution of compound XXI in a solvent, e.g.
tetrahydrofuran, can thereafter be subjected to an
alkali metal hydroxide such as lithium hydroxide to
afford a compound of the formula
XXII O
~ o - CH2 - CH2 - ~ C - OH.
Compound XXII and a chlorinating agent, e.g. oxalyl
chloride in a solvent, e.g. benzene, is treated
with a catalytic amount of dimethylformamide under
nitrogen at a temperature within the range of from
about 0C to about 25C. The acid chloride
so-formed can then be dissolved in, for instance,
tetrahydrofuran and subjected to a solution of
methylhydroxylamine hydrochloride and triethylamine
to afford a compound having the formula
XXIII O OH
~ o - CH2 - CH2 - ~ - C - N - CH3
that i6, a compound of formula I wherein X is
oxygen, Y iB oxygen and Z i~ aralkyl.
To obtain compounds of the invention wherein
X is a single bond, Y is NR, R is hydrogen, n = 0
and Z is phenyl, a benzoate of the formula
~304390
Q1~198
XXIV o
NH2 --~ C--OCH3
can be reacted with benzyl bromide in the presence
of anhydrous sodium bicarbonate and a solvent, e.g.
dry HMPA, under nitrogen at a temperature within
the range of from about 0C to 70C to afford a
compound of the formula
XXV
11
~ CH2--NH --~ C--OCH3 .
Compound XXV can be reacted with a halogenated
phenylalkane of the formula
XXVI ~ (CH2)m - Halogen
(e.g. l-bromo-3-phenylpropane for the case where m
is 3) in a molar ratio of from about 1:1 in the
presence of a dry organic solvent, e.g.
tetrahydrofuran and a base such as sodium hydride
or n-butyllithium in hexane. This reaction, which
2S can be carried out at a temperature within the
range of from about 0C to about 25C, affords a
compound of the formula
XXVII o
~ (CH2)m - N - ~ C - OCH3
~ CH2
By subjecting compound XXVII to an alkali metal
hydroxide, such as lithium hydroxide in the
-- 13Q43~0
QA198
-12-
presence of water and an organic solvent, e.g.
dioxane or methanol, a product of the formula
XXVIII o
~ CH2)m - N - ~ C - OH
(~}CH2
is obtained.
The carboxylic acid XXVIII can thereafter be
treated with oxalyl chloride in the presence of a
catalytic amount of dimethylformamide and a
solvent, e.g. benzene, to produce
XXIX o
~ (CH2)m - N - ~ C - Cl
@~ CH2
Compound XXIX can be reacted with the compound of
formula IX in the presence o triethylamine, an
organic solvent, e.g. tetrahydrofuran and water at
a temperature within the range between about 0C
and 25C to afford compound
xxx OR2
~ (CH2)m - N ~ C - N - Rl
~ CH2
This compound can be subjected to 5% palladium on
carbon in the presence of hydrogen and an organic
I
3~
QA198
-13-
solvent, such as methanol, to produce the compound
of the invention
XXXI OR2
~ (CH2)m--NH ~ N--Rl
that is, a compound of formula I wherein X is a
~ingle bond, Y is NR, R is hydrogen, Z is phenyl
and n = 0.
The compounds of the invention are
~5-lipoxygenase inhibitors and prevent leukotriene
C4 formation in macrophages (Samuelsson, B.,
Science, Vol. 220, p. 568-575, 1983). The
lS administration of compounds of this invention to
humans or animals provides a method for treating
allergy of a reagin or non-reagin nature. Asthma
is preferably treated but any allergy wherein
leukotrienes are thought to be involved as
pharmacological mediators of anaphylaxis can be
treated. For example, the compounds of this
invention can be used for treatment of such
conditions as allergic rhinitis, food allergy and
urticaria as well as asthma and psoriasis.
An effective but essentially non-toxic
quantity of the compound is employed in treatment.
The compounds of the invention can be
administered orally, parenterally or topically to
variou~ mammalian species known to be subject to
such maladies, e.g., humans, cattle, horses, cats,
dogs, and the like in an effective amount within
the dosage range of about 1 to 100 mg/kg,
preferably about 1 to 50 mg/kg and especially about
2 to 25 mg/kg on a regimen in single or 2 to 4
divided daily doses.
13tg43~0
QA198
-14-
The active substance can be utilized in a
composition such as tablet, capsule, solution,
suspension cream, ointment or lotion containing
about 5 to about 5000 mg per unit of dosage of a
compound or mixture of compounds of formula I.
They may be compounded in conventional matter with
a physiologically acceptable vehicle or carrier,
excipient, binder, preservative, stabilizer,
flavor, etc. as called for by accepted
pharmaceutical practice. Also as indicated in the
discussion above, certain members additionally
serve as intermediateæ for other members of the
group.
The following examples represent preferred
embodiments of the present invention.
~31~
QA19
-15-
Example 1
N-HydroxY-N-methyl-4-[3-(phenylamino)-
propyl]benzamide
A. 4-~Hydroxy Propyl)benzoic acid
1.7M n-butyllithium in hexane (19.4 ml, 33
mmole) was added dropwise to a chilled (-78C) and
stirred solution of diisopropylamine (4.63 ml, 33
mmole) in 20 ml of dry tetrahydrofuran (hereinafter
THF). After 20 minutes, a solution of p-toluic
acid (2.042 g, 15 mmole) in 20 ml of dry THF was
added dropwise. After stirring at -78C for
ansther 1.5 hours, 4 ml of HMPA was added followed
immediately by a solution of ethylene oxide (2.99
g, 67.9 mmole) in 10 ml of dry THF. The resulting
solution was stirred at -78C for 2 hours, treated
with 5% hydrochloric acid and warmed up to room
temperature, the THF being removed in vacuo. The
aqueous solution was saturated with sodium chloride
and extracted three times with ethyl ether. The
combined ether extracts were concentrated from 300
to 100 ml and extracted with a 0.5N sodium
hydroxide solution. This extract was acidified
with 10% hydrochloric acid and extracted three
times with ethyl ether. The ether extracts were
dried over anhydrous magnesium sulfate and
evaporated to a residue. This residue was
chromatographed on a silica gel column, eluting
successively with dichloromethane-ethyl acetate
(9:1 and 1:1), ethyl acetate and
dichloromethane-methanol (9:1) to give 750 mg of
the title A compound, with consistent spectral
data.
13~
QA198
-16-
B. 4-(3-Hydroxy propYl)benzoic acid, methvl
ester
To a solution of title A compound (750 mg,
4.16 mmole) in a mixture of 50 ml of
dichloromethane and 10 ml of methanol was added a
solution of ethereal diazomethane until a yellow
color persisted. After stirring for 30 minutes,
the excess diazomethane was destroyed by a few
drops of glacial acetic acid. The solvent was
evaporated in vacuo to give 800 mg of title B
compound, with consistent spectral data.
C. 3-[(4-MethoxY carbonyl)phenyll-pro~anol
p-toluene sulfonic acid ester
A solution of title B compound (2S0 mg, 1.29
mmole) and p-toluenesulfonoyl chloride (493 mg,
2.59 mmole) in 7 ml of dry pyridine was stirred at
room temperature under nitrogen for 4 hours. The
resulting solution was poured into a cold 10%
hydrochloric acid solution, saturated with sodium
chloride and extracted three times with ethyl
ether. The combined ether extracts were dried over
anhydrous magnesium sulfate and evaporated to give
386 mg of title C compound, with consistent
spectral data.5 D. [N-[3(4-MethoxY carbonyl)Phenyl]propvl]
aniline
A mixture of title C compound (1.0 g, 2.87
mmole), aniline (267 mg, 2.87 mmole) and anhydrous
sodium bicarbonate (360 mg, 4.3 mmole) in 7 ml of
dry EMPA was stirred at 85C under nitrogen for 7
hours. The resulting solution was cooled to room
temperature, diluted with 25 ml of water and
extracted with ethyl ether. The ether extract was
washed several times with water, dried over
anhydrous magnesium sulfate and evaporated to
~3~
QA198
-17-
produce an oil. The oil was chromatographed on a
silica gel column to give 500 mg of title D
compound, with consistent spectral data, as an oil.
E. [N-[N-[3-(4-MethoxY carbonYl)phenvl]-
~ropvllbenzYl] aniline
A mixture of title D compound (1.1 g, 4,08
mmole), benzyl bromide (768 mg, 4~49 mmole) and
anhydrous sodium bicarbonate (515 mg, 6.13 mmole)
in 15 ml of dry HMPA was stirred at 70C under N2
for 3 hours. The resulting solution was cooled to
room temperature, poured into 50 ml of cold water
and extracted twice with ethyl ether. The combined
extracts were washed, dried over magnesium sulfate
and evaporated to give an oil which was
chromatographed to yield 1.1 g of title E compound,
with consistent spectral data, as a solid.
F. ~N[N-[3-(4-CarboxY)phenyl]~ro~vllbenzyl
aniline
A mixture of title E compound (1.1 g, 3.06
mmole) and lithium hydroxide (1.0 g) in a mixture
of 20 ml of dioxane and 10 ml of water was refluxed
under nitrogen for 2 hours. The resulting solution
was cooled to room temperature, adjusted to a pH of
5.5 with 5% hydrochloric acid and most of the
dioxane was removed in vacuo. The residual slurry
was saturated with sodium chloride and extracted
three times with ethyl acetate. The combined
extracts were dried over anhydrous magnesium
sulfate and evaporated to give 1.05 g of title F
compound, with consistent spectral data, as a
solid.
G. [N-~N-[3-(4-Chlorocarbonyl)phenYl~pro~yl]-
benzyl] aniline
To a chilled and stirred solution of title F
compound (1.05 g, 3.04 mmole) in a mixture of 25 ml
- ~L3(~
QA198
-18-
of dry benzene and 0.3 ml of dry dimethylformamide
(hereinafter DMF) at 0C, under nitrogen, was added
dropwise oxalyl chloride (1.5 ml, 17.19 mmole).
Thereafter, the solution was stirred at room
temperature under nitrogen for 1.5 hours. The
solvent was evaporated by a stream of nitrogen.
The residue was dried in vacuo at room temperature
for 1 hour to afford 1.06 g of title G compound,
with consistent spectral data.
H. N-HYdroxy-N-methvl-4-[3-(N-benzYl-N-phenyl
amino)-~ropYllbenzamide
To a stirred solution of
N-methylhydroxylamine hydrochloride (600 mg, 7.18
mmole) in a mixture of lS ml of THF and 5 ml of
water was added triethylamine (4.5 ml, 3.23 mmole).
A solution of title F compound (1.06 g, 2.91 mmole)
in 20 ml of dry THF was then added dropwise. The
resulting solution was stirred for 16 hours,
adjusted to a pH of 5.5 (with 5% hydrochloric
acid), most of the THF removed in vacuo, saturated
with sodium chloride and extracted three times with
ethyl ether. The combined extracts were washed
with dilute brine, dried and evaporated to give a
gum. The gum was chromatographed to give 920 mg of
title H compound, with consistent spectral data, as
an oil.
I. N-Hydroxy-N-methyl-4-[3-(Phenylamino)-
propyl]benzamide
A mixture of title H compound (920 mg, 2.46
mmole) and 5% palladium on carbon (100 mg) in 75 ml
of methanol was hydrogenated at room temperature
under atmospheric pressure for 2 hours. The
resulting mixture was filtered, washing with
methanol. The filtrate was concentrated ln vacuo
and chromatographed to afford 250 mg of title I
13~
QAl 98
--19--
compound, with consistent spectral data, m.p.
93-94C .
~L3~3~1
QA198
-20-
Example 2
N-Hydroxy-4-[3[(4-hYdroxYDhenYl)aminol-
pro~yl]-N-methvlbenzamide
A. 4-(3-BromoDropyl)-benzoic acid
A complex of triphenyl phosphine (3.16 g, 12
mmole) and N-bromosuccinimide (2.14 g, 12 mmole)
was prepared by stirring these in 35 ml of benzene
in an ice bath for 10 minutes and at room
temperature for 1 hour. A solution of 4-(3-hydroxy
propyl)-benzoic acid (1.08 g, 6.0 mmole), prepared
as described in Example 1, step A, in 15 ml of dry
methylene chloride was added to the complex and the
stirring was continued for 30 minutes. The
so-formed mixture was then concentrated, diluted
with 50 ml of ethyl ether and a solution of sodium
carbonate (1.27 g, 12 mmole) in 50 ml of water and
stirred vigorously. The ethyl ether layer was
separated and the aqueous layer was extracted again
with ethyl ether. The aqueous layer was acidified
(with 10% hydrochloric acid) and extracted twice
with ethyl ether. These extracts were combined,
washed, dried, evaporated and chromatographed to
afford 1.2 g of title A compound, with consistent
spectral data, as a colorless solid, m.p.
116-117CC.
B. 4-(3-Bromopro~yl)-ben~y~ chloride
To a cooled and stirred solution of title A
compound ( sao mg, 2.05 mmole) in 15 ml of dry
benzene was added oxalyl chloride (0.6 ml) followed
dropwise by a solution of dry DMF (0.2 ml) in dry
benzene (Z.0 ml). Following a vigorous gas
evolution, the mixture was stirred at room
temperature for 1 hour, evaporated and dried to
afford 570 mg of title B compound, with consistent
spectral data, as a gummy solid.
QA198
21-
C. 0-Benzyl-N-methyl hydroxylamine
To a stirred solution of 0-benzylhydroxyl
amine hydrochloride (1.59 g, 10 mmole) in 15 ml of
dry HMPA containing dry sodium bicarbonate (3.36 g,
40 mmole) was added methyl iodide (1.5 g, 11
mmole). After 5 hours the resulting mixture was
diluted with 30 ml of water and 20 ml of brine and
extracted three times with ethyl ether. The
extracts were combined, washed, dried and
evaporated to produce the crude product as an oil.
The oil was chromatographed to afford 800 mg of
title C compound, with consistent spectral data.
D. N-BenzYloxy~N-methYl-4-(3-bromoPropyl)
benzamide
A solution of title C compound (548 mg~ 4.0
mmole) in dry THF (lO ml) containing triethylamine
(1.1 ml, 8.0 mmole) was cooled and stirred in an
ice bath. A solution of title C compound (570 mg,
~2.05 mmole, crude) in dry THF (10 ml) was added.
A deep purple color developed. After 1 hour, the
mixture was diluted with 10% hydrochloric acid (25
ml) and brine (75 ml and extracted three times with
ethyl ether. The extracts were combined, washed
with brine, dried and evaporated to afford a dark
pink colored oil. The oil was chromatographed on a
column of silica gel to give 690 mg of title D
compound, with consistent spectral data, as a
light-purple-colored oil.
E. N-Benzvloxv-4-[3-[(4-hYdroxYPhenyl)amino]-
Eropyll-N-methyl benzamide
A stirred solution of title D compound (690
mg, 1.97 mmole) in dry HMPA (8.0 ml) containing a
suspension of dry NaHCO3 (50g mg, 6.0 mmole) was
mixed with p-aminophenol (654 mg, 6.0 mmole) and
heated under an atmosphere of nitrogen in a bath at
13(~4390
QA198
-22-
75 for 1.0 hour. The mixture was then cooled to
room temperature, diluted with water (50 ml) and
extracted twice with ethyl ethex. The extracts
were combined, washed with water, dried and
evaporated to afford the crude product as an oil.
The oil was chromatographed on a column of silica
gel to give 670 mg of title E compound, with a
consistent spectral data, a slightly colored thick
oil.
F. N-Hvdroxy-4-[3-[~4-h~droxyPhenyl)amino]
ProPyl]-N-methylbenzamide
A solution of title E compound (630 mg, 1.67
mmole) in methanol (30 ml) containing 5% palladium
on carbon (50 mg) was stirred under an atmosphere
of hydrogen for 1 hour. It was then filtered
through a bed of celite, washing with small amounts
of methanol. The filtrate and the washings were
combined and evaporated to afford a thick oil. The
oil was crystallized from ethyl acetate:hexane
(7:3) followed by drying to afford 320 mg of title
F compound, with consistent spectral data, as a
brownish-gray solid, m.p. 152-153C.
,,~ 1304390
QA198
-23-
Example 3
N-Hvdrox~-N-methyl-4-(2-phenoxyethoxy)
benzamide
A. Mixture of D-hYdroxYbenzoic acid and
4-(2-Phenoxy ethoxy)benzoic acid
A mixture of 50% NaH/paraffin (960 mg, 90
mmole), dry DMF (35 ml) and p-hydroxybenzoic acid
(1.2 g, 10 mmole) was heated in a bath at 120 for
30 minutes resulting in a thick white solid. After
dilution with more DMF (20 ml),
alpha-bromophenetole (2.01 g, 20 mmole) was added
and the heating continued for another 18 hours.
Water (5.0 ml) and solid sodium hydroxide (500 mg)
were added and the mixture was heated again for 15
minutes. Most of the DMF was then removed by
distillation in vacuo. The residue was diluted
with water (150 ml) and extracted twice with ether.
The extracts were discarded. The aqueous layer was
acidified with concentrated hydrochloric acid and
extracted three times with ethyl acetate. The
extracts were combined, washed with brine, dried
and evaporated to afford 1.4 g of a mixture of
p-hydroxybenzoic acid and 4-(2-phenoxy
ethoxy)benzoic acid, with consistent spectral data,
(1:3) a~ a solid.
B. 4-(2-Phenoxy ethoxy) enzoic acid, methyl
_ster
The mixture from step A (1.4 g) was dissolved
in a mixture of methanol (10 ml) and chloroform (40
ml) and a slight excess of a solution of
diazomethane in ether was added resulting in a very
fast reaction. The solution was then evaporated to
dryness. The residue was dissolved in ether (100
ml) and stirred vigorously with lN sodium hydroxide
(50 ml) for 1 hour. The ether layer was separated,
~31~43~
QA198
-24-
washed once with water (10 ml), dried and
evaporated to afford 1.0 g of title B compound,
with consistent spectral data, as a solid, m.p.
92-93C.
C. 4-(2-Phenoxy ethoxy)benzoic acid
A solution of title B compound (1.0 g, 3.98
mmole) in THF (25 ml) containing lN lithium
hydroxide (15 ml) was refluxed under stirring in an
atmosphere of nitrogen for 24 hours. The mixture
was then concentrated in vacuo, diluted with water
(100 ml) and acidified with concentrated
hydrochloric acid. The so-treated ~aterial was
isolated by filtration, washed with water, dried
and evaporated to afford 900 mg of title C
compound, with consistent spectral data, as a
solid, m.p. 198-199C.
D. N-HYdroxv-N-methvl-4-(2-phenoxyethoxY)-
benzamide
A solution of title C compound (300 mg, 1.16
mmole) and oxalyl chloride (1.5 ml, 16.9 mmole) in
dry benzene (7.5 ml) was cooled down to 0, treated
with dry DMF and stirred at 0 for 30 minutes under
nitrogen and at room temperature for one hour. The
excess oxalyl chloride and solvent were removed and
the res:idual solid dried in vacuo for one hour.
This acid chloridP was dissolved in dry THF (2.1
ml) and added dropwise with stirring into a cold
solution of 98% methylhydroxylamine hydrochloride
(204.3 mg, 2.40 mmole) and triethylamine (0.6 ml,
4.88 mmole) in THF (4.5 ml) and water (4.5 ml).
The mixture was stirred at 0 for 30 minutes and at
room temperature for 5 hours, diluted with water
(15 ml) and extracted twice with dichloromethane
(80 ml). The combined organic extracts were washed
with lN hydrochloric acid (15 ml), 5% sodium
`` ~3~ 9~
QA198
-25-
bicarbonate (8 ml) and brine (12 ml), dried,
filtered and evaporated to dryness giving 700 mg of
the title compound as a solid, with consistent
spectral data, m.p. 134-136.
~L3~
QA198
-26-
Exam~le 4
N-HYdroxy-N-methvl-4-[(3-phenylpropyl)aminol-
benzamide
A. N-[(4-Methoxy carbonyl)phenyl benzylamine
A mixture of methyl-4-aminobenzoate (4.535 g,
30 mmole), benzyl bromide (5.134 g, 30 mmole) and
anhydrous sodium bicarbonate (3.78 g, 45 mmole) in
20 ml of dry HMPA as stirred at 75 under nitrogen
for 6 hours. The resulting reaction mixture was
poured into 200 ml of cold water and extracted
twice with ethyl ether. The combined ether
extracts were washed several times with water,
dried over anhydrous magnesium sulfate and
evaporated in vacuo to give a solid. This was
chromatographed on a silica gel column to give 6.2
g of title A compound, with consistent spectral
data, as a solid.
B. N-[(4-Methoxv carbonyl)phenyll-N-[(3-phenyl)-
ProPyl 1 -benzYlamine
A stirred solution of dry diisopropylamine
(1.0 ml, 7.14 mmole) in dry THF (40 ml) was cooled
to -78 under nitrogen and 1.65M n-butyllithium in
hexane (4.33 ml, 7.14 mmole) was added dropwise.
After 20 minutes a solution of title A compound
(1.206 g, 5 mmole) in 20 ml of dry THF was added
dropwise. The mixture was stirred at -78 for 30
minutes and then gradually warmed up to 0.
l-Bromo-3-phenylpropane (2 ml) was then added
immediately, followed by dry HMPA (2 ml). The
mixture was then warmed up to room temperature and
stirred under nitrogen for 16 hours. Water was
added and the THF was substantially removed
in vacuo. The residual slurry was extracted twice
with ethyl ether. The combined ether extracts were
washed several times with water, dried over
-" ~3~
QA198
-27-
anhydrous magnesium sulfate and evaporated in vacuo
to give a gum. This was chromatographed on silica
gel to give 1.03 g of title B compound, with
consistent spectral data.
C. N-[(4-Carboxy)~henYll-N-[(3-Phenyl)~ropYll-
benzylamine
A mixture of title B compound (500 mg, 1.39
mmole) and lithium hydroxide (440 mg, 18.3 mmole)
in a mixture of water (5 ml) and dioxane (20 ml)
was refluxed under nitrogen for two hours. The
resulting reaction mixture was cooled to room
temperature, adjusted to pH = 5.0 with 5%
hydrochloric acid, saturated with sodium chloride
and extracted twice with ethyl ether. The combined
ether extracts were dried over anhydrous magnesium
sulfate and evaporated in vacuo to give 450 mg of
title C compound, with consistent spectral data.
D. N-[(4-Chlorocarbonyl)Phenyll-N-[(3-phenyl)-
propyl]-benzylamine
To a chilled and stirred solution of title C
compound (200 mg, 0.578 mmole) in a mixture of DMF
(2 drops) and benzene (3.5 ml) at 0 was added
dropwise oxalyl chloride (0.3 ml, 3.44 mmole).
After the addition was complete, the solution was
gradually warmed to room temperature and stirred
under nitrogen for 1 hour. The solvent was
evaporated. The residue was dried in vacuo at room
temperature for 1 hour to give 205 mg of title D
compound, with consistent spectral data.
E. N-Hvdroxv-N-methvl-4-[[(3-PhenYl~propyl-
N-benzyl]-amino]-benzamide
To a solution of N-methyl hydroxylamine
hydrochloride (115 mg, 1.37 mmole) and
triethylamine (0.85 ml, 6.10 mmole) in a mixture of
THF (3 ml) and water (1 ml) was added dropwise a
. 13~3g~
QA198
-28
solution of title E compound (205 mg, 0.56 mmole)
in 3 ml of dry THF. The solution was stirred at
room temperature under nitrogen for a few minutes,
acidified with 5% hydrochloric acid to pH = 5.5,
saturated with sodium chlorlde and extracted three
times with ethyl ether. The combined ether
extracts were dried over anhydrous magnesium
sulfate and evaporated in vacuo to give a gum.
This was chromatographed on a silica gel column to
give 185 mg of title E compound, with consistent
spectral data.
F. N-HvdroxY-N-methYl-4-[(3-~henYl~ro~Yl)-
aminol-benzamide
A mixture of title E compound (95 mg, 0.254
mmole) and 5% palladium on carbon (30 mg) in 10 ml
of methanol was hydrogenated at room temperature
under one atmospheric pressure for 1.5 hours. The
resulting mixture was filtered through a bed of
celite and washed with methanol. The filtrate and
washing were combined and concentrated in vacuo to
give 70 mg of N-Hydroxy-N-methyl-4-[(3-phenyl-
propyl)amino]-benzamide, with consistent spectral
~data, m.p. 107-108.
~3~
QA198
-29-
Exam~les 5 to 20
The following additional compounds within the
scope of the present invention may be prepared by
employing the teachings as outlined above and in
S the working examples.
o OR2
C - N - R
z - X - (CH2)m - Y - (CH2)n - ~
~13Q439~
QA198
--30--
5 ~'`I _l o o
Ei
1,
~ I
I . I I !
o ~ U~ = o U~
X~^ = o U~ U~= o o
I
U~
, ~ .... , .. - . , .
13~439~1
-31- QA198
o ~
10 o~ ~ [~ 8,,, 8"
flf ~ frfN
1~
~ b b t~ ~
~ . ~
~ ~ f
a~ o ,~ ~
~ ~ l ,
. ... . . .
QA198
--32--
10 ~
lS p~
~ b
X ~ O
,~
130~390 QA198
--33--
c:
~ ~ ~ o
~, ~ ~ ~
~ ' .
15" P ~ P
~ b ~ ~
30 X Z I z
l~i ~ o
B