Note: Descriptions are shown in the official language in which they were submitted.
-- 1 --
4-16177/16336/~/CGC 1235/1257
User-Activated Transdermal Therapeutic System
Field of the Invention
-
The instant invention relates to improved transdermal drug delivery
systems. In the systems of the invention, the active agent i8 in a
form which a) does not migrate beyond the limits of its reservoir,
and/or b) is less active, less toxic, and/or more stable than the
form of the active agent which would otherwi6e be administered by
the transdermal route until such time as the system is activated by
the user. Activation is achieved by contacting an activating
substance with the active agent reservoir and/or the active agent
reservoir contents, which activating substance has been physically
separated from the active agent and active agent reservoir.
Back round of the Invention
g _
Transdermal drug delivery systems have, in recent years, become an
increasingly important means of administ0ring drugs. Such systems
offor advantages which are clearly not achievable by other modes of
administration such as avoidance of the gastro-intestinal tract and
"first-pass" through the liver, application close to the site of
action, sustained action which can readily be ad~usted, etc.
Clearly then, such systems will become of even greater significance
in the future.
Typical transdermal sygtems currently known are disclosed in U.S.
patents 3,598,122; ~,598,123; 3,742,951; 3,797,494; 3,948,254;
3,996~934; 4,284,444; and 4,597,961. These systems fall essentially
into two catagories, the "matrix" reservoir type and the "membrane
bag" reservoir type.
Each type has some kind of backing material, a drug reservoir, and
an adhesive. The backing material is inert to the drug (or drug
formulation) and adhesive and does not permit any of the drug
formulation to migrate through lt.
In matrix type systems, the drug reservoir ~s a matrix in which the
drug i8 dispersed and through which lt may migrate by diffusion or
mlcroporous flow. The matrix material may slmultaneously act as an
adhesive as well; in which case only an occlusive, removable
covering is required ~o complete the system. When the matrix
material i8 not an adhesive, a suitable adhesive is also necessary
to mount the matrix on the backing material as well as to the
removable occlusive covering. Alternatives to adhesives to secure
the "matrix" to the backing materlal and removable occlusive
covering include compression fitting and "hot melting" including
thermal impulse and ultrasonic welding.
In "membrane bag" type systems, a drug permeable membrane is mounted
on the backing layer to define a pouch (either by 8dhe~1ve,
compression fitting or hot melting) or two membranes are sealed
together to define a "bag" which 18 mounted on the backlng with a
suitable adheslve. Adhesive is also requlred on the bag's surfsce
distal to the back:Lng layer to afflx an occlusive removable
covering.
In each of these systems the drug contalned ln the system ls, at all
relevant times, capable of crossing all of the system components
which would be interposed between the drug and the removeable,
occlusive covering, or the patient's skin.
~;~O~i3~3~
While these known systems are quite useful, they also have severe
drawbacks and limitations to their use. One of the most important
drawbacks of the known transdermal systems is intimately related to
the properties which make the route of administration possible at
all, the ability to permeate intact skin. Because the active agent
can (or the formulation containing it permits it to) permeate intact
skln, extreme caution must be used in the manufacturing process.
This problem is of even greater concern when tha active agent has a
high vapor pressure resulting in vapor settling on clothes,
uncovered skin or elsewhere.
Another problem of known transdermal systems is that frequently the
permeating form of the drug is not suitably stable; therefore, the
~helf "life" of the system would be too short to be commercially
practical. A third problem encountered by the known transdermal
systems is the problem of "drug leakage", primarily through the
adhesive. The drug must be able to migrate through or around the
adhesive. Since it can, it will redistribute itself from the
reservoir into the adhesive, and if the adhesives (and permeable
membrane) have edges which are not surrounded by an occlusive
covering, drug loss results.
In addition, transdermal systems of the art are limited to
regulating drug delivery by only a few, very limited means; By
varying drug concentration, membrane or matrix material snd their
thickness, and by using flux enhancers. However, once the parameters
are chosen, only a single release rate results per system. Moreover,
the adhesive between the reservoir and the removeable, occlusive
covering absorbs a portion of the drug. In this case, an initial
"burst" effect is observed. The amount of drug initially delivered
is higher and then tapers off to a sustained release level.
~3~ 14
4 21489-7302
Another pro~lem encountered with known transdermal devices is the
uncertainty to replace the devlce. Dosing of any medication, by
almost any route of administration is largely based on estimation
and~or approximation, especially with respect to ambulatory
patients and long term medication.
Therefore, the invention seeks to provide a transdermal
system which overcomes these and other defects.
The invention also seeks to provide a transdermal system
which can be manufactured with greater safety with a broader range
of active agents than previously possible.
This inventlon further seeks to provide a transdermal
system which minlmizes rlsks to the user during its application to
the skin.
The invention also seeks to provide a tran~dermal system
which has greater s~orage stability than the known systems.
The invention seeks to provide a change in the device
perceptible to the user to indicate the system no longer contains
an adequate drug supply.
The invention further ~eeks to deliver topical drugs to
the skin in accordance with the foregoing.
Summary of the Inventlon
These and other objects are achieved by the instant
inventlon which is an improved transdermal system having at least
two physically distinct reservoirs.
The invention provides a therapeutlc substance non-
releasing drug delivery system having a removable protective layer
thereon, said system comprising
(a) a therapeutic substance reservoir containing a
~3~ $~
21489-7302
~herapeutic agent or precursor of said therapeutic agent in a
first form which cannot permeate i-rom said therapeutic
reservoir through said system to the surface of said system
which is or was in contact with said removable protective
layer;
(b) an activating agent reservoir containing a system
actlvating agent;
(c) a burstable seal or membrane between (a) and (b) which
is impermeable to said system activating agent and said
therapeutic agent or precursor of said therapeutic agent; and
(d) an occlusive backing layer; wherein upon bursting of
said burstable Real or said burstable membrane, said system
activating agent activates said system by contacting and
converting said therapeutic agent or precursor of said
therapeutic agent from said first form into a therapeutic
agent second form which second form migrates from sald
therapeutic agent or precursor of said therapeutic reservoir
to said surface of said system which is or was in contact
with said removable protective layer whereby said system
becomes a therapeutlc substance releasing delivery system.
The invention also provides a therapeutic substance non-
releasing drug delivery system having a removable protectlve layer
thereon, said system comprlsing
~a) a therapeutic substance reservoir containing a thera-
peutic agent in a ~orm which cannot permeate from said thera-
peutic reservoir through said system to the surface of said
system which is or was in contact with said removable
protective layer;
13~538~
5a 21489-7302
(b~ an activatinq agent reservoir containing a system
activating agent;
(c) a burstable membrane or burstable seal between said
activating agent reservoir and the portions of said æystem
through which said therapeutic agent must migrate in order to
reach said surface which is or was in contact with said
removable protectlve layer but through which said therapeutic
agent cannot permeate; and
(d) an occlusive backing layer; wherein, upon bursting of
said burstable membrane or burstable seal, said activating
agent activates sald system by contactlng said portlons
through which sald therapeutic agent must migrate but cannot
migrate to reach the surface of said system which is or was
in contact with said removable protection layer and modifying
such portions into a form through which sald therapeutlc
agent migrates, whereby ~aid therapeutlc agent permeates
through said system from said therapeutic reservoir to said
surface of said system which is or was in contact with said
removable protective layer and said system becomes a
therapeutic substance releasing delivery system.
Brief De~c ~ n ~ the Drawings
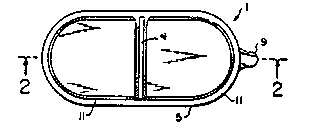
Flgure 1 is a top view of one embodiment of the
invention having the respective reservoirs side by side.
Figures 2 and 3 are cross-sectional views of Figure 1
along line 2-2.
Figures ~, 4 and 5 are cross-sectional views of other
embodiments of the invention having the reservoirs stacked, rather
than side by side.
130~i3~4
5b 21489-7302
Figures 5 and 7 are cross~sectional vlews of still other
embodiments of the invention havin0 more than the two reservoirs
shown in the other Figures.
Figure 8 is a cross-section of an embodiment of a
membrane activation system according to the invention.
Detailed Descri~t,i.,on of the Drawinqs
Figures 1 and 2 represent a first embodiment of the
invention. As shown, the bottom most layer 8 ls a removable
protective layer which is taken off from the transdermal patch 1
by a user or other person just prior to applying the remainder of
the patch to the patient's skln. The remainder of the patch,
comprising components 2-7 and 9 ls applied to the patient as a
unik, with first adhesive layer 7 contacting the skin. Adhesive
layer 7 is also in contact with rate controlling membrane 6.
Impermeable backiny
'~9
~L3~5;~84
membrane 5 is sealed to the rate controlled membrane 6, and together
membranes 5 and 6 deEine two reservoir areas 2 and 3 therebetween.
The two re~ervoir areas are separated by the pressure sensitive
seal 4.
One of rexervoir areax 2 and 3 contains an activating xubstance
whlle the other contains a form of a therapeutic agent or a
precursor thereof.
First tab 9' is a portion of the protective layer 8 which is not
coated with adhesive and ~uts out from the rest of patch 1 so as to
facilitate removal of protective layer 8. Second tab 9 is al30 not
coated with adhesive and is used to remove the patch from the
patient's skin.
Thix embodiment is typlcally prepared as follows:
A silanlzed polyester (mylar film on which silicone layer has been
polymerized) or other suitable material treated with a releasing
agent, approximately 75 micron thick, ix uxed as removable layer 8.
Onto thix ix caxt a contact adhesive, layer 7, typically a polyiso-
butylene xolution. This is further laminated to a control
membrane 6, if de6ired, about 100 microns thick. Ethylene-vinyl
acetate is quite suitable for this membrane. Next, the contents of
reservoirs 2 and 3 are dispensed, in a suitable form, on the rate
controlling membrane and a suitable backing layer (ahout 80 micron)
having a heat sealable coating on one surface ls placed over the
reservoir contents, coated side againDt the contents, and the devlce
i6 heat sealed around the perimeter, seal 11, and between the two
reservoirs, heat seal 4. The dimensions of heat seal 4 LPreferablY
about 0.5 to about 2.0, more preferably about 0.5 to about 1.0 mm
wide~ and the seal(s) around the perimeter of the patch are such
that seAl 4 will selectively burst under force applied by the
"user", advantageously at about 10 pounds of force to about
50 pounds of force. The minimum force to burst a burstable seal may
be as high as about 20 pounds, preferably 17, more preferably 14,
~L36~S3~3~
most preferably 10 pounds of applied fo}ce. Throughout this specifi-
cation, and claims the term "applied force", unless otherwise
characterl~ed, means the total force ultimately translated to the
burstable portions of the system.
As a practical matter, the burstable heat seal 4 must be capable of
burstlng under force which will be commonly applied by those using
or applying the system. Preferably, the maximum applied force
required to burst the burstable seal is about 40 pounds, more
preferably 30 pounds, most preferably 20 pounds. The only real
limitation within these bounds is that the non-burstable seals and
membranes be capable of maintaining their integrity at the applied
forces. Hence, such non-burstable seals and membranes must be of
sufficient size and material to remain intact under the forces
applied to the system. Preferably, the non-burstable portions of the
systelll are of such size and materials HO that they can withstand a
Eorce preferably at least 1.5, more preferably at lsast 2.0, most
preferably 2.5, times that required to burst the burstable seal.
In Figure 3, layer 7 is also in contact with permeable membrane 6',
and if present, impermeable membrane 6. If the monolithic matri~ has
adhesive as a part thereof, no additional adhesive is needed. If
not, a thin layer of adhesive sufficient to adhere the monolithic
layer to the rest of the system (and the patient) is also required
on the appropriate surfaces. This coating, if present, is not
separately shown in the figures, but considered to be the edglng of
layer 7 as shown. Impermeable backing membrane 5 is Healed to
permeable membrans 6' and impermeable membrane 6, and together
membranes 5, 6 and 6' define two reservoir areas 2 and 3 there-
between. The two reservoir areas are separated by the pressure
sensitive seal 4.
Reservoir area 2 contains an activating substance while area 3 is
empty. The agent to be activated is distributed in monolithic
layer 7, either throughout the entire area or only in that region
covering area 3. ~lternatively, membranes 6 and 6' may be of ths
13~
same material provided it i8 impermeable to two substances, which
substances, when mixed, generate an activating substance which can
permeate membrane 6-6'. In that event, one of the activating sùb-
stance precursors are contained in area 2 and the other in area 3.
First tab 9' is a portion of the protective layer 8 which is not
coated with adhesive and juts out from the rest of patch 1 so as to
facilitate removal of protective layer 8. Second tab 9 is also not
coated with adhesive and i8 used to remove the patch from the
patient's skin. It is irrelevant which portion, around the perimeter
of the patch, as viewed in Figure 1 has tabs 9 and 9'. Tab 9 can be
an extension of either membrane 6 or 6'.
As viewed from the top (see Figure 1), the patch overall perimeter
(exclusive of the tab) can be of any desirable configuration.
However, that shown in Figure 1 and circular are preferable.
This embodiment is typically prepared as follows:
A silanized polyester (or other suitable material treated with a
releasing agent), approximately 75 micron thick, is used as remov-
able layer 8. Onto thia i8 cast the drug containing also monolithic
layer 7 containing contact adhesive, typically a polyisobutylene
containing solution. This is further laminated to membranes 6-6', if
desired, about 100 microns thick. Ethylene-vinyl acetate is quite
suitable for this membrane but other~ known in the art will be
apparent. Next, the contents of reservoir 2 (ant 3 if any) i8
dispensed, in a suitable form, on membranes 6-6', and a suitable
backing layer about 80 micron) having a heat sealable coating on one
surface is placed over the reservoir contents, coated side against
the contents, and the device is heat sealed around the perimeter,
seal 11, and between the two reservoirs, heat seal 4.
The dlmensions of heat seal 4 and its properties are analogous to
the embodiment of Fig 2.
A second embodlment is shown in Figure 4 (seal 11 and tabs 9 and 9'
not being shown) wherein the reservoir areas 2 and 3 are on top of
another and separated by pressure 6ensitive, non-permeable mem-
brane 10. The pressure sensitive, non-permeable membrane 10 shown in
this embodiment i8 prepared by laminatlng a perforated sheet of
membrane material 10" to a continous sheet of membrane material 10';
but it may also be a single layer continuous material which is
unaltered or depth slit as desired. The limitations in the preceding
paragraph regarding applied force are applicable here as well,
pressure sensitive membrane 10 replacing burstable heat seal 4 in
the description there. Advantageously, the continuous material 10'
is about 10 to about 50 microns and the perforated material 10"
independently about 10 to about 100 microns thick. More specifi-
cally, the continuous material 10' is suitably 50, preferably 41,
more preferably 33, and most preferably 25 microns thick; while the
perforated material 10" is suitably 100, preferably about 83, more
preferably 66 and most preferably 50 microns thick. Alternatively, a
continuous sheet of material can be 'depth slit' to a suitable
degree. These dimensions are given when the laminate membrane
materials consist of ethylene/ vinyl acetate copolymers. Vse of
different laminate materials having different strengths will lead to
slightly different relative dimensions of thickness of the continu-
OU8 and perforated materials. However, it should be realized that
any degree of selectively re-inforcing only portions of the burst-
able membrane aid in the performance of the system.
The above laminate is placed over the contents of reservoir 2, with
the weakened surface of the laminate preferably facing reservolr 2,
the contents of reservoir 3 and backing layer 5 applied, and the
entlre assembly sealed.
Suitable alternative materials and dlmensions for layers 5-9 are
known in the art of transdermal application as apparent from the
aforementioned U.S. patents. Layer 10 can be selected from any
s~litable membrane material which is known to maintain a separation
~3~ $~
-- 10 --
between the contents of reservoir 2 and reservoir 3. Most preferable
layer 10 is a polyolefin (for example ethylene/vinyl acetate or
polyethylene).
Alternative embodiments include those where rate controlling
membrane 6 is either unnecessary or undesired and thus eliminated.
In these situations, either reservoir 3 is a solid or a matrix
containing the therapeutic agent. In another embodiment, reservoir 3
can be dispersed through an adhesive. In such an arrangement,
membrane 6 is eliminated and reservoir 3 and adhesive 7 are a single
layer.
In any event, when membrane 10 is present, the burstable membrane is
no greater than about 20 % to 80 %, preferably about 20 % to about
50 % as thick as any other membrane made of the same material ln the
system.
A further embodiment is shown in Figure 5 (seal 11 and tabs 9 and 9'
not being shown) wherein the reservoir areas 2 and 3 are one on top
of another and separated by pressure sensitive, non-permeable
membrane 10. The pressure sensitive, non-permeable membrane 10 shown
in this embodiment is a single layer of continuous material; however
it may be depth slit or a laminate of a continuous sheet and
perforated sheet of membrane materlal. The limitations in the
preceding paragraph regarding applied force are applicable here as
well, pressure sen~itive membrane 10 replacing burstable s~al 4 in
the description there.
Advantageously, in the laminate, the continuous material i~ about
10 to about S0 microns thick. More specifically, the continuous
material is suitably 50, preferably 41, more preferably 33, and most
preferably 25 microns thick. In addition, the continuous sheet of
material can be 'depth slit' to a ~uitable degree. These dimensions
are given when the laminate membrane materials are ethylene/vinyl
acetate copolymers.
13~5;~
The above laminate, when used, is placed over the contents of
reservoir 3 (if any), with the weakened surface of the laminate
preferably facing reservoir 3, the contents of reservoil- 2 and
backing layer 5 applied, and the entire assembly sealed. In this
system, reservoir area 3 is empty and reservoir 2 contains an
activating substance or reservoir 2 and 3 contain substances that,
when mixed, generate an activating substance. In such an embodiment
having substances in both areas 2 and 3, membrane 10 must be
impermeable to both, while membrane 6 should be impermeable to the
substance in reservoir 3 if it is capable of activating the active
agent precursor, but otherwise, membrane 6 need not be impermeable.
In any embodiment where reservolr 2 contains an activating agent
(not a precursor) and membrane 10 i9 a laminate of a continuous
sheet of impermeable membrane and a perforated sheet of a membrane,
reservoir 3 (other than the perforation space), reservoir 3's
contents, and membrane 6 can be eliminated. In other words, the
perforated side of laminate layer 10 is in direct contact with
monolithic solid matrix layer 7.
As one of ordinary skill will be aware, other embodiments include
combinations of features of the foregoing embodiments. One such
variation is the embodiment of Figures 6 and 7. These differ from
Figures 3, 4 and 5 in that reservoir~t 2 and 3 have been split into
two compartmenta each 2', 2", 3' ant 3", by nonburstable, non-
permeable membranes (or nonburstable seals) 12 and membrane lO i8
shown in the laminate form as layers 10' and 10". Membrane (or seal)
12 meets the same requirements for nonburstabillty as set forth
earlier for other nonbur~table seals or membranes in the system.
Such systems can be utilized to administer more than one therapeutic
agent at a time when those agents are either chemically incompat-
ible, require incompatible activating substances, should not come in
contact with the activating substance of the other therapeutic
agent, or require different flux enhancers. Still other variations
of construction and utilities for the more complex systems will be
apparent to those of ordinary skill. Of course, as stated above,
$~
- 12 -
where desired, empty reservoir areas (such as 3' and 3" in Figure 7)
in appropriate circumstances (other than the "perforation space")
and membrane 6 can be eliminated if desired.
A further variation of the embodiments described above is the
inclusion of a user perceptible timing indicator to alert patients
or those administering the patches to patients that the transdermal
device has been exhausted, is near exhaustion, or is otherwise
depleted to an extent that desired delivery characteristics are no
longer being met. The timing device can be a non-permeable indicator
which changes color over time once activation of the system has
occu}red, a non-permeable colored ingredient which has been
encapsulated with the encapsulating material degrading over time
once activation has occurred, or most advantageously, a non-perme-
able color changing indicator which color change results from the
depletion of the active agent itself.
~enerally, the user bursts the barrier (seal 4 or membrane 10)
immediately before or preferably after applying the patch to the
skin. This now allows the contents of reservoirs 2 and 3 to come
into contact, whereby the system is activated. Usually, the
therapeutic agent i8 in a non-active form which must be altered for
the desired transdermal delivery and the activating substance
transforms the non-active therapeutic agent into the active species.
However, it is not critical that the therapeutic agent be altered.
It is only required that until the barrier between the reservoirs ls
breached, the system as a whole be in the inactlve state. As such,
systems wherein the activating substance alters the permeabillty
characteristics of the layers between reservoir 2 and skin 90 that
the unchanged therapeutic agent can then migrate to and through the
skin are also within the scope of the inventlon.
As noted above, the crltical feature of the lnvention is that until
the activating substance is brought into contact with the inactive
form of the therapeutic agent and/or the barrier between the
therapeutic agent and the removeable occlusive layer or skin, the
13~5;;~4
system is essentially inactive. Once this contact has been made, the
system is activated and drug flow from the reservoir to the skin
begins.
When a heat seal is used as barrier between the reservoirs. heat
seal 4 should be significantly thinner than the perimeter seals so
that seal 4 is preferentially burst when placed under force. In
order to insure that heat seal 4 does not inadvertantly burst under
normal handling, it should be of sufficient thickness to resist
forces of up to about 5 to about 15 pounds applied force, preferably
about 10 pounds to about 15 pounds, more preferably 10 pounds of
applied force. Advantageously, upon the application of forces in
excess of about 5 to about 20, preferably about 10 to about 15, more
preferably about 10 pounds, seal 4 will burst. However, seal 4 may
resi6t greater forces, as set forth earlier, if desired.
When a membrane barrier is used, such as membrane 10, it can be
constructed of the same or different materials as the other
membranes of the system. If membrane 10 i9 of the same material as
the other membranes, the unreinforced area should be significantly
thinner than other membranes to insure selective bursting of barrier
membrane 10. If desired, the reinforcing portion of barrier
membrane 10 may be omitted, but it is most preferably present. If a
different membrane material la aelected for the unrelnforced portion
of barrier membrane 10, it can be of any spproprlate thlckness which
will preferentlally burst vls-a-vis the other membranes and seals
when sub;ected to the applied forces in the range of 10 to 50 pounds
as set out above. As noted above wlth respect to seal 4, barrier
membrane 10, whether relnforced or not, must be reslllent enough to
reslst bursting unlntentlonally under normal handling condltions.
There are a variety of inactive or precursing forms of the thera-
peutic agent and correspondlng activatlng substance suitable for the
instant invention. These include the therapeutic agent being a
powder, a crystal, in an ionized form, being bound to an ion
exchange resin or covalently coupled via labile linkages to an
5;~34
- 14 -
lmmobilizing moiety, being trapped in a polymer matrix, being
encapsulated with an appropriate material or being in the form of a
precursor or prodrug. Many other forms will be apparent to those of
ordinary skill and are within the scope of the invention. Prefer-
ably, this inactive form of the therapeutic agent will not traverse
the skin and/or at least one barrier of the system between the
therapeutic agent and the skin (or removable, protective layer 8).
This may be exemplified by an application of an acidic drug, where
the ioni~ed species penetrates to a slight degree, but the free acid
permeates through the appropriate barrier to a much larger degree
and freely permeates through the skin. Upon application, but before
activation, only a very low dose would be delivered. Upon acti-
vation, the much larger desired dose would reach the patient.
The activating substances are any appropriate substances which
change the therapeutic agent form into one which will deliver the
desired dose at the desired rate to the patient. These include
solvents such as water, alcohol, etc; pH regulators such as buffers,
acids, or bases which liberate the corresponding acidic or basic
therapeutic agents which act as free acids or bases on the skin,
salt solutions to elute the drug; enzymes or catalysts to cleave
labile linkages, swelling agents to open microencapsulation pores;
appLopriate reactive species to generate the drug from the prodrug
or precursor; etc.
One alternative of the invention in which it is not necessary to
alter the therapeutic agent form is in the case of a drug which
requires a flux enhancer to permeate across intact skin. Until
activation, the flux enhancer is kept in the second reservoir. Upon
activation, the separating barrier is breached and the flux enhancer
can now aid in drug delivery.
In other embodiments, the barrier between the drug and the skin (or
removeable protective layer 8) is altered, instead of the thera-
peutic agent, to achieve activation. Such a system is exemplified by
Figure 8. In this Figure, permeable membrane 6 might be a xerogel or
~3~-3~
- 15 -
ionic gel which is not permeable to the drug or drug formulation
until it is hydrated. Compartment B would contain water or buffer in
a water impermeable casing of membrane A and A'. Depending upon the
desired characteristics~ activation could be achieved by selectively
breaking the sidewalls of compartment B (walls A') and hydrating
xerogel or increasing hydration of the ionic gel membrane 6. Once
hydrated, the pores in membrane 6 will allow the passage of the drug
in reservoir 2. A typical example is a crosslinked polyacrylic acid
membrane 6 and a basic activating agent. When walls A' are broken,
the polyacrylic acid pores open under the action of the basic
activating agent so that the drug to be administered can migrate
through the system.
In the mono]ithic systems de3cribed above, the actlvating agent can,
instead of altering drug from or solubilizing the drug found in the
monolithic layer, alter the characteristics of the monolithic layer
itself. For example, a drug which itself is unaffected by the
actlvating agent can be immobilized within the monolithic layer due
to pore size. So long as the pore size is small, the drug cannot
migrate, but otherwise, migration and skin permeation would be
possible. Upon contact of the activating substance with the mono-
lithic layer matrix, the matrix swells, expanding pores, and allows
the drug to migrate.
While virtually any drug which can be administered transdermally
(see for example US patents 3,598,122; 3,598,123; 3,742,951;
3,797,494; 3,948,254; 3,996,934; 4,284,444; and 4,597,961; etc.)
with the present system, it is especially useful to use the present
invention to administer a drug selected from: antitubercular
agents, such as isoniazid and rifampin; analgesic~ such as fentanyl
snd sufentanyl; muscle relaxants, such as baclofen; ~-adrenergic
receptor agonists and antiasthmatics, such as theophylline,
formoterol, and terbutaline; steroids, such as estradiol,
progesterone, met'nyltestosterone, and desoxycorticosterone; anti-
cholinergics, such as scopolamine and methscopolamine; vasodi1ators,
such as nitroglycerine; antihypertensives, such as metoprolol;
~3~ 4
antihistamines, such as triprolidine, tripelenamine, and diphen-
hydramine; cholinergic agents, such as arecoline; CNS stimulants,
such as methylphenidate and nikethimide; angiotensin converting
enzyme inhibitors, such as 3-l(5-amino-1-carboxy)pentyl-amino~-l-
carboxymethyl-2,3,4,5-tetrahydro-lH-l-benzazepine-2-one; nicotine,
physostigmine, and naloxone. The only limitation to use of this
system for a drug for transdermal use is that the drug have at least
one form which permeates through the skin and any barriers of the
system between the drug reservoir and the skin. If a topical drug is
being administered, the only restriction is that there be at least
one form of the drug which can migrate through the system barriers
between the drug reservoir and the skin.
A preferred claas of drugs for use in the system of the invention
i9: fentanyl, sufentanyl, terbutaline, formoterol, theophylline,
estradiol, progesterone, scopolamine, nitroglycerine, triprolidine,
tripelenamine, diphenhydramine, arecoline, nicotine, and 3-1(5-
amino-l-carboxy)pentyl-amino~-1-carboxymethyl-2,3,4,5-tetrahydro-lH-
l-benzazepine-2-one. A still more preferred group of drugs for use
in the invention includes: arecoline, nicotlne, progesterone,
triprolidine, diphenhydrsmine, formoterol, scopolamine, nitro-
glycerine and estradiol. A most preferred drug for administration
with the invention is selected from arecoline, nicotine, scopol-
amine, nitroglycerine and estradiol.
The invention will be further understood in connection with the
following Examples which do not limit, but only exemplify, the
invention.
Example 1: Membrane System
This Example is based on Figures 1 and 2. The transdermal system
described has an active drug releasing surface of 10 cm~ and is
manufactured according to the invention as fo1lows: A polyiso-
butylene based contact adhesive dissolved in hexane is cast onto a
75 micron thick film of sylani~ed polyester. After drying of the
adhesive, the adhesive side of the assembly is laminated to a
100 micron thick membrane of ethylene-vinyl acetate (EVA) copolymer
leaving a strip at one side free of adhesive. The contents of
reservoir 2, containing the drug, and reservoir 3, containing the
base activator, are simultaneously dispensed side by side on the EVA
copolymer side of the described laminate in the center of
reservoirs 2 and 3. Reservoir 2 contains 183.6 mg of an Arecoline
Hydrobromide ointment consisting of 58.5 % Arecoline HBr, 1.7 %
Carbopol~ 934P (as a gelling agent) and 39.8 % of water. (183.6 mg
of this ointment contains 107.4~mg or 0.455 mmole of Arecoline H~r).
Reservoir 3 contains 121.3 mg of a potassium carbonate ointment
consisting of 51.88 % of Potassium carbonate, 1.57 % of CARbOPOL 934
(as a gelling agent) and 46.58 % of water. (121.3 mg of this
ointment contains 62.39 mg or 0.455 mmole of potassium carbonate).
A 80 micron thick backing film (5) of polyester with an ~VA heat
sealable coating is laid over the dispensed portions of reservoir
ointments 2 and 3, heat sealed at the perimeter and through the
center, separating reservoirs 2 and 3. The perimeter consists of a
non-destructive seal while seal 4 consists of a pressure sensitive
seal breahing at 10 psi.
The system is activated by the user by bursting seal 4 and mixing
the contents of reservoir 2 and 3. The membrane impermeable salt
form of the drug is thus converted by the base activator into a
membrane permeable form, the free base. The device has an active
drug releasing surface of 10 cm2 and delivers 22.5 mg of free base
within 24 hours in vitro.
~xample 2: Solid Matrix
A transdermal drug delivery system according to the invention, as
described in Figure 4, is prepared as follows:
A solid matrix drug reservoir (3) is prepared by extruding ethylene
vinyl acetate (EVA) copolymer mixed with Acrecoline Hydrobromide as
an about 200 micron thick film, thus forming a porous monolithic
matrix containing 50 to 60 % drug by weight. Drug/polymer discs,
- 18 -
corresponding to desired system surface areas, are then punched from
the extruded film. A pressure sensitive membrane (10 in Figure 3) is
prepared by laminating a previously perforated EVA film ~about
50 microns thick) with a continuous EVA film (about 10 microns
thlck) to form a net membrane laminate (60 microns thick) consisting
of a structurally reinforced film with nonreinforced areas corre~
sponding to the degree of perforation in the laminated film. As
mechanical pressure is applied across the membrane, the non-rein-
forced perforation selectively ruptures. An alternative technique to
prepare a weakened seam in a membrane is to depth slit an EVA mem-
brane (about 70 microns thick) to produce structurally weakened
points about 60 microns deep within the film. As pressure is applied
across the film the controlled depth sllt regions will selectively
r~lpture. An activator ointment (2 ln Figure 4) is dispensed onto the
surface of the pressure sensitive membrane (10 in Figure 4) and a
liquid form filled seal is made between the pressure sensitive
membrane and a backing film (S in Figure 4). A solid matrix drug/-
polymers disc (3 in Figure 4) is then positioned on the membrane
surface of a rate controlling membrane/adhesive laminate ~6 and 7
respectively) and an outer destructive seal is made between the rate
controlling membrarle (6 in figure 4) through the pressure sensltive
membrane (10 in Figure 4) to the backing layer. The system is
activated by applying pressure to the top of backing film 5, thus
rupturing pressure sensitive membrane 2. The activator ointment is
then drawn into the solid drugtpolymer matrix 3 by c~pillary tension
and osmotic pressure, thereby converting impermeable Arecoline HBr
to permeable Areco]ine free base. Materials, drug forms, activators
and rate control membranes described in the previous Example are
applicable to this solid matrix system design.
Exarnple 3:
This Example is based on Figures 1 and 2. The transdermal system
described has an active drug releasing surface of 10 cm~ and is
manufactured according to the invention as follows: A silicone base
adhesive is hot melted onto a 75 micron thick film of flurocarbon
treated polyester. The adhesive side of the assembly is laminated to
-- 19 -
a 75 micron membrane of ethylene-vinyl acetate (EVA) copolymer. The
contents of reservoir 2, containing the drug, reservoir 3, con-
taining the base activator, are simultaneously dispensed side by
side on the EVA copolymer's side of the described laminate in the
center of reservoirs 2 and 3. Reservoir 2 contains 157.7 mg of a
Nicotine 2HCl ointment consisting of 44.9 % Nicotine 2HCl, 3.8 %
Carbopol 934P (as a gelling agent) and 48.7 ~O of water. (157.7 mg of
this ointment contains 70.8 mg or 0~301 mmole of Nicotine 2HCl).
Reservoir 3 contains 226 mg of a sodium hydroxide ointment consist-
ing of 14.2 ~/O sodium hydroxide, 2.7 % Carbopol 934P (as a gelling
agent) and 83.1 % of water, (226 mg of this ointment contains
32.1 mg of 0.802 mmoles of sodium hydroxide).
A 80 micron thick backing film 5 of polyester with an EVA heat
sealable coating is laid over the dispen3ed portions of reservoir
ointments 2 and 3, heat sealed at the perimeter and through the
center, separatlng reservoirs 2 and 3. The perimeter consists of
a non-destructive seal while seal 4 consists of a pressure sensitive
seal breaking at 10 psi.
The system is activated by the user by bursting seal 4 and mixing
the contents of reservoir 2 and 3. The membrane impermeable salt
form of the drug is thus converted by the base activator into a
membrane permeable form, the free base. The device has an active
drug releasing surface of 10 cm~ and delivers 16.5 mg of free base
within 24 hours in vitro.
ExamFle 4-
A transdermal drug delivery sy6tem according to the invention,
essentially as in Figure 5 (membrane 6 and reservoir 3 are
eliminated), is prepared as follows:
A solid matrix monolithic drug reservoir ls prepared by extruding
poly-ethylene vinyl acetate (EVA) mixed with Arecoline Hydrobromide
as an about 200 micron thick film, thus forming a porous monolithic
matrix containing 50 to 60 æ drug by weight0 Drug/polymer discs,
~L3~
- 20
corresponding to desired system surface areas, are then punched from
the extruded film. A pressure sensitive membrane (10 in figure 3) is
prepared by laminating a previously perforated EVA film (about
50 microns thick) with a continuous EVA film (about 10 microng
thick) to form a net membrane laminate (60 microns thick) consisting
of a structu}ally reinforced film with nonreinforced areas corres-
ponding to the degree of perforation in the laminated film. As
mechanical pressure i8 applied accross the membrane, the non-
reinforced perforation selectively ruptures. (An alternate technique
to prepare a weakened seam in a membrane is to depth slit an
EVA membrane (about 70 microns thick~ to produce structurally
weakened points about 60 microns deep within the film. As pressure
is applied across the film the controlled depth slit regions will
selectively rupture. An activator ointment (at area 2 in figure 3)
comprislng 121.3 mg of K2C03 ointment consisting of 51.88 ~/0 K2CO,t,
1.57 ~0 carbopol 934P (a gelling agent) ant 46.58 % water, i8
dispensed onto the surface of the pressure sensitive membrane (10 in
Figure 3) and a non-destructive seal iB made between the pressure
sensitive membrane and a backing film (5 in figure 3) of polyester
having an EVA heat sealable coating. The solid matrix drug/polymers
disc is then coated with adhesive (disc plus adhesive is represented
by 7 in Figure 3) and positioned on the perforated or depth slit
surface of membrane 10. Finally a 75 micron thick film of sylanized
polyester (8 in Figure 3) is placed over the expoued adhe~ive
surface of the solid matrix drug/polyeuter disc, The system is
activated by applying pressure to the top of bac~ing film 5, thus
rupturing pressure sensitive membrane 10. The activator ointment is
then drawn into the solid drug/polymer matrix by capillary tension
and osmotic pressure, thereby converting impermeable Acoline HBr to
permeable Arecoline free baue.