Note: Descriptions are shown in the official language in which they were submitted.
3~7
TITL~ P~-2309
IMPROVED SILVER-BASED ELECT~OSTATlC ~ ~N~5lNC~y~
BACKGROUND OF_TH~ _NVE~ TION
Thl6 inventio~ relatefi to elec~rostatic
~rinti~g and, more par~icularly, ~o a~ i~proved
electrostatic printin~ ~a~ter adapted ~or the u~e o
co~ven~ional ~ilYer halide ~hotogra~hic technigue~
during ~rep~ration of the ~a6teY ~or pri~ti~g.
; Ele~tro~tatic p~inti~g i6 ~ell-know~ ~n the
art and ha6 been propo~ed ae a~ alternatiYe ~o ot~er
: ~ri~ting te~hnique6. In one method o~ electEosta~ic
; prin~ing, one f ir~ ~repare~ a "~a~er~ tha~ i~
capable o~ ~electively holding electro~ta~ic ~harge$
to for~ the desi~ed i~age. The master i~ expo~ed to
a corona di6charye that forms a latent elec~rostatic
image, and contacted with dry or liquid toner of the
oppo6ite electrostatic charge to develoe the i~age.
The toned image i~ then tran~ferred ~o a sub~rate,
typically paperO where the toner i6 fu6ed ev fix ~he
image, a~d the ~a~ter i~ retur~ed for the ~e~t
printing cycleO
It has been 6ugge6ted in U.S. Patent
4.069,759 that an i~roved electro6ta~ic p~i~ting
~a~ter ca~ be fabricated by disper~ing a ~onve~tio~al
6ilve~ halide photographic ~alt in an in6ulating
polymer (e.g., gelati~)~ and coa~ing the di~persion
on a conducting substrate. The coating i8 expo6ed
: i~agewi6e, and i~ developed to ~au~e ~he exposed
6ilve~ halide to be reduced to metalli~ ~ilver~ The
30 u~expo6ed ~ilveE halide ~ then di~601Yed and ~emoved
from the coating to fix the i~age. ~hile the ~aster
6ugge6eed in U.S. Patent 4,069,7Sg offers many advan-
tage6, and per~it6 the u~e of ~onven~ib~al aqueous
~ er halide photographic che~istry whe~ gelatin iB
35 ~ele~ted a6 the in6ulating polym2r, it has bee~ Pound
.
~ t7
tha~ gelatin is tGo highly 6en~itive to humidi~y to
hav~ prac~ical application in a typical workplace.
~elatin rapidly ab60rbs ~oi6ture ro~ the ai~ and at
~odera~e to high humiditi~6 no lo~gel fun~tio~s a~ a~
in~ulaSing medium, bu~ provide~ a ~onclucti~e path
~hat g~ound~ ~ur ace ~hargei impoced on the master
during the electro~ta~ic prin~ing ~roce~.
Thu6. ~here i~ a need for ~n improved
electro~tatic printing ma6ter that will offer ~he
advantage6 of being ba~ed on conventional aqueou6
~ilver halide photoyraphic chemi6try and provide
6upe~ior in~ula~ing propertie~ u~der relative
humidity condition6 commonly encountered duri~g
printing.
SUMMARY OF THE TNVENTION
Thi~ invention provides a photosensitive
compo~ition adapted or u6e in preparing an elec~ro
æt~ti~ printing ma6teL. the ~ompo6ieion con6~6~ing
e~entially of a siilYer halide phstog~aphic ~alt
: di6persed in an in6ulating pol~meric binder ~ha~ i6
~wellable in aqueou6 photographic proc~ ing solu-
tion~ having a pH higher tha~ approxi~a~ely ~-1/2~
and Letain~ 6ignifi~ant in6ulating prope~ie~ under
relative hu~idity condition6 normally enrou~ered
during the printing proce~6. The composition ha~
an insulation value 6uch that it will ~upport an
apparent macro6copic electric field of ~t least ~ive
~5) volt6/~icron, a~ mea~ured by an electro~tatic
6urface voltage probe two (2) 6econd~ following full
charging of it6 surface that ha~ been allowed to
equilibrate at 50% relative humidity at 20C for an
hour. common photographic gelatin, practically the
only medium conventionally u6ed for wet proce66ing.
3s hold~ approximately one (l) volt/micron or le6E after
6~37
equilib~ation u~der the6e te6~ condition~. 5ince the
binder i~ swellable under pH condi~io~s high0r than
approxi~ately 8-1~2, ConVeA~iOnal aqu~ou~ fiilver
halide developi~g 801ution6 can be u6ed to proce66
the ~a~ter for u~e in electro6ea~ic prinei~g.
Copolyme~s of acrylic o~ ~ethac~ylic a~id having
a~id number6 in the range of 70 to 160 are a p~e-
ferred bi~dar that ~ay be 6elected in p~acticing the
inve~ion. The silver halide~binder co~position i6
typically coated onto a condu~ting ~ubstra~e, which
~ay be mounted on a flexible ~upport, for u6e a6 an
ele~tro~tatic ma6ter. After ~he ~a6ter i6 imaged
~ith actinic light. the ma6ter i~ developed to
contain a ~ er image using conventional aqueous
6ilver halide developing and ~ixing chemistry.
In a 6econd embodi~ent. a diffu6io~ transfer
film i8 p~epared by coating the poly~eric binder which
contain6 development nuclei onto a conduc~ive 6upport,
and o~ercoa~ing ~he binder with a co~ventional 6ilver
halide photographic emul6ion. ~he photosen~i~ive
element i~ expo6ed and then developed using ~onven-
tional difu6ion tran~fer technique~ to p~ovide an
imaged elec~rostatic ~a6ter.
A6 u6ed herein. the term ~electrostatic
~aster~ refer6 to the fil~ element that will be u6ed
for elec~ro~tatic printing, whether the fil~ element
contai~s 6ilve~ pa~ticle~ in the form of the de6ired
i~age, and ~hu~ i~ ready for ~he printing proce66, or
contain~ eilve~ halide particle6 ~hat yet have to be
e~posed and/or developed.
BRIEF DESCRIPTION OF THE DRAWINGS
, ,
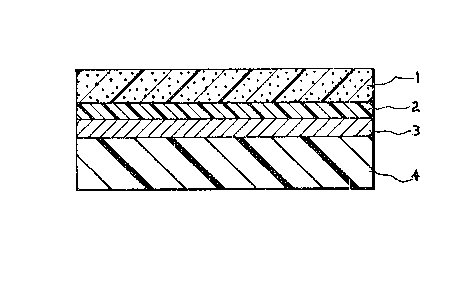
Figure 1 i6 a ~chematic 6ectional view of an
electro6tatic printing ~a6ter in which a 6ilver halide
photogLap~ic 6alt i~ di~per6ed in the insulating
binder to form ~hoto6en6itive layer 1.
1137
Figure 2 6how~ th~ ~a6ter of ~ig. 1 in which
a l~te~t i~age ha~ been formed and developed.
FiguEe 3 show~ ~he master of Fig. 2 after
the image has been fixed.
Pigure 4 show~ the master of 'Fi~. 3 after
bei~g charged.
~igure 5 illu~trate~ the ~a6~er of Fig. 4 in
which toner ~article6 have been attracted ~o the
charged 6urface.
Figure 6 is a çchematic ~ec~ional view of a
~econd embodiment in ~hich the photo~en6i~ive layrr
i8 a diffu6ion t~an6fer film.
Figure 7 6how6 the embodiment of Fig. 6 in
which the diffusion tran6fer fil~ ha6 bee~ imaged and
development has commenced.
Figure 8 8~0Wfi the embodiment o~ Fig. 7
after developmen~ is complete.
Figur~ 9 ~how~ t~e embodiment of Fig. B
a~ter the ~hotosensitive layer 8 has bee~ remo~ed, at
~; 20 ~hich time it i6 ~eady ~o be used as an electro6tatic
master.
:~.
D TAlLED DESCRIPTION OF THE INVENTION
~he u6e of conventional aqueous silvee
halide ehotographic ch~mis~ry ideally ser~es ~ha
require~ent6 for the preparation of electrostatic
printing master~, par~icularly when high refiolution
B reguired for high-quality half-tone or con~inuous-
to~e aeplication6. Sharp image resolution can be
3G obtained due to the fine qrain size of 6ilver that
may be obtained when using aqueous photographic
~hemistry well known i~ the art.
ln~ulating binder6 ~hat ~ay be ~ele~ted in
practi~ing the invention are "swellable~ in aqueou~
3~ 601utions having a pH higher than approxi~ately
3~7
8-1/2, typieally in the range of 9 ~o 1~, that a~e
com~on ~o conven~ional aqu~ou~ developing ~olueion~
u6ed in ~ilver halide photo~raphy. ~y "~wellable" it
i6 ~eant ~hat the binder readily ~ake~ up watel, and
indeed 6well~ i~ thi~ pH range ~imilar to gelatin.
~he~ using preferred polymer6 de~cribed hereinaftQrO
6welli~ accompll~hed by ionizing acidic groups
~u~ually carborylic acid group6 ~hat are chemically
bonded to the in~ulating binder) by ba6ic solution6
at a pH o~ approximately 8.5 or higheE. This
characteri6tic permit6 the aqueous developer
(reduci~g) ~olution to come in~o intimate contact
wi~h the ~il~er hal~de. When negative wozking 6ilver
halide emul6ion6 are used, the expo~ed silver halide
5 i6 eeduced ~y developer ~olution6 to metallic ~ilver
and complexing agents di6601ve the u~expo6ed ~ilver
~alide salt. When po~itive working ~ilver halide
emul~ions are used (e.g. tho~e prepared by such
well-kno~n ~echniques a~ 601ariza~ion or ~hemical
fogging) the unexpo~ed 6ilver halide 1~ re~uced to
metallic silveE and the ~xpo~ed ~ilver halide
removed.
I~ the embodime~ des~ribed in grea~er
detail hereinafter in which negative workinq ~il~er
halide is di6per6ed in the i~ulati~g binder~ ~ro-
vided by ~he invention, developer above approximately
pH 8.5 6welle the binder and reduces expo6ed ~ilver
halide ~o ~etallic ~ilve~ ~nd complexing agent6,
usually in a fixer eolu~ion, remove unexpo6ed ~ilver
halide. I~ ~he diffu6ion transfer em~odi~ent where
negative working photosensit~ve 6ilver halide ~6 in
an emulsion layer (u6ually gelatin) that i6 ~eparate
from the insulating binder containing a fine di6per-
~ion of development nu~lei, developer solu~ion having
a pH above appeoxi~ately 8.5 swell6 both t~e e~ul~ion
3'~
layer and insulaeing binder layer provided by this
invention~ ~hereby developing tAe expo~d 6ilve~
h~lide ~o ~etallic silver in the emul~ion layer and
difisolvi~g ~he unexpo6ed ~ilYer halide with complexing
agentfi (i'6ilver 601Yents"3. The complexed unexpo6ed
6ilver halide then dif~u6es into the ~wollen binder
l~yer wherein t~e s~lver ions are selectively Ieduced
to 6ilver ~etal on the development nu~'Lei.
Although the in6ulaei~g binders are
lQ 6wellable in the developiny 601ution, ~he in6ula~ing
properties do ~ot drastically deteriorate a6 ehose of
g~latin do under typical humidity conditions en~oun-
tered i~ the ~orkplace. As a consequence, the binders
will retain an applied cha~ge durin~ slectrostatic
lS printing and it i6 not nece66ary to provide ~pecial
humidity control~ or dry the ma6te.r be~ore each
printing cycle. a6 would be ~eces6ary u6ing a gelatin
bind~r 0
The binder~ generally are characte~ized a6
bei~g capable of 6up~0rti~g an apparent macroscopic
electric ~ie}d of at lea~t 5 volt~ per micron. and
preferably at lea6~ 30 volts per micron, as mea~ured
by a~ electros~atic 6urface voltage probe two (2)
second6 ollowing ~ull charging of the 6urface af~er
the surface has been allowed ~o equilibra~e. and thus
ab60Eb moi~ture, at 50% relative humidity and 20OC.
Equilibration for testing purpose6 will nor~ally
occur within approxima~ely 60 minutes. In contrast,
gelatin is significantly l~ferior and exhibits an
apparen~ ~acro6copic electric field in the order of
approximately one ~l~ volt per micron or le86 under
thi~ test procedure.
Ie has been found that ~ynthetic polymers
having an acid number o app~oximaeely 70 to 160 are
par~i~ularly u~eful in praclicing the invention. A
~3~
preferred cla~6 of poly~er~ contain~ 10 ~o 25~ by
weigh~ of ~rylic or meehacrylic acid ~o impart
swellabili~y. ~he polymer typically ~ill al~o
con~aih ~tyrene. or oth~r aromatic ~ono~ers, ~hat
S are ~ot compatible with water, and ~hu~ resder the
polymer le~ hydrophilic to moi~ur~ in the air~
Generally, the polymer will al~o con~ain ~ono~er6.
~uch a6 appropriate acrylic or methacrylic e~ter6,
~hat ~ontribute to fil~ clarity, flexibili~y, eough
ne~, eroces6ibility. e~c. Other co~ono~ers~ ~uch a~
alken~6 havi~g 2 to 12 carbon ato~ aloolefin6,
vinyl acetate, vinyl ether6 having 3 to 12 carbon
atomsO methacrylamide. and the like can be similarly
u~eful.
Preferred polymer~ ~re copolymer~ ~ontaini~g
~yrene and acrylic or ~ethacrylic acid mono~ers, and
preferably also an acrylic or ~ethacrylic e6ter
mo~omer. Polymer6 containing 25 to 35% by weight
styrene, 10 to 25~ by ~eiyht acrylic or me~hacrylic
ac;d, with ~e remainder compri6i~g a~rylic or
~ethacrylic e~teL6, are pa~ticularly pre erred. The
~ole~ular weight of the preferred copolymer~ will
typically b~ in the range of 25,000 to 150,000.
'rhese polymer~ a~e compatible with silver ~alide
di~per~ion~, will forr~ teasonably durable film6
that have clarity, and are readily available f~om
commercial ~ou~ce6, or can be made using conventional
technique6 6uch a6 free radical polymerization in
~u~pen~ion or emul~ion. Equi~alent polymer~ that
will be useful in practicing the invention will be
readily apparent to ~ho~e ~killed in the art. These
polyme~s include acrylic acid and ~thacrylic acid
polymer6 and copoly~er~, and include commercially
available polymer6 ~uch as Ca~bo6et~ 525 an~ Car~s6et~
526 ~anufactured by 9. F. Goodri~h Company, and
Joncryl~ ~7 manufactured by John60n ~ Johnson.
A preferred cla~6 of polymer6 consti~ute6
terpolymer~ and ~etrapolymer~ of (1) a ~tyrene-type
~onomer, (2) a~ a~rylate-type ~o~omer, ~nd (3~ an
un6atu~a~ed carboxyl con~aining ~ono~er. The ~ir~t
~omponen~ lend~ hardne~s and moisture re~istance to
the polymer, the ~econd, flexib~lity and pla~ticity
to the polymer backbone; and the third, alkali-
wellability. The ~tyrene-type ~onomer will
~y~ically be 6tyrene, an alpha-6ubstituted ~tyrene
havi~g a 1 to S carbon alkyl group, and tho~e wherein
th~ benzene ring ha6 f~nctional 6ubstitu~ed group~
6uch a6 ~itro. alko~y, acyl. carboxy, 6ulpho, or halo.
wi~h 6imple compound~ ~uch as 6tyrene, alphamethyl
6tyrene, para-~ethyl 6tyrene and para-t-bu~yl styrene
being prefeered. The acrylate-type component i.ncludes
alkyl and hydroxyalkyl acrylate6 and methacrylate6
wherein the alkyl group ha6 from 1 to 12, preferably
~rom 1 to 6 carbon atoms 6uch a6 methyl methacryla~e,
ethyl~ethacrylate, ethyl acrylate, hydroxypropyl
methacryl~te, ~ydroxyethyl methacrylate and hydroxy-
~thyl aerylate, and mixtu~e6 ~hereof. The un~aturated
carboxyl-containing ~onomer will typically be a
mono~er having from ~ to 15 ~arbon atom6, prefeeably
3 to 6. and includes cinnamic acid. ~ro~onic acid,
sorbic a~id, i~aconic acid, maleic acid, fu~aric
acid, or more preîerably acrylic or methacryli~ acid,
theie corre~ponding hal~ e6ter or the corresponding
anhydr ide .
~hen thi6 clas6 o polymer i~ ~elected in
~racticing the invention~ ~he ratio of ~he three
monome~ component6 i6 ~elected ~uch that the eonduc-
tive film element ha6 the Pollowing propertie6 ~he
~ilver halide. when incorporated into the conductive
Pilm ~lement, i8 proce~ible by conventio~ai aqueou6
photographic technique6: the electro6~a~ic ma6ter
~3~3~7
~ade ~heIef~om ~e~ain6 applied cha~ge6 in the
~onsilve~ ~ ea6 under am~ieRt relative humidi~y
condition6; and the electro~ta~ic ~a~ter i5 flexible
and durable, but not ~acky. Typi~al proportions u6ed
to a~hieve the~e result~ are shown in Table lo
TABLE 1
Binder Com~onent Broad Range Preferred Ra~g~
~ei~t %) (weiqht~
~tyrene-type 10-50 25-35
10 As~ylate-type 0-85 40-65
Ca~boxylic 5-50 10-2
acid-type
Poly~e~ within ~hi6 class also generally offer the
~dva~tage o~ being insensitive to I60par~, the
co~mGnly u6ed cacrier employed in liquid toning
~y8 te~6.
In~ulating polymeric binders described above
are made by conventional free-radical polyme i2ation
~20 ~ech~ique6~ a6 illu~trated in the examples. The6e
:polyme~ are ~oluble in basic solution~ and can be
coated fro~ aqueou6 solutions of triethylamine.
a~onia~ or ~o~as~ium hydroxide, and ~he like. The~e
polymers are ~ompatible with silver halide disper6ions
and will fo~m rea60nably durable films ~hat have
clar~ty. It may be de~ired to modify the binder
(cros61ink, ha~den, plasticize. adju~t acidity, etc.)
prio~ to aqueous photographic processing, and thereby
~ont~ol ~welling or improve durabili~y. Various
modifying agents may be added for these pu~poses.
~ypical modifying agent~ include aldehydes, multi-
functional aziridine6, and epoxides. The diglycidyl
ethe~ of 1.4-butanediol is a preferred modifying
;agent for this class o~ poly~ers in prac~icing ~he
3~ invention.
' .
~ ~}~ 37
Equivalent polymer6 that achieve the balance
of propertie~ de~c~ibed above will be apparent to
thofie skilled i~ ~he art. and ~ay be selected in
practicing the invention.
S The light 6en6itive 6ilver halide 6elec~ed
for ~i6per6ion in the binder can be any of ~he
well-known 6alt6 u6ed in pho~ographic application6.
Representati~e u6eful 6alt6 include 6ilver ~hloride,
~ilver bromide. 6il~er iodide, silye ~hlorobromide,
~ilver iodobromide, and 6ilver ~hloroiodobromide,
either ~ingly or in ~ix~ure~. Precipitation of the
halide i~ carried out in conventional ~anner in
gela~in. The amount o~ gelatin pre~ent 6hould be
limited~ or 6ub6equently reduced by rinsin~, to avoid
d~feating purpo~e6 of the invention. Generally,
levels of gelatin a6 high a6 3 to 15 gram6 per mole
of 6ilver can be tolerated in the elect~o~tatic
printing ma6ter6. without adver6e effect.
: Grain ~ize di~tribution and ~en6itizatio~ o~
the 6ilver halide can be controlled to adapt the
~ilver halide6 for ~he 6alec~ed cla~6 of pho~ographic
proce~a, includi~g general continuou6 tone, X-ray,
lithographic, microphotographic, direct posi~ive, and
t~e like. Ordinarily, the 6ilver sale di~per6ion~
will be ~en~itized with ~o~ventional co~pound6 ~uch
a~ sulur, gold, rhodlum, selenium and the like, or
with o~ganic sen6itizing dye6 6uch a~ cyanine, 1.1'-
diethyl-4,4'-cy~nine iodide, methine and polymethine
cyanine dyes, kryptocyanine6, ~erocyanine~, and the
like. Other additive6 commonly employed in 6ilver
halide photographi~ com206ition6, may al60 be pre6en~
if de6ired.
To prepare the di6per~ion of ~ilver halide
in the in6ulating polymeric binder, the binder i6
conveniently fir~t di6~01ved in an aqueou6 ~olution
- . ~
`fil5~
containing amine6. 6uch a6 a~onia or triethyl amine.
If de~ired, a~ alcohol, 6uch aç me~ha~ol, ethanol, or
i60propanol, ~ay be added ~o aid in 601ubilizing the
polymer. ~e~one6, ~uch a~ methyl e~hyl keto~e, may
5 be u6ed a~ a co~olvent. ~ aqueou6 di6persio~ of the
silver halide ~al~ hen added to the di~ olved
binder in the de6ired quanti~ie6. The re6peceive
portion~ o silver halide tD binder will depend o~
detail6 of the application~ but ~ill generally be
10 6uch that 6urface of the ma~te~ immediately above the
developed 6ilYer will di~charge ~iqnificantly fa~er
tha~ area6 devoid of ~ilver. ~eigh~ ratio~ of 6ilver
to polymeri~ binder in the ranys of 0.5:1 to 3:1 will
typically provide u6eful recult6. A p~eferred range
i~ 1.7:1 to 2.3:1.
The polymeric binder containing ehe 6ilver
halide i6 usually applied to a conductive substrate
a6 a 601ution or di6persion in a carrier ~olvent,
u~ually a~ aqueou6 601ution co~taining ba6ic amine6
o~ 60dium o~ pota6sium hydro~ide a~ de6cribed above.
The eoa~in~ procedure may be any conven~ional one
including 6p~ayin~, brus~i~g. applying by a roller or
a~ er~ion coater, flowing over the ~urface, picki~g
up by immersion, ~pin coatiny. air-knife coa~ing,
wire~bar coating or any other 6uitable mean6. The
film thicknes6 can be adju6ted accordingly and after
; drying i~ u6ually abou~ O.OZ to about 0.3 mil6
~0.5-7.5 ~icron~). p~eerably about 0.04 to about
0.20 ~il~ -5.~ micron6). Depending on the
application, the conductive 6upport may be a ~etal
~late, such a~ aluminum. copper. zinc, 6ilver or the
like; a conductive polymeric film; a 6uppor~ such a6
paper, gla6~. synthetic re6in and the like which ha6
.~ been coated with a metal, metal oxide. or ~etal
3s halide by vapor depo6i~ion or chemical depo6ition:
.
11
-
- ~ 3~ 3 ~
a ~uppor~ which has been coated with a conductive
~olymer: or a 6uppor~ which ha~ been Goated wieh a
polyme~ic binder con~aining a metal, metal oxide~
~etal halide, conduc~ive polyme~, carbon, or other
conductive filler~.
In addi~ion to Gomponen~ deficrib@d abo~e,
various ~onven~ional photogLaphi~ ~dditive~, e.g.,
de~eloping aqent6. ~uper additive~, antifoggant6,
~oating aid6 6uch a6 6~ponin, alkylaryl6ulfonic acid6
lo or ~ul~oalkylæuccinic aeid6; plasticizers 6uch
glycerol or l,5-pen~anediol: anti~tatic agent6: agen~
to p~eve~t the Por~ation of s~ot~: antihalation dye6;
underlayer~, ~ubbing or backing laye~; a~d the like
~ay be added ~o the master a~ appropriate. Po6it~ve
image6 ~ay be obtained by rever6al proce66ing of the
6ilver halide u6ing either light fogging or a
chemical fogging agent: or by u~ing 6ilver halide
emul6ion6 that give direct po6itive image6 UBiDg the
prefoggi~g technique. Di~ect po~i~ive emul6ion6 have
been de6cribed in Leer~maker U.5. Pat. No. 2,184,0l3.
Illing6wor~h U.S. Pat. No. 3,50l,307 and el6ewhere.
Referring now ~o the drawing6. Figure 1
depic~ an elect~ostatic printinq ma~ter in which
hoto6en~itive layer l con~ain6 ~en6itized ~ilve~
halide di~per~ed in t~e in~ula~i~g polymeric binder
in acco~dance with the invention. Layer 1 i6
generally between 0.5 and 7.5 ~i~ron6 in thickne~,
but ~he thickne6~ can be decrea6ed or increa6ed for
~he 6peeific intended application. A thin layer 2
of an adhe6ion promoter ~uch a6 gelatin, which i6
o~ional, aid6 adherence of the phoeo~en~i~ive layer
to the ~ondue~ing 6ub~trate 3, whi~h in ~uen i~
mounted on 6uppo~ting 6u~strate ~
The ~a6ter i~ expo6ed imagewi6e u6ing any
of the proceduce6 commonly u6ed with ~ilver halide
.
12
~L3~ l37
13
pho~ographic material6. such a~ by imaging with
ac~ini~ light, a cathode ray ~ube, or la~er. ~or
- ne~ative-working emul6ion6 ehe latent image 5 i~
then developed by reduc~ng ~he expo6ed ~ilver halide
parti~le6 to metallic 6ilver u6ing a con~entional
aqueou6 developing solueion. a6 illu6~rated in
Figure 2. A conventional aqueou~ fixing solution,
such a~ 60dium ~hio6ulfate, i6 ~hen u~ed to remoY~
the unexpo6~d 6ilver halide particles, as illu~trated
~o in ~igure 3. The deYeloped ma6~er i6 then ready for
tbe electEo6tati~ printi~g proce66.
Figure 4 illu6trate~ t~e master of Fiqure 3
after it has been cha~ged by a corona discharge ~hat
depo~ited po6itive charge6 6 on the ma6ter 6urface.
The area of the film that contain6 ~ilver S pro~ide~
a pathway for overlying charges to pa66 to ground,
thus forming a latent image of charges that remain on
: the master ~urface. Al~ernatively. charging can be
accompli~hed with the u6e of a negative corona
di~charge, 6hielded ~orotron~ ~corotron, radioactive
60urce. ~ontact electrode6 ~uch a6 eleet~ically
bia6ed 6emiconductive rubber roller~, and ehe like.
The latent i~age i6 ~hen develop~d witb
liquid or dry toner 7 of the oppo~ite polariey, a6
:~ 2~ illu6trated ~n Figure 5. Cascade, ma~e~ic bru6h,
powder cloud, liquid, r~agne-dry and we~ting develop-
ment te~hnique6 are 6uitable. Repre~enta~ive dry
~oner~ that may be u6ed include Kodak Ektaprint K
toner, Hitachi HI-Toner ~MT-414, Cano~ NP-350F ~oner,
30 and To6hiba T-50P toner. Exa~ple& of ~uitable liquid
toner6 are Savin 24 toner~ Canon LBP ~one~ and Jame~
River Graphics TlB18 toner. The laten~ image 60
developed ("toned") i6 tran6ferred to the usual
~ub6trate, typically paper. where it i6 fixed in
3S conventional ~a6hion.
.
13
37
1'1
Figure6 6 through 9 illu6trate a 8econd
embodi~en~ wherQi~ co~ventional diffu6ion ~an6fe
technique~ 6u~h a ~ho6e de~cribed in U.S. Patent
2.352.104 and 2.983.606. are uæed to prepare a~
i~aged electro~tatic priAting mas~er of di~persed
~ilve~ in the insulating synthetic bincle~ p~eviou~ly
de~cYibed. In thi~ embodimen~, th~ in~ula~i~g
6yntheti~ bi~der 9, approximately 0.25 ~o 3 microns
in thick~e~6, eontain~ di6per6e~ developmen~ ~u~ai~
10 a~d a photo6en6itive layer 8 containi~g 6ilver halide
~altg di6peL~ed i~ a hydrophilic colloid ~ha~ o~erlay6
the binder, wherein the ratio of 6il~er to binder 9
~ 1 to 5:1. A conductive layer 3 and 6ub6trate 4
are employed a6 hereinbefore de6c~ibed. Suitable
15 development nu~lei are well-known in the art, and
typically will be (1) a metal, 6uch a6 ~ilver. gold,
and ~hodium; (2) 6ulfide6, 6elenide6, telluride6~
poly6ulfide6, or polyselenide6 of metal6 including
6ilver, zinc, chromiu~. gallium, iron, cadmium cobalt,
20 nickel, ~anga~ese, lead, ~ti~ony, bi6~u~h. arsenic,
Opp8r, rhodium. palladium, platinum, lanthanum, and
ti~aniu~: (3) easily reducible 6il~e~ ~alt6 which
f4r~ 6ilver nuclei duLing proce~ g, ~uch as ~ilver
nitrate o~ 6ilver cit~ate: (4~ inorganic 6alt6 which
25 ~eac~ with ~he i~coming diffu~ing ~ilver salt~ to
form nuclei; and ~5) organic ~ompound6 which (a~
contain a labile 6ulfur atom and ~hich therefore lead
~o the formation of 6ulfide ~uclei during pcoce6~ing,
in~luding mercaptans, xanthate~, thioaeetamide.
30 di~hiooxamide, and dithiob~urate or (b~ are reducing
agent6 6uch hydrazine derivative~ or 6ilane~ and give
ri~e to 6ilver nuclei when evaporated on~o æilicic
acid6 or baeium 6ulfate. Likewi6e ~he hydrophilic
colloid can be any of the sub~tance~ commonly used
in diffu6ion tran6fer proce66e6, 6uch a6 gelatin.
phthala~ed gelatin, cellulo~e deri~fa~iveG ~uch a~
~arboxymethylcellulo~e and hydroxylaethylcellulos0.
and other hydrophilic high molecular weighc colloidal
6ub~ance~ ~uch a6 dextrin, ~oluble 6tar~h. polyvinyl
5 alcohol, or polyfityrene~ulfonic acid.
P~ef~rring ~co Figure 7. photo~en6itive layer
~ i~ imaged in con~rentional fa~hion to form a laten~
image with the ~ensi'cized ~ilver halide. For
negative-working e7~ul~ion6 tlle photo6en6i~cive l~yer
10 is then treated with a develoeing agent ~hat redu~eE;
tlle exposed ~ilver halide ~o meealli~ silver, in area
10. and a~ aqueou6 solvent compo~ition ~hat ~onvertF,
6ilver halide in the unexpo~ed area~ to form a 601uble
6ilver halide ~omplex that diffu~e6 into the binder
15 of layer 9 whe~e ie contact~ the development nu~}ei
and i6 reduced to insoluble ~ilver particle6 11,
forming a silver image. Layer ~ i6 ~hen removed as
illustrated in Figure 9. ~e6ulting in an electro-
~tatic ma~ter that i~ ready fo~ printin~ in corlven-
20 tional manneE. Developing bath6 for the diffu~iontran6er proce6~ are ~ell known in the art and are
- de~ibed, for exa~ple, in
Diffu~ion Proce66e6 by ~ndre P~ott and E:dith ~eyde
(~ocal Pres~, 1972 ) and Modern Pl~ol:o~raPhi~
Proces6ina, trol. 2 by Grant Hai6t ~iley. 1979~.
~ 5any additional embodiment~ will be e~ident
to tho~e skilled in the art. For example, a po6itive-
working silver halide emul6ion san be u~ed in sonjunc-
tion wi~h ~ch~ diffu6ion tran6Per coating 8 illu~trated
30 in Figure6 6 throu~h 9, and 'che expo6ed 6ilver halide
~an be complexed in aqueou~ 601uti on6 to dif fu6e into
the ~n6ulating binder layer 9, where it i6 reduced by
the development nuclei ~co form the de~ired silver
image. Similarly, a 6eparate pho'co~en6itive ~ lm ~an
35 be employed ~ n lieu o coatis~g 8, and brought into
37
16
operative a660ciation with ~he in~ulating bi~der 9
before or a~ter i~aging~ a6 i~ photo~echanical
tran~fer. The photo6en6itive ~ilver halide emul~ion
laye~ or coating 8~ and the ln~ula~i~g polyme~i~
binder layer 9 may al60 contain compou~ld6 commonly
u6ed in di~fu~ion ~ran~fer ~y~tem6 provided t~at
the ~pecific ingredient doe~ ~ot adverEiely a~fec~
in6ula~ing propertie~ of ~he binder or eonduc~iYe
propertie~ of ~he 6ilver-containing are!a 11 o the
10 elec~ro~tatic printing ma~ter. Thu~, appropriate
an~ifogging agent6. ~uch a6 tetraaza~nclene~ and
; ~ercaptotetrazole6, ~oating aid6. 6uch a6 6aponin
and polyalkylene oxides, hardening agent6, ~uch a~
ormaldehyde and chrome alum, and pla6ticizer6 may be
lS employed if de6ired. The sub6trate 4 ~180 c~n be
tran6parent if the ma~ter i6 to be u6ed a6 a photo-
tool or or graphic art~ applications.
Variou6 conventional method6 can be 6elected
for toning the electrostatic pr~nting ma~er. If ehe
toner particle~ a~e electrically conductive and
: ecsentially neutral, or charged oppo~ite of the
latent image, they will adhe~e ~o the charged latent
imageO I~ the toner i6 ~harged wi~h the 6ame polarity
a~ the charged latent image, the ~oner will adhere ~o
the uncharged portion. A developme~t electrode can
be u~ed to improve the quality of the toned image:
i.e., to facilitate uniform toning of 601id image
areafi having latent electro~tatic charge and to pre-
vent background ~oning i~ image areas that contain no
charge. Tranfifer of the toned image to the de6ired
6ub trate. ~ypically paper. can be as~i~ted by u6ing
; a corona di~charge of oppo6ite polarity o~ the
opposite ~ide of the ~ub~traee. Alternatively, toner
tran&fer can be accompli6hed with a conduc~ive roller
that i8 electrically bia6ed, adhe6ive fil~ and paper.
16
17
and the like. ~he toner ~mage ~hu~ ~ran6~erred can
be fixed by a teehnigue ~onven~ionally known in the
art. V6ually, hea~ing ~ixation, solvent ixat~on.
pre6~ure fixatio~ and the like are e~ployed. If
~e~e~6aly, the 6urface of the ~a~ter may be eleaned
by u~i~g a ~leaning ~ean~ ~uch a6 a b~ush, cloth, a
blade, a vacuum knife and the like ~o ~e~ove the
remaining toner i~age.
Electrostatic printi~g ~a6ter6 offee ~everal
advantage~ oYer tho~e described in the prior art.
Since conventional aqueou~ development and fixi~g
~echnique~ !emove byproduc~ ~ha~ are ~oluble in ~he
601ution u~ed for tho~e purpo6e~, the ~a~ter does not
~on~ain byproduct that ~ight interfer with the
lS insulating propertie6 of the binder or conductive
path of the developed sil~er image, a 6ituation that
may be encounte~ed using the dry 6ilver halide
~ developmen~ technique~ de~cribed in U.S. Patent
: 4,069,759. Al~o. the in6ulating property of the
bi~der6 6~1ected in accordance with the invention i8
le66 sen6i~ive to moi~ture which ca~ in~erfese with
the electrosta~ic printin~ proce6~, and ~hu6 the
~a~ter can be u~ed repetitively or after ~torage
~ithout ~he ~eed to heat the ma6ter ~o remove
moi~tuLe o~ to unde~Sake 6pe~ial humidity contsol~.
Hiqh re~olutio~ may ~e obtained uBing the
elect~o~tatic prin~ing ma~ters provided by the
inventlonO aohieving re6ult~ comparable to that
ob~ained in high-quality lithogLaphic. flexog~aphic,
and letter pre66 prin~ing. ~hile half-tone imaging
will ~ormally be ~elec~ed for the6e ~pplication6, it
i6 po~ible to ~ailor a ma~e~ for con~inu4u6 tc~e
application6 ~ince ~he den6ity of developed ~ilver
will ~a!y with intensity of light u6ed to image the
film, a6 in ~onventional pho~ography.
17
~3,,~
1~ ,
The following example6 ~ur~her illu6~rate
variDu6 embodiment6 o the ~nven~io~, and a~e ~ot to
be co~6~rued eo limit i~. O~her embodi~ent6 will be
apparent ~o those ~killed in the art. In the
example~,.all part6 and percentage~ are by veiyht,
and all ~empera~ure6 are i~ degree6 Cel6iu~, unle~s
otherwi6e ~ated.
Unle66 otherwi~e 6~ated, the ~ilver halide
emul6ion6 were negative working and ~en6i~ized with
gold and 6ulfur-~ontaining compound~ in a ~o~ventional
~anner~ The ~ilver chloride wa6 doped with 0~13
millimole6 of RhC13 per mole of ~ilver.
P~eParation of Polvmers
lS The general pcocedure for the preparation of
the polymer6 is illu~trated by the p~epara~ion of
Polymer A [~tyrene/methyl methacrylate/ethyl acrylate/
~ethacrylic acid in a 30/10/40/20 weight ratiol a~
given below.
To a five liter fla~k fit~ed wit~ a high
6peed 6tirrer, a reflux co~denser. an additio~ funnel
and a thermometer were charged 7aR grams of deioniz~d
water, 5 gram6 of Duponol WAQE (60dium lauryl ~ul-
fate), 35.2 grams of 6tylene. 11.7 gram~ of methyl
methacryla~e, 46.9 grams of ethyl acrylate. 23.4
gram~ o~ ~ethacrylic acid, and 0.5 grams of octyl
mercaptan. The flask wa~ purged with nitrogen and
~eated to 60C and held for 15 minute6. Fercou6
ammonium ~ulfa~e, 0.02 grams, ammonium persulfate,
0.2~ gram . and 60dium bi6ulfiSe, 0.28 gram~, were
: added to the 1a~k while the mix~ure wa6 emul~ified
and maintained at 69-74~C. A ~ixture of 316.5 gram6
of 6tyrene, 105.5 grams of methyl methacrylate, 422
grams of ethyl acrylate, 211 gram6 of metha~rylic
acid and S.10 gcam~ of octyl mercaptan wa6 a~ded tc
lB
19
~he flask over a period of 140 ~inute6 while a 601u-
~io~ con~aini~g 2.06 gram~ D~ a~monium p~ula~e,
0~52 qIa~6 o 60dium bi6ulfi~e and 19.4 g~am~ of
Duponol ~A~E in 1000 gram~ of deionized wa~er was
al~o addad over the 140 mlnut~6. Polymerization was
continued for an additional hour and the emulsion wa~
allowed to cool 610wly to ambient temperature. A 5%
~alcium ace~a~e ~olu~ion ~a6 added whe~eupon the
poly~er coagulated. It wa6 ~trained ro~ exce~6
10 wate~, wa6he~ and fileered repeatedly wi~h deionized
wate~ un~il the fil~ra~e became cl~ar, and vacuum
dried. Polymer6 B I were prepar~d in a ~i~ilar
~anner. The polyme~ compo6itions and acid number6
are give~ in ~he Table 2 below. The acid numbeI6 ~re
defined a~ the milligra~ of pota~6iu~ hydroxide
neutralized eer gram of polymer a6 determined ~y
~o~entiometric titr~tion.
TABLE 2
_ ~onomer~a
~y~ 5 ~MA EA EMA A~ ~AA ~N
A 30 10 40 20124
B 25 40 20 ~594
C 27 60 13B0
~ 25 D 30 53 17100
:~ E 25 21 30 24151
F 35 13 28 24152
G 25 40 20 15 81
H 51 Z9 20135
I 45 40 1597
a S 2 6tyrene
~MA - me~yl methacrylate
EA ~ ethyl acrylate
AA ~ acrylic acid
MAA ~ ~ethacrylic acid
AN - acid number
19
.
. .,
l3~
Example~ 1-11 demon6~rate ~he charge retention of the
different polyme~ when used with difer~nt 6ilYer
halide6 and at different ~ilver halide to polyme~
ratios.
S
Exam~le~ 1-6
A 601ution wa6 made fEom the following
ingredient6:
polymera 0.5 gram~
triethylamine 0.3 grams
wa~er 3.2 gra~
a Example 1 = Polymee A
~xample 2 = Polymer B
Example 3 = Polymer C
E~ample 4 = Polymer D
Example 5 ~ Polymer E
E~ample 6 = Polymer F
To this solution wa~ added with s~i~ring 12.5 gram~
of a 15.1% ~olu~ion of a silver chloride emul6io~
~AgCl grain~ doped with 0.13 millimoled of ~hC13 per
mole of AgCl and w~h a median edge length of 0.13 to
0.17 micron~) containing 3.3 gra~6 of ~ela~in per
mole of ~ilver chloride. ~he dispe~ion wa6 coated
~5 onto a co~per-clad polye6ter ba~e by doctor knife.
~he dried films were 2.4 micron~ thick and had 90
milligram6 of silver chloride per 6quare decimet~r,
with a ~ilver ion to polymer ratio of 2.8 to 1. The
unexposed fiim~ were tray-proce~ed according ~o the
following procedure: 1 minute in a commercial litho-
graphic de~eloper (CUFD, ~. I. du Pon~ de ~mour6 and
Company) at 32.2C, 30 6econd6 in 30~ 60dium thio-
~ulfate fixer and 15 6econd~ in 2~ acetic a~id 6tOp
both at 25C. followed by cold water wa~hing and
drying at 125C for 10 minute6. The proces~ed
.3a?6~...3~t7
~ 1
film6 were mounted on a fla~ plate, ~he copper layer
connected to ground. and eguilibrated at 24~C and the
qiven relative humidity for one hour. They ~ere then
corona ~haLged (with a double wire corotron~ at ~.2
kv. Cha~glng wa6 6topped (at ti~e =Q) and ~he cha~ge
allowed ~o de~ay. Electrosta~ic voIeasle~ were
de~ee~ined with the u6e of an ele~tro~t:atic 6u~fa~e
pro~e. The re6ults~ in voltage6 per mi.cron, are
~ummarized in the Table 3 below.
: ~ABLE 3
--
Example
~9~ 1 Z 3 4 5 6
RH=23~
2 55 55 41 6~ 39 63
53 53 39 59 3s 58
51 52 37 54 36 53
; RH=50%
. 20 2 37 36 30 27 20 44
31 35 26 16 25 38
~ 60 26 33 23 11 ~2 33
:
Example6 7 10
A solution wa~ made from the following
25 ingredient6:
polymera 32.7 grams
trie~hylamine 11.5 grams
water 131 grams
Example 7 3 Polymec G
30 ~xample ~ . Polymer H
Example 9 ~ Polymer I
~xample 10 - RESYN 28-1300 ~National Sta~ch co~)~
carboxylated poly(vinyl aceta~e) with
acid number of 67.
21
:
fi.
22
To this solution was added with stirring 74.2 grams of the silver chloride as in Examples 1-6
but containing 33.3 grams of gelatin per mole of silver chloride. The dispersion was coated
on copper-clad polyes~er base as in the previous examples. The dried film had a thickness
of 4 microns with a silver weight of 80 milligrams per square decimeter. The ratio of silver
ion to binder was 1.15 to 1. Films were developed in a commercial X-ray film developer
(MXD. E.I. du Pont de Nemours and Company~ and fixer (thiosulfate) at ambient
temperature. They were treated with 2% acetic acid, water-rinsed and dried at 125C for 10
minutes. After equilibration at 24C and 37% relatively humidity, the processed films were
corona charged as described in the previous examples. The results, in voltages per micron,
are summarized in Table 4 below.
TABLE 4
EXAMPLE
Time(Sec) 7 8 9 10
2 62 22 55 6
53 13 33 5
49 10 25 5
120 44 7 20 4
Example 11
Example 9 was repeated except that a silver iodobromide emulsion AgBrO 985 lo ol5 with an
average grain volume of 0.0185 cubic microns~ containing 13.3 grams of gelatin per mole
of silver halide was substituted for the silver chloride. The dry film had a coating thickness
of 4 microns and contained 80 milligrams of silver halide per square decimeter. The ratio
of silver ion to polymer was l.lS to 1.
23
The film wa6 p~oce~6ed and charged as in Exameles
7 10. A~ 2~oc and 37~ rel~tive humidi~y, ~he
elec~ro6eatic voltage6 held per microm i~ the
polymer area~ were ~0, 56, ~7~ and 40 Yolts per
5 mi~ron a~ 2, 30, 600 and 120 6e~0nd~ re6pe~tively.
Exa~ple~ 12-17 demon6~rate the u6e o
different conductive 6ub6trate6 with l~wo different
insulatiag polymer6.
0 ExamDle 12
Polymer J tmetha~rylamide/~e~hyl ~etha~rylic
acid~e~hyl a~rylate/ methacrylic acid in a 4.2~42.8
43/10 ratiol wa~ prepared a6 ~ollow~: a mix~ure o~
4.2 gram6 of ~ethacrylamide. 42.B grams methyl
~ethacrylate, ~3 gram6 ethyl acrylate, 10 ~ra~6
methaccyli~ acid and 0.1 gra~ VA~0 64 initiator
~azobi6i~0butyronlt~ile) in 666 gram6 t-butanol wa6
heated at reflux under a nitrogen atmo6phere for two
hour~. A~othe~ 0.1 grams of VAZ0 wa6 added, refluxlng
co~ti~ued ~or two hour6, two ~ore addition6 made of
0.1 g~am~ of VAZ0, and ~eflu6i~g continued to a ~otal
eeac~io~ ti~e of a hour6. The polym~r wa6 precipi-
tated i~ cold ~ater, rin6ed with water. and dried to
a white æowder
A 601ution wa6 2ade of the following
i~gredient6:
Polymer J S.0 gram6
triethylamine 0. 5 gr8m6
water 3~.0 grams
To S gram6 of ~he polymer 601ution wa6 added with
~tirring 9.9 gram6 of an ortho-~en6itized ~ilver
iodobromide emul~ion a6 i~ Example 11 in whi~h ~e
gelatin content wa6 13 grams of gelatin per ~ole of
6ilver halide and the 6ilve~ halide content ~a6
11.~ The di6persion wafi ~oa~ed under red ~afeligh~
23
2~
condi~ion~ onto aluminum using a wire-wound bar ~o
give, af~er drying, a coating of 6.0 ~icron~.
The coa~ing wa~ handled and proce~sed under
rad ~afeligh~6. Image6 were prepared by ~ontac~
5 expo~ure ~o hai~one and re601ution targe~s in a
vacuu~ fra~e u~ing a ~ung6ten lamp at 56 in~he6 ~la~p
output ~ 10 foo~ candl~ lZ inche6 rom the bulb)~
Thi6 exampl2 wa6 expo~ed one ~econd, Sray developed
for 1 minute under ni~ogen at~o6phere i6 the
10 following develope~
O.Olt pota66ium b~omide
0.05~ ~odium sul~ite
1.00~ hydroxyla~ine hydrochloride
0.01~ Dimezone-S
1.00% hydroquinone
5.40~ pota66ium carbonate
S.40% pota66ium bicarbonate
deionized wate~
It wa6 the~ fixed 2 minute6. 6topped 2 minu~es in 2%
20 a~etic acid, rin6ed 2 ~inute~ in distilled water all
at 26C, blown dry, and heated 1 ~inutes at 125C.
The lmage con6i6t~ of black 6ilver image
vhere the coating wa6 expo6ed and a white background
where unexp~6ed. Re~olu~ion wa~ at lea6t 101 line
~5 pai~ per milli~ete~. Charge acceptance and dark
d~cay ~ere determined u6ing a ~onloe Model 276A
s~atic charge analyzer. The expo6ed area6 read
ini~ial acceptance of R VOltfi which i~ the Eame as an
alu~inum blank, and did not decay over 60 second6:
the unexposed area6 initially accepted 153 volt6
which decayed to 100 volt¢ at lD 6econd6, 92 volts at
20 ~econd6, 75 volt6 at ~0 ~econds. Thi~ diffe~ence
in charge be~ween the expo~ed and unexpo~ed area~ is
u6eful ~o~ electro6tatic ~oning.
24
rhe elec~eostatic ma~ter wa~ charged with a
po6itive corona to ~aximum acceptance char~e while
~he aluminum ~upport wa~ electrically grounded. After
a few 6econd6 decay the ground wa6 di~onnected and
5 the plate immersed in a di6per6ion of .nega~i~ely
charged black toner particle6 in 1~oea~0, a nonpolar
; hydrocarbon liquid having a Kauri-buta,nol value of
about 27, Exxon Core~ Tone~ wa6 at~racted ~o the
white non-~ilver part6 of the image making the
10 overall ~a6~er look black. I~ wa6 then rin~ed gently
~n a t~ay of I60par~, drained, ~ewet with I60pa~0.
covered wi~h paper, and passed under the po6i~ive
corona to a66i6t toner tran6fer to paper. The image
~ran ferred normally (toner tran6fersed where the
15 master wa6 6ilver-free) and had 6 line pair~millimeter
resolution when the ma~ter ~tayed wet with Isopar~
throughout.
Exampl~ 13
The procedure in Example 12 wa6 repeated
with the following exceptions: the emul~ion W~6
coated on~o copper-clad Kapton~ (polyimide ~ilm.
E. I. du Pont de Ne~our6 and Company) ~o achieve a
thickne~6 of 5.7 micron~:,and the proce6sed film wa6
Z5 heated for 5 minut~6 at 125C. The fini6hed electro-
6tatic ma6ter thu6 prepared wa6 mounted on a Savin
770 copier drum and charged and toned, the image
transferred to paper a6 in Example 12, to obtain
100-150 copie6 o~ black eoner image with resolution
of 20 line pair6 per millimeter.
ExamDle-l4
; Exa~ple 12 wa~ repeated except tha~ 9.9
gram6 o~ polymer ~olution wa6 u6ed, re6ulting in a
~ilvec ion to polymer ratio of 0.5~ to 1; and the
2s
26
di~persion wa~ coaeed on coppe~-clad ~apton~ with a
~oa~ing thickne66 of 5.7 ~icron6. The ~ub6equent
treatment wa6 the ~a~e a6 in Example 13. The ~a6ter
appeared to char~e and tone better wit,h ~he higher
: 5 petcen~ polymer (Example 1~), but the image coating
had a grea~er ee~dency to delaminate.
Example 15
2xample 12 wa~ repeatQd except ~hat the
10 conductive 6ub6trate u6ed wa6 aluminized Mylar~
(polye~ter film, Eo I.-du Pont de Nemour6 and
Co~pany~. Thi6 re~ulted in an intact image with
no noticeable anchorage or quality problems.
15 Example 16
PDlymer ~ wa6 prepared in the ~ame manner as
Polymer J, but u~ing 4.2 gcam6 of methacrylamide.
21.8 gramfi methyl ~ethacryla~e. 64 gram~ ethyl
acrylate, and 10 gram6 ~ethacryl~c acid. The film~
20 were prepared, imaged, proceG6ed, ~harged and toned
a6 in Example 12. Charge accepta~ce initially was
55 volt~; at 10 ~econd6 i~ wa~ 16 VOl~B.
xa~e 17 ,
Thi6 example used the 6ame coating and
~ proce~6ing a6 Example 13 except ~hat the image wa6
: heated fo 10 minute~ at 125C. A coating thickne66
of 1.8 ~icron~ wa6 achieved. Image area6 that air
dried before heating (A) were 60mewhat cloudy: areas
hat were wet when placed in the ove~ (B) were ~ran6-
paren~ after heating. The black ~ilver image had
re~olution of 228 line pair6 per millimeter. The
charge acceptance and decay of the ima~e wa~ deter-
~ined on a Monroe 276A Static Charge Analy~er at
- ~5 variou6 relative humiditie6 a6 6hown in the Table 5
below. The data are in volt6 per ~icron.
Z~
~l3~
27
TA~LE 5
~elative 0 lO 20 30
Humidity ~econd6 6econds6econd6 6econd6
4~ ~ 75 S~ 53 47
B 50 40 34 32
20% A ~0 50 43 3
B 4~ 32 27 24
35% ~ 5~ 2~ 23 19
B 36 17 13 11
49& A 53 24 1~ 15
B 35 13 9
63% A 18 5 2
B 11 2 - -
1572~ A 21 - - _
B 9
The copper layer of the electrofitatic ma6ter
of Example 17 was electcically grounded a~d ~he image
po6itively charged with a corona unde~ ambient condi-
tion6. Af~er a few second6 the grounded image wa~submerged in a toner bath consi6ting o~ negatively
charged toner particle~ opa~, drained. lightly
rinsed with I~opar~ and the wat i~a~e tran~fer~ed to
aper with the help of a ~egative corona behind the
paper. The toner image wa6 positi~e with ~e6pect to
the original image, negative with respect to the
master, and re601ution wa6 16 line pair6 per ~illi-
~eter. The electro6tatic ~aster was recharygd and
toned and the ~oner image allowed to dry. Clear
; 30 adhesive tape picked the toner of~ the master to give
a clean positive image with re~pect to the original.
with resolution of ~0 line pair6 per milli~eter.
~5
27
. ~
3~
ExamPle~ 1~=24
The6e example~ contra6t the propertie6 of
film6 fo~med by disper6ing a silver 6alt in gelatin
binder6 to those formed by disp~r6ing the 6ame 6ilver
alt in the improved in6ula~ion media of ~he pre6~n~
invention. In all ca6e6 ~he 6ilver 6alt u6ed wa~
AgCl g~ain~ doped w;th 0.~3 millimole6 o~ ~hC13 ~e~
~ole of ~gCl with and with a median edge length of
0.13 to 0.17 mieron6. The charge retention wa6
10 mea6ured ater developing the unexeo~ed film~.
. .
(i~ Fil~s with gelatin binder~
~ ~ilve~ chloride di6per~ion wa6 prepared by
adding 3610 gram6 of ~ilver chloride curd6 (qrain6
doped with 0.13 millimole6 o~ RhCl3 per mole of AgCl
and with a median edge length of 0.13 to 0.17 micron~)
~ontai~ing 13.3 gram6 of gelatin per mole of AgCl ~o
; 3045 gram6 of wa~er, adju6ting the pH to 6.7 with 130
gram6 o 0.1 N sodium hyd~oxide, heating and 6tirring
~ 20 for one hour a~ 45C and adding 214 gram~ of a ~olu-
: tion made up by mixing 165.2 g 0.1 N ~odium hyroxide.
32.1 grams tetraazaindene stabilizer~ and 16.7 gra~
water.
tetraazaindene = ~-hydroxy-2-methyl-
2~ ~1,2,4~triazolet2,3-b]pyrimidine
Gelaein was 6wollen in water at 20C and
then dissolved in additiona~. water a~ 50C to give a
lS w~% 60lution. 295 grams o the gelatin 601ution
was then added to 705 grams of the ~gCl solution to
~ake a net 17.63 wt% AgCl emul6ion. Formaldehyde
hardener wa6 added at a concentraeion o 5 gram6
formaldehyde per 1000 gram6 emul6ion. The e~ul~ion
wa6 coated onto an indium tin oxide coated polye~te~
6ubstrate (6urface re6i6tivi~y of abou~ 500 oh~ pe~
~3~
29
square, 5 mil tbick polye~ter ba6e~ u~ing a lab
coater. The f ilm6 were tray proce6~ed u6ing standard
reagents ln the following ~eguence: develope~. stop,
~ix, stop, Lin~e~ dry. The gelatin6 ul;ed a~d the
5 coating thickne~es after ~roce66ing obta~ned ~e
~ummariz~d in ~able 6.
. (ii) Films with improved polymeric bi~der~
A 601ution wa& made from the following
ingredie~t~:
polymer 2.00 gra~
water 10.44 gram6
i~opropanol 3.20 gram6
pota66ium hydroxide0. 30 gram6
pota~sium bicarborlate 0.06 g~a~s
acid violet 520 dye0.10 qram6
: To ~his solution was added with ~tirring 54 gram~ o~
AgCl curd~ containing 10 grams gelatin per ~ole of
AgCl. The di~per~ion was coa~ed on~o a gel-~ubbed
20 indium tin oxide coated polye6ta~ 6u}~6trate (6urfaçe
re6i6tivity of about 500 ohm~ ~er 6quare~ 5 mil thick
polye~ter ~ase~ u6ing a wire-wound rod. The ~ilm~
were ~roce~ed following the procedure de6cribed ~o~
the gelatin ~ilms. The polymers u~ed and the coati~g
weight obtained are summarized in Table 6.
i5
29
;.
3~
TABLE 6
COa~Ci ng
ThiCkne~6
EXamP1 e ~Bi nde r
~,18 Z68~deiOn1Zed ge1atin 5 . 2
19 26B8deiOniZed ge1atin 1. B
20ROU6~e1Ot ILLS rlOn-
deitln1Zed ge1a~in 5.
21RQU6~e10t ILLS non-
deionized gelatin 2. 8
22Polymer A 1. 6
23Polymer E o. 9
24Polymer
a af~er proces~ing
(iii)Determin.a~ion oiE charge retention
Sample~ of the above f ilm~ were mounted on
20 an aluminu~ ~late and electrical conneceior~ fcom the
conductave ~ndium tin oxide ~ub~tra~e to ground ~a6
made ~ith ~e u6e o~ condu~ive coe~er tape. Th:e
lms wer~ eguilibratea in a glove box at a givell
relativs humidity a6 mea6ured with an O~ega hand held
25 hygrometer (Model RH-201~ for one hour and then
corona cha~ged with a double wice corotron. 5 kV
being applied ~o the corotrsn. Yoltage~ were
de~er~isled with the u6e of an electrostaeic 6ucface
Yoltage erobe. The re6ult~ are ~ummacized in terms
30 0~ volts per micron i n Table 7 .
,
~ .
TABLE_7
Time ~ec) la 19 20 2122 23 2
T=24C RH=l:Lt
~ 3~ 6113 ~5123 12~ 9g
lB 27 3 1591 97 69
13 19 2 1081 07 59
Example
Ti~e (sec) lB 1920 21 22 23 24
T=23C ~H=30~
2 4 10 1 3 B6 89 51
lS 1 3 0 0 41 49 27
1530 0 2 0 0 33 41 21
_ _ Exam~le _ ._
Time (6ec) 18 19 20 2122 23 24
T=22C RH=4BS
Z 1 1 0 1 37 34 21
0 0 0 0 13 17 9
0 0 0 0 8 13 6
Films with gelatin binder6 were heated to
determine the effect on the electro6tatic propertle~.
Films in Examples 18-~1 were dried at lOO~C for 10
minutes and then condicioned aC 4B% relati~e humidity
for 1 or 10 minutes after which electrostatic data
were obtained. These data in volts per ~icron aLe
summarized below in Table 8.
31
, .
.3~7
3Z
TABL 8
19 ?_ 2l
1 1~ 1 10
Tame ( ~ec 3 ~in ~in ~in ~in rnin Dlin ~in Dlin
2 2~ 5 ~ 4 6 1 15
0 3 1 1 0 2 0
3~ 5 ~ 1 1 0 0 1 0
(iv) Toning re~ult6
10~ilm~ rom E~cample6 18-24 were toned with
liquid electro6tatic toner containing ~arbon black
pigment il~ a ~nodif ied Savin 870 copying machine unde~
identical conditions~ t~le temperature wa6 19C and
the relative humidity wa~ 48%. Time from ~orona
15 shargi~g to toning was 15 secon~6 . The double wi re
corotron wa~ bia~ed at 6 kV and the development
elect~ode wa~ maintained at ground ~otential.
T~an6fer o~ the toller f!om the film ~urface to off6et
enainel paper wa~ aecompli6hed with the u6e of a bias
20 tran~fer roll. Once tran6~erred to ~ape~. the toner
wa~ thermally fu6ed at 100C in an oven. E~eflection
~` optical den6ity rneafiurernent6 were D~ade with the u~e
a Maebe~h RD9~8 den6itometer and are given in the
~' Table 9 below.
:~ 25
TABLE 9
Exa~Ple A,mbien~ Heateda
18 0.02 0.56
1~ 0 . ~2 O. 30
~ 20 0 . 02 0 . ~0
21 0.02 0.27
22 :L. 51
1. 3~
24 1.11
35 aE~eated îor10 minute6 at 100C ~ollowed by
1 mir~ute ~ondi~ioning ~t ambient condition~
' p~ior to toning.
~.~3~it;37
33
Example~ 26 and 27 illu~trate the u6e of a
commer~ial resin as the in~ulating polymeric bi~der.
In 35 grams of water was dis~ol~ed 2.5 y~a~6
of Carbo6~t~ 526 (sopolymer of ethyl acrylate/me~hyl/
metha~yla~e~acrylic a~id in a 17/71/lZ ~atio, ~. F.
Goodrich Co.) and 0.59 grams of triethylamine. Equal
a~ou~ts of the ~olymer 601utio~ and ~ilve~ halide
emulsion of Example 12 weLe ble~ded and coated at
: 60 milligram6 per 6quare ~ecime~e~ on copper clad
Kapto~0. Expo6ure and development following the
procedu~e in ~xample 12 re~ulted in a black ~ilver
image wi~h a clear background with good re601ution.
Charging and charge decay fitudie6 as a function of
~elative humidity were conducted on coating6 o~ pu~e
Carbo6et~ 5Z6 at 36.90 milligrams per ~quare deci-
meter on copper under the 6ame condition~ a~ Example
17. At 4 to 72t relative humidity Carboset0 526
held charge at least as well o~ better than Polymer J
" of Example 12.
:``
ExamPle-2-6
ample ZS wa~ ~epeated using Carbo~et~ 525
(copolymer of ethyl acrylate~methyl methacrylate/
acrylic acid in a 56/37/7 ratio. B. F. Goodrich,
Co.). An image wa6 produced. however it wa~ weaker
than tha~ ~f Example 2~.
Example 27
A film wa~ prepared a~ in Example 1 except
- ~hat ~he AgCl emulsion contained 13.3 gram6 of
gelatin pe~ ~ole of AgCl and the final coa~ing weight
was 120 milligrams pe~ ~qua~e decimeter. The film
wa~ expo~ed and proce~fied in ~XD (E. ~. du Pont
3~
34
de Nemour6 and Csmpany. Inc.~ rapid acce~ Xray film
developer 60 as to get a variet~ o~ amounts of 6ilver
developed. DeYelopment wa6 determined by a Pan~lyzer
4000 (Panametric6, di~i6ion of E6terline Corp.).
S Surface re6i~ance an ~he ~ilver ~maye area6 was
mea6ured with a Pluka 77 ~ulti~eter ~John Fluke Mfg.
Co., Inc.) between ~WQ probe6 1 centil~eter apar~.
Acc~pta~ce voltage in the 6ilver i~age acea~ wa~
mea6ured on a Moncoe 276A s~a~ic te6t metec. The
re6ul~ are given belQw in Table 10.
TAB~E 10
Acceptance
SilverRe6i6tance Voltage
oh~6 ~ ~volt~)
~5
: 100 70 4
96 1000 7
92 200 6
82 107 25
20 47 __ 88
3~ -- 155
9 -- 262
Ex ample 28
Indium tin oxide coated ~ylar~ (polyester
film) wa6 coated with a 1.~ milligram per ~quare
deci~eter 6ubbing o~ polyvinylidine chlo~ide ~e6in at
200 f~et per minute with a fountain air knife coater.
and heat cet at 170C at 20 pm giving a residence
time of 8 ~inute6. Thi~ wa6 ove~coated with a
gelatin layec at 0.~ 1.0 milligrams per 6quace
decimeter at 200 pm with a ~ountain air knife and
heat relaxed at 145C at 4s ~pm gi~ing a ~esidence
time o 3.5 minute~.
34
~3~ 7
A ~olution of Polymer E wa6 prepared by
~dding ~o 231~ gram6 o water with 6t:Lrriag: 454
gram6 i~opropyl alcohol ~95~), 450 gra~ methyl ethyl
ketoneO and 13~ gram6 pota~6ium hy~roxide pellet6.
To thi~ ~olu~ion ~a6 added with rapid ~e~rr~ng C00
gram6 of Polymer ~ r$n~ waæ ~ontinued until it
: wa6 ~06tly di~olved ~15 minutes). To ~his wa~ added
: 54 grams of potassium bicarbonaee. A ~ilver chloride
di6per~ion wa6 prepared by addi~g 361~ gra~6 of ~ilver
halide curds (g~ain6 doped with 0.13 ~illimole6 of
RhC13 per mole of AgC~- and wi~h a median edge l~ngeh
of 0.13 to 0.17 mi~ron6) containing 10 gram6 gelatin
per mole of ~lver chloride to 2300 gram6 of w~ter
and ad~u6ting ~he pH to 6.7 by the addition of 130
gram6 o~ 0.1 N 60dium hydroxide and 15 gram~ of 0.1 N
~ulfuric acid. Thi~ wa~ heated ~or 1 hour at 45C
and ~14 gram6 of a solution made up o~ 386 gra~6 of
0.1 N ~odium hydro~ide, 75 gram6 of tetraazaindene
~tabilizer, and 39 gram6 water was added. Thi6 wa~
20 diluted to ~5~ 6ilver ~hloride with ~14 ~ram6 water.
To 630 gram6 of ~he 25% AgCl ~olu~ion wa~
added ~lowly with 6tirrin~ 247 grams of the polymer
~ ol~tion (15%). Before coating 6.7 gram~ of ~PI-REZ
: 5022 (diglycidyl ether of 1,4-butanediol, Celanese
25 Corp.) wa6 added and coated onto the above treated
indium tin oxide ~ylar~ 6heet at 15 ntilligram6 per
6quare decimeter polymer coating weight u6ing a lab
coaee~ at 60 f p~t. Thi6 w~t6 dried for 30 6econd~ at
10C, 60 ~econd~ at 30C, and 60 6econd6 a~ 50C.
30 Total dry coating weight was 103 ~illigram6 per
6qtlare decimeter.
Afeer ~xpo6ure and development as in Exam-
ple6 18-24 the developed expo~ed 6ilver image had
~urface re6i6tance of 50-100 ohm~ and acceptanee
35 vol~age of i volt a6 mea~ured 2 ~econd~ after
1 R~ 3 7
36
~harging. The u~expo~ed non-silver part of the
image had an accep~ance voltage of 242 VoltB a8
mea6ur~d 2 second6 ~~er charging. 206 volt6 a~ter
15 second6l and 190 volt~ after ~0 ~econd~ a~ 19%
relative humidity. Toni~g in a modified 5avin 870
Ofice Copier as de~cribed in Example 18-24 ~ave
5-98% dots and 150 lines per ~illime~er re olution.
The i~age ~ran~ferred to paper had a DmaX of 2.4
a ~ in f 0-03
ExamPle ?9
.
I~ thi~ example the inven~ion i6 illu6trated
by a diffu~ion tranGfer film. To ~he follo~ing
~olution
water 3116 gram~
am~onium hydroxide (29~) B4 gram6
i~opropyl alcohol (9~%) 400 gram~
~ wa6 added with inten~e stir~ing 400 grams of ground
: Polymer A. Thi6 ~olueion wa~ left un6tirred until
polymer dissolved to~e~nigh~). To 1720 gra~6 of the
polymer ~olution wa~ added over 1 minute with rapid
etirri~g 600 grams of a 2~ 601ution of zinc 6ul~ate:
then added over 5 ~e~ond~ with ~tirring 210 gram6 of
a 1.06Z% ~olution of ~odiu~ 6ulfide: then over 30
6econd6 added 520 grams of z.ss~ 601ution of a~id
violet ~20 (antihalation dye). Thi~ wa6 diluted to
4~ by the addition of 1250 gram6 of water~ Before
coating, 31 g~am~ of EPI-E~EZ 5022 (diglycidyl ether
o~ 1, 4-butan*diol ) wa~ added . Th~ ~olution was
coated using a fountain air-knife at ~he following
condition6: 200 fpm, 4 inch air knife pre6~ure: onto
5 mil ~hick ~ylar~ (polye~e~) previou61y 6puttered
wit~ indium-tin oxide. Thi~ wa6 d~ied a~ 85C. This
f ilm was subsequen~cly heat relaxed on a ~epara~e pa
s at 145C and 45 fpm giving a ~e6idence time of 3.5
36
minut*6 a~ 145C. rhis wa~ overco~ted with a blue-
6en6itized ~amera ~peed high contrast emul6ion o~
g o.~BrO.195I005 (average grain volume ~ 0.01 cubic
micron~ di6per6ed 2:1 in gelatin u6ing a bar coater
at 80 ~pm. The inal ~inder layer coating weight wa6
.3 ~illigram6 per ~quare decimeter: ~he emul6ion
layer wa~ 73.6 milligram6 per 6quare decimetel. The
ratio of 6ilver ion ~o eolymec wa6 3.0 ~o 1. The
film wa~ expo6ed and developed with very lietle
agitation ~or 1 minute in ~g~a CP297B ~gfa-GaeYert)
diffusion t~an6 er developer at 2~C, agitated for
1 ~inute i~ 10~ acetic acid stop 601ution at 28C
re~oving much of the gelatin top layer. rinsed i~
15C wa~e~, and dried at coom tempera~ure.
The unexpo6ed area6 gave developed silver
in the ~olymecic binder layer with 6urface resi6tance
of 20-35 ohm6 and accep'cance voltage of o YOlt6. The
expose~ area~ were 6ilver-free in the eolysQeric
bi~der layer and af~er charging. ehe accep~an~e
20 ele~ric ield at 38~ relative humidity was 150
volt6 at 2 second6: 10~ tlolt~ a~c 15 6econds: 91
volts at 30 6econds . Tonislg in a modif ied Savia
870 copying machine a~ de6cribed in Example6 18-24
gaYe 4-98~ halftone dot6/150 line per inch halftone.
The DmaX was 2-5 and the Dmin was
ExamPle~ 30~31
~he~e example~ contra~t the propertie~ of
30 difu6ion tran6fer film~ which contain ei~her gelat;n
or a styr~ne-acrylic tetrapolymer a6 the binder ~n
ehe receptor layer.
37
~L3~ 3 7
38
(i) Diffu~io~ ~ran6fer film with qelatin binder in
~he ~eceptor ~ayer ~Exampl2 30)
60 gram~ of Rou66elo~ Il16 g~lati~ ~ere
added to 1360 milliliters o~ deionized wa~er and
allowed to 8~iL a~ room temperature with ~a6~ agita-
tion ~or 20 minutes. The su~pe~sion wa~ heaeed ~o
52C for 30 ~inute~ and the~ ~ooled ~o 35C. 106
: milliliterg of a 0.15 M zinc sulfate 601ution and
6 ~illilite~s of a 0.15 ~ iron(II) 6ulfa~e ~olution
were added o~er a 1 minut~ interval. 336 ~illil~ers
of a 0.05 M ~odium ~ulfide eolution wa6 added through
a~ orifi~e 60 ~hat the addition ti~e wa6 approximat~ly
2 minute~. The following aqueous solution~ ~ere the~
added:
15% 601ution 0~ Polystep B-27
(S~epan Chemical Co.) 60 ~1
1.33 M formaldehyde 40 ml
O.Z64 M chro~ium po~as~ium
ulfate 40 ml
The ~olution wa~ immediately ~oated onto ~he conduc-
~ive ~ide of indium tin oxide ~oated ~ylar~ a~ a
coati~g weight between 0.7 and 1.0 gra~6 per ~guare
meter of ~ela~in.
ZS An ortho ~en6i~ized camera ~peed high con-
trast emul6ion of AgC10 7BrO 3 (average gcai~ volume
approximately 0.025 ~ubic micron6) wa6 coated onto
~he gelatin layer at a ~ilver coa~ing weight o~ 3.1
gca~6 per ~guare meter. The emulsion contained no
30 hardener.
The multilayer film wa6 expo6ed i~agewi6e
wi~h a ~ung6ten ligh~ and de~eloped in Co~e~ial
AgfA PMT developer ~Type CP297B) for 60 second6 at
approximately 20C with lit~le agi~ation. The
35 emulsion layer was then removed with pre6~urized
3~
~a3~
39
water at 38C. The 6ample was wa6hed for 2 ~inutes
in 38C water a~d dried at roo~ ee~perature.
(ii) Diffu6io~ ~ran~fer film~ wi~h ~Improved
: 5 poly~eri~ binder~ ~Example 31)
To a ~olution o~ 4.0 g~am6 o~E Poly~er ~ and
2.5 gram6 of tr1ethyla~i~e in 80 gr~ls o wa~er wa~
added over 1 minute ~ milliliter~ of a 4% aqueous
601utio~ 0~ Zi~ 6ulate. then o~er 5 ~econd~ 19.2
~illiliter~ o a 0.23% aqueou6 Rolutio~ of sodium
~ulfide. ~fter ~tirri~g 5 ~inute6 the precipi~ate
wa~ iltered off and the 601ution containing the zi~c
ulide nuclei wa6 coa~ed on the conductive ~ide
(surface re~i6tivity ~ 500 ohm6 per ~quare~ o~ indium
lS ti~ oxide coated ~ylar~ to give 7 milligramfi per
~qua~e decimeter clean colorles6 polymeric receptor
l ayer with 1% zinc 6ulfide nuclei. Thi6 W2~ hea~ed
at 125C ~or 10 minute6 to i~prove adhe~ion to the
~onductive ~ub6~rate.
A blue-6en6iti~ed camera-6~eed high con~ra~t
conclu~io~ of AgC10 80BrO.l9sIO-0o5 ~g
volu~e of 0.01 cubic micron~) di~per6ed 2:1 in gela-
tin wa6 coated without hardener over the polymeric
receptor layer at ~ ~oating weight of 69 milligram6
2S per 6quare decimeterO
The ~ultilayer coatinq wa~ expo6ed imagewi6e
with light and developed in ehe ~o~meLcial ~odak PMT-D
developer (East~an ~odak Co., Chicago. Ill.) ~odified
wi~h 12~5~ pota66ium hydrcxide and 5~ pota~iu~
carbGnate for 60 cecond6 at 28C wi~h lietle
agita~ion. The developed ima~e wa~ agitat~d 30
6econd~ in 10~ acetic acid 6top ~olution a~ 28~
removing mo~t of the top ~elatin laye~. The black
po~itive diffusion tran~fer image in ~he receptor
layer remained on the conducting support and wa~
39
~3~ t7
rin~d ree o gelatin and 1006e 6ilve~ re~idue6 wi~h
40C water, dried. heated 5 minutes at 125C to clean
ou~ vola~ile contaminant~. The i~age had D~aX of
3.0-3.5 and low D~in.
The receptor areas co re6~0nding to unexpo6ed
image had ~.8 ~alligram6 per zquare deci~eter finely
divided black zilver ~etal di.6perzed i~ 6.S milligram6
per ~quare decimeter polymer matrix. The ratio o~
~ilver to polymer of 1.34 to 1 i6 above the thLeshold
of about 1.2 and the 6urface re6istance in 6ilver
contai~ing area6 wa6 ~ery low. 5 to 14 oh~s. The
area~ corte~ponding to the expo6ed image were fairly
clean~ nearly colo~le~6 and had 6urface lesiztance of
gceate~ than 107 ohm~. The ma~ter wa~ toned on a
modified 5avin B70 copying machine as in Examples
18-24. With a 50 volt development electLode po~en-
tial the background of the toner image transferred
to paper ~orre6pondi~g eo the silver area6 of t~e
ma6ter) wa6 completely clean of toner and with
halftone dot6 of 2-95~/150 line per inch halftone.
(iii) ~lectroztatic data
Data were obtained for the difu6ion transfer
25 film6 in Examples 30-31 at various relative humidites
: according to the procedure described for Examples
18-24. The temperature was 22C in all cases. The
~e~ult6 in volt6 per mi~ron are summa~ized in Table
11 .
.~ 3~
3~
41
TABLE 11
_E:xamP l e
Time ~sec2 30 31
2 1~ ~5
0 17
0 13
lG
E~H ~ 30%
2 ~ 34
lS 0 17
RH ~ 49%
2 t~ 2 3
0 12
~: 2~
0 8
The diffu~ion t~an~fer Pilm wieh gelatin a6
binder, Example 30, wa6 heated at 100C f or 10 Dlinutes
25 followed by conditioning at ~85i rela~iv~ humidity fo~
1 or 10 ~inutes. The electro~tatic da~a, in ~rolt~
eer micron, obtained immediately af ter ~onditiQning
are given in T~ble 12 below.
~ . :
3 0 TABLE 12
onditioninq Time
Time ( sec ) 1 ~in 10 ~in
2 35 15
41
~ L-~
3~7
42
~iv) ~oning re6ult6
Film~ from Example630 31 were toned at
21C and 43t relative hu~idity a6 in Example6 18-24
Reflection optical den6itie~ mea~ured a6 ~n Example~
lB-24 are given in ~he ~able 13 below.
TABL 13
Optical Den~itY _
~ mbient Hea~eda
0.00 0.73
31 1.~3
Heated at 100C for 10 minute6 followed by
conditioning for 1 ~inute at ambient condi~ions.
ExamPle 32
The ~olution of polymer E con~aining ZnS
dev~loement nuclei a6 de~cribed fo~ Example 3~ was
coated on gela~in 6ubbed polyefiter ~ilm at 2~ milli-
gram6 per square decime~er giving a cl~ar colorle~scsating. ~ piece of Kodak P~T ~egaeive Paper was
expo6ed i~agewi6e. The expo~ed PMT paper and
receptor polymerJnuclei coating were fed into the.
nip o a la~inator with the eaper emul6ion 6ide
facing the nuclei coating and the 6hee~ ~pread
apart. ~odak PM~-D developer wa~ applied at the nip
between the ~hee~. the 6heet6 were we~ laminated
together at 1 meter per minu~e under light nip
pre6~ure, the lamina~e wa6 held 30 6econd6 at roo~
ee~perature and then the 6hee~ were 6epar~ted to
gîve a black po6i~ive i~age of D ~ax 0.7 and D ~in
0.02 in the receptor coating and a strong negative
~mage on the PM~ paper. ~his illu6tra~e6 ~he well
known photomechanical tran6fer proce6s and can be
u6ed to prepare a ~ilver i~age in polymer E.
.
42