Note: Descriptions are shown in the official language in which they were submitted.
6~3
-- 1 --
A FERVESCENCE COMPOSITION CONTAINING 15-KETO-PGES
This invention relates to a fervescence composition
containing 15-keto-prostaglandin E and its dexivatives.
Development of a drug to assist patients to recover
from hypothermia occurring after hypothermic operations~ e.g.
5 operations oE the cardiovasculum and brain surgery, hypo-
thermia caused by a decline in basal metabolism, e.g. thyroid
hormone hyposecretion, hypothermia caused by brain disorders
or hypothermia caused by serious bleeding and consciousness
disorders resulting from, for example, traffic accidents as
10 well as the development of a drug to prevent hypothermia has
been desired.
Prostaglandins are known to possess varicus pharma-
cological activities, antip]atelet aggregation activity, myo-
metr;um stimulating activity, antiulcer activity and the like.
15 Among the prostaglandins, prostaglandin E2 is known to have
fervescence activity. However, it is not suitable for use as
a drug for recovering body temperature since it strongly de-
creases blood pressure at the same time. On the other hand,
15-keto-prostaglandin E (noted as 15-keto-PGE hereafter) and
13,14-dihydro-15-keto-prostaglandin E (noted as 13,14-dihydro-
15-keto-PGE hereinafter) are known as substances naturally
produced by an enzyme in the metabolism of prostaglandin E
(noted as PGE hereinafter~ in the living body.
" ~3~g~
-- 2 --
These 15-keto-PGE compounds have been considered to be
physiologically and pharmacologically inactive substances
(Acta Physiologica Scandinavica, Vol~ 66, pp 509 (1966)). It
has never been recognized that these 15-keto-PGE compounds
have fervescense activity.
In drawings which illustrate preferred embodiments
of the present invention:
Fig. 1 shows body temperature change before and
after administration of test drug 24,
(13,14-dihydro-15-keto-PGE~ ethyl ester) to a rat loaded
with bloodletting;
Fig. 2 shows body temperature change before and
after administration of test drug 8,
(13,14-dihydro-6,15-diketo-~GEl ethyl ester) to a rat
loaded with bloodletting.
Figs. 3 - 13 are n.m.r. charts of
13,14-dihydro-15-keto-PGEs of the present invention.
This invention provides a fervescence composition
containîng 15-keto-prostaglandin E and its derivatives
2~ (referred to as 15-keto-PGEs hereinafter), and including
their derivatives which are separated from any substantial
blood pressure decreasing activity. Esters on the end
carboxyl group of the a-chain of 15-keto-PGEs show
fervescence activity even ~y peripheral administration.
g
- 3 - ,
In one embodiment the invention provides a ferves-
cence composition comprising a 15-keto-PGE as an active
ingredient.
In this invention, 15-keto-PGEs include 15-keto-PGEs
in which the carbon atom at the 15-position is carbonyl, and
13,1.4-dihydro-15-keto PGEs in which the bond between the
carbon atoms at the 13- and 14-position is saturated and the
carbon atom at the 15-position is carbonyl. Therefore, this
invention includes every prostaglandin E as long as it is in
the 15 keto or 13, l4-dihydro-15-keto prostaglandin skeleton
structure form ~nd it is not limited by other additional
ske~.eton structures or substituents~
In the present specification 15-keto-PGEs are
expressed according to the following nomenclature. The
15-keto-PGEs have the following basic structure:
~ , ~ COOH ( a-chain)
I 0 ~2 0 ~ ~ -chain)
~ )EI o
and the position number of the carbon atom constituting
the a-chain, the ~-chain and the five membered ring in the
prostaglandin skel.eton structure is used as it is in the
nomenclature. That is, the position number of the carbon
atoms i.n the skeleton structure are numbered from the
- carbon atom constituting the carboxylic acid at the
terminal. position of the a-chain through the five membered
ring to the ~-chain i.e. 1 to 7 are attached to the carbon
atoms in the ~-chain in this order, 8 - 12 are attached to
the carbon atoms in the five membered ring, and 13 - 20 are
attached to the carbon atoms in the ~-chain. In a compound
where the carbon number in the ~-chain is less than 7 the
position numbers from 2 to 7 are simply eliminated in this
order without any change in the position numbers of the other
carbons. In other words 15-keto-PGEs having 6 carbon atoms
in the ~chain have no position 2, i.e. 15-keto-PGEs of such
compounds are not renamed as 14 keto-PGEs. In the case where
carbon atoms are added to the ~-chain, the added carbons are
nominated as a substituent on the carbon at position number 2
without any change of the position numbers of the other
carbons. Therefore, l5-keto-PGEs having 8 carbon atoms in
the ~-chain are nominated as 15-keto-2-decarboxy-2-acetic
acid-PGEs. In the case where the number of carbons in the
~chain decreases, the position number is nominated as
reducing it from the carbon at position number 20, one by
one. In the case where the number of carbon atoms in the
~-chain increases, the increased carbon chain is nominated
as a substituent on the carbon at position number 20. That
is, 15-keto-PGEs having 10 carbon atoms in the ~-chain are
nominated as 15 keto-20-ethyl-PGEs.
The above formula expresses a sp~ci~ic configu-
ration which is a most typical one, and in this specifi-
cation compounds having such a configuration are expressed
`` ~L3~
without any description of it.
PGEs have a hydroxy group on the carbon atom at the
~ pos~ti.on in general, but in the present specification the
term "PGEs" includes prostaglandins having groups other than
S the hydroxyl group of the normal PGE. Such PGEs are called
ll-dehydroxy-ll-substituent-PGEs, for instance, ll-dehydroxy-
ll-methyl-PGEs in the case where the substituent is a methyl
groupO
PGEs are classified as PGEl and PGE2 according
10 to the bonds between the carbon atoms at position 5, 6 and 7.
PGEl and its derivatives (referred to as PGEls
hereinafter) refer to the group of compounds in which the
bond between the carbon atoms at position 5 and~6, and at
positions b and 7 are each a single bond. PGE2 and its
15 derivatives (referred to as P~E2s hereinafter) refer to the
group of compounds in which the bond between the carbon atoms
at positions 5 and G is a trans-double bond. Therefore, PGEs
having the structure -CH2-C(O~-CH2- are nominated as
6-keto-PGEls, and PGEs having the structure ~CH2-C-C-
20 are called 5,6-dehydro-PGE2s. 6 5
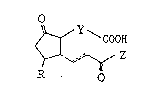
The ~ervescence activity is remarkably expressed in
15-keto-PGEs represented by the following formula:
~ \ COOH
~Z
R O
~3~
-- 6
wherein R is a hydroxyl group, a hydroxyalkyl group, or an
a]kyl group; ~ is a saturated or unsaturated hydro~arbon
moiety having 2 - 6 carbon atoms wherein a portion of the
carbon atoms constituting the hydrocarbon moiety may be
carbonyl or a portion of the hydrogen atoms constituting the
hydrocarbon moiety may be substituted with other atoms or
groups; Z is a saturated or unsaturated hydrocarbon moiety
which may constitute a straight chain or a ring, wherein a
portion of the hydrogen atoms of the hydrocarbon moiety may
be substituted with other atoms or groups; or
physiologically acceptable salts thereof or esters which are
esterified at the terminal carboxyl group.
Y represents a saturated or an unsaturated
hydrocarbon moiety having 2 - 6 carbon atoms, and includes an
aliphatic hydrocarbon, e.g. an alkyl group, an alkenyl group,
an alkynyl group and the like. Y is preferably a hydrocarbon
chain having 6 carbon atoms.
Examples of PGEs in which Y is an unsaturated
hydrocarbon moiety are PGE2s, 5,6-dehydro-PGE2s, PGEs in
which the bond between position 2 and position 3 is
unsaturated, and the like.
A portion o the carbon atoms constituting the
hydrocarbon moiety represented by Y may be carbonyls, whose
typical examples are 6-keto-PGEls in which the carbon atom
25 at the 6-position is carbonyl.
The hydrocarbon moiety represented by Y may be
substituted with other atoms or groups, for example, halogen
-- 7
atoms, e.g. a fluorine atom, a chlorine atom, typically a
fluorine atom; an alkyl group, e.g. methyl, ethyl; a
hydroxyl groùp and the like. Typical examples of such
substituents are 15-keto-PGEs having an alkyl group on the
carbon atom at the 3-position.
% represents a saturated or an unsaturated
hydrocarbon moiety having 1 - 10 carbon atoms. The
hydrocarbon moiety may be an aliphatic hydrocarbon or a
cyclic hydrocarbon itself or in part. The hydrocarbon moiety
represented by Z may be substituted with other atoms or
groups.
The number of carbon atoms in Z is preferably 3 - 7
in a straight chain. PGEs in wh;ch Z has 5 carbon atoms
correspond to typical PGs. Therefore, the PGEs in which the
carbon numbers of the hydrocarbon moiety represented by Z are
6 or more than 6 are nominated as PGEs having a substituent
on the carbon atom at 20-position. That is, PGEs in which
the number of carbon atoms in z is 6 are nominated as
20-methyl-PGEs.
Though the hydrocarbon moiety represented by Z may
hav~ substituents at any position, a saturated hydrocarbon is
more preferable. Examples of the hydrocarbon moiety having a
cyclic ring are cyclopentyl or cyclohexyl containing the
carbon atom at position 16 or position 17 itself as a ring
constituting member.
The hydrocarbon moiety represented by z may be
substituted with other atoms or groups, for example, halogen
-
, ' ' . .
~$~
-- 8
atoms, e.g. a fluorine atom or a chlorine atom; an alkyl
group, e.g. ~ethyl, ethyl, isopropyl, isopropenyl; an
alkoxy group, e.g. methoxy, ethoxy; a hydroxyl group; a
phenyl group; a phenoxy group and the like. The position of
5 the substituent may preferably be the carbon atom at position
16, 17, 19 and/or 20, but it is not restricted to only these
positions. Examples of preferred compounds include ones
which have one or two, different or identical atom(s) and/or
groups, for example, halogen atoms, e.g. a fluorine atom,
10 an alkyl group, e.g. methyl, ethyl; an aromatic group which
may have substituents, e.g. phenyl, benzyl, phenoxy; a
hydroxyl group on the carbon atom at position 1~. Other
examples of preferred compounds include those which have a
cycloalkyl group, e.g. cyclopentyl, cyclohexyl which
15 contains the carbon atom at position 15 as a constituent of
the cyclic ring; an alkyl group, e.g. methyl, ethyl on the
carbon atom at position 17 or 19; an alkyl group, e~g.
methyl, ethyl, isopropyl, isopropenyl; an alkoxy group,
e.g. methoxy, ethoxy, propoxy on the carbon atom at
20 position 20.
The generic name, PGE~ is used to represent com-
pounds having a prostanoic acid structure in which the carbon
atom at the ll-position has a hydroxyl group, and the carbon
atom at the 9~position is carbonyl. In the present specifi-
25 cation a prostanoic acid compound in which the hydroxyl groupon the carbon atom at the ll-position is substituted with a
hydroxyalkyl group or an alkyl group is also called a PGE.
Therefore, the 15-keto-PGEs o~ the present invention
~3~6~
include compounds in which R in general formula (I~
represents a hydroxyalkyl group or an alkyl group, for
example, a hydroxy group, e.g. hydroxymethyl, l-hydroxyethyl,
2-hydroxyethy], l-methyl-l-hydroxyethyl; an alkyl group,
5 e.g. methyl, ethyl.
The steric configuration of R with respect to the
carbon atom at the Jl-position may be ~ or ~ or a mixture
thereof.
~ he 15-keto-PGEs of the present invention may be in
10 the form of a physiologically acceptable salt or ester which
is esterified at the terminal carboxyl group.
The cation to be used for producing such salts may
be an alkaline metal, e.g. sodium and potassium; an alkaline
earth metal, e.g. calcium or magnesium; amines, e.g. methyl-
15 amine, dimethylamine, cyclopentylamine, benzylamine, piperi-
dine, monoethanolamine, diethanolamine, monomethylmono-
ethanolamine, tromethamine, lysine, ammonium,
tetraalkylammonium salts an~ the like.
~Iseful esters of 15-keto-PGEs may include saturated or
20 unsaturated lower alkyl esters which may have a branched chain,
e.g. methyl, ethyl, propyl, n-butyl, isopropyl, t-butyl,
2-ethy]hexyl, allyl and the like; aliphatic cyclLc esters,
e.g. cyclopropyl, cyclopentyl, cyclohexyl and the like;
aromatic esters which may have substituents, e.g. benæyl,
25 phenyl and the like; hydroxyalkyl or alkoxyalkyl esters, e.g.
hydroxyethyl, hydroxyisopropyl, methoxyethyl, ethoxyethyl,
methoxyisopropyl and the like; trialkylsilyl esters e.g.
6~
1 o
trimethylsilyl, triethylsilyl and the like; heterocyclic
esters, e.g. tetrahydropyranyl and the like. Preferred esters
for the present invention include a lower alkyl ester which may
have a branched chain, or instance, methyl, ethyl, propyl,
n butyl, isopropyl, t-butyl; a benzyl ester;. a hydroxyalkyl
ester, e.g. hydroxyethyl, hydroxyisopropyl; and the like.
15-keto-PGEs of the present invention may include
various kinds of isomers, e.g. tautomeric isomers, optical
isomers, geometric isomers and the like. As an example of such
isomers there is exemplified a tautomeric isomer between the
hydroxyl group on the carbon atom at the ll-position and the
carbonyl group on the carbon atom at the 15-position of
15-keto-PGEs. The latter tautomeric isomer is likely to occur
in 15-keto-PGEs having an electron attractive group, e~g. a
fluorine atom, at the 16-position.
A hemiacetal, a tautomeric isomer between the hydroxyl
group on the carbon atom at the ll-position and the carbonyl
group on the carbon atom at the 15-position, may sometimes be
formed, and an equilibrium mixture of the compound with R being
20 a hydroxyl group and a hemiacetal may be given. Such an
equilibrium mixture or the tautomeric isomer is also included
in the 15-keto-PGEs of the present invention.
A most preferable group of 15-keto PGEs of the present
invention is the group of 15-keto~PGEs haviny a double bond
between the carbon atoms at the 5- and 6-position or forming a
carbonyl group at the 6 position. Another pre~erable group of
15-keto-PGEs of the present invention is one having 8 - 10
carbon ato~s in the ~-chain. Another preferable group of
15-keto~PGEs of the present invention is one which has a
halogen atom or atoms or an alkyl substituent or substituents
5 at the 16-position. Another preferable group includes
15-keto PGEs which have a lower alkyl, especially a methyl
substituent at the 19-position of the ~-chain having more than
7 carbon atoms in the skeleton chain.
Concrete examples of the most preferable 15-keto-PGEs
are 13,14-dihydro-15-keto-19-methyl-PGE2 alkyl ester, 13,14-
dihydro-l5-keto-l6R~s-fluoro-pGE2 alkyl ester, 13,14-
dihydro-15-keto-16R,S-fluoro-ll-dehydroxy-llR-methyl-PGE2
alkyl ester, 13,14-dihydro-15-keto 20-methyl-PGE2 alkyl
ester, 13,14-dihydro-15-keto-16R,S-methyl-PGE2 alkyl ester,
13,14-dihydro-6,15-diketo-19-methyl-PGEl alkyl ester, 13,14-
dihydro-6,15-diketo-16R,S-fluoro-PGEl alkyl ester, 13,14-
dihydro~6,15-diketo-16~,S-~luoro-ll-dehydroxy-llR-methyl-
PGEl alkyl ester, 13,14-dihydro~5,15-diketo-20-methyl-PGE
alkyl ester and 13,14-dihydro-6,15-diketo-16R,S-methyl-PGE
alkyl ester.
In the present specificatio~ the PGEs are named based
on a prostanoic acid skeleton, butthey can be named according
to IUPAC nomenclature, accordin~ to whlch, for instance,
PGE, is nominated as 7-{(lR,2R,3R~-3-hydroxy-2-[(E)-(3S)-3-
hydroxy-1-actenyl3-5-oxo-cyclopen~yl~-heptanoic acid; PGE2
is nominated as (Z)-7-~(lP~,2R,3R,~-3-hydroxy-2-[(E)-(3S)-3-
hydroxy-l-octenyl3-5-oxo-cyclopentyl}-hept-5-enoic acid;
.. . ..
. .
- 12 -
13,14-dihydro-15-keto-16R,S-fluoro-PGE2 is nominated as (Z)-
7-{(lR,2R,3R)-3-hydroxy-2-~4R,4S)-4-fluoro-3-oxo-1-octyl]-
5-oxo-cyclopentyl}-he~t-5-enoic acid; 13,14-dihydro-15-keto-
20-ethyl-11-dehydroxy-llR-methyl PGE2 methyl ester is
5 nominated as methyl7-{(lR,2S,3R)-3-methyl-2~[3-oxo-1-decyl]-
5-oxo-cyelopentyl}-hept-5-enoate; and 13,14-dihydro-6,15-
diketo-l9-methyl-PGE~ ethyl ester is nominated as ethyl 7-
{(lR,2R,3R)-3-hydroxy-2-(7-methyl-3-oxo-1-octyl)-5-oxo-
cyclopentyl}-6-oxo-heptanoate.
The 15-keto-PGEs show fer~e~ence activity by
intracerebroventricular administration whether as carboxylic
acid type compounds or carboxy ester type compounds.
However, the carboxylic acid type compounds show no
fervescence activity by peripheral administration , e.g.
intravenous injection or oral administration. On the other
hand, carboxy ester type compounds show fervescence activity
even when administered peripherally. ~he ester compound in
which R is a lower alk~l group, e.g. a methyl or ethyl group
shows the strongest fervescence activity.
Improvements in body mechanisms, e.g. increases in
body temperature are brought on to the patient even when the
patient is out of normal conditions for example, in a state
of shock brought on by bleeding. Namely, the compound is
effective for the patient~out of homeostasis or under
anesthesia.
The 15-keto-PGEs used in this invention may be
- 13 -
prepared, for example, by the method noted in Japanese Patent
Application No. 18326/1988.
A practical preparation of the 13,14-dihydro-15-keto
PGEs involves the following steps; as shown in synthesis
chart (I), reaction of the aldehyde (2) prepared by the
Collins oxidation of commercially available Corey lactone (1~
with dimethyl ~2-oxoheptyl)phosphate anion to give a,~-unsacu-
rated ketone (3), reaction of the ~, ~- unsaturated ketone
t3) to the corresponding saturated ketone (4), protection of
the carbonyl group of ~he ketone t43 with a diol to the
corresponding ketal (5), and deprotection of the
p-phenylbenzoyl group to give the corresponding alcohol (6~
followed by protection of the newly generated hydroxy group
with dihydropyrane to give the corresponding tetrapyranyl
ether (7). According to the above process, a precursor of
PGEs o wh;ch ~chain is a 13,14-dihydro-15-keto-alkyl group
is prepared.
Using the above tetrapyranyl ether (7),
6-keto-PGEls (15) in which the group constituting the
carbon atoms of the 5-, 6- and 7-positions is
-CH2-C(O)-CH2-, may be prepared in the following steps;
reduction of the tetrapyranyl ether (7) with, for example,
diisobutyl aluminum hydride to give the corresponding
lactol (8), reaction of the lactol (8), with the ylide
generated from ~4-carboxybutyl)triphenyl phosphonium
bromide followed by esterifica~ion, cyclization between the
double bond between the carbon atoms at the 5- and 6-
,.~ .. . .
3~6~
positions and the hydroxvl group on the carbon atom at the9-position with NBS or iodine to give the halogenated
compound (11), dehalogenation of the compound (11) with, for
example, DBU to give the 6-keto compound (13) followed by
5 Jones oxidation and removal of the protecting groups.
Furthermore, PGE2s (19) of which a group
constituting the carbon atoms at the 5-,6- and 7-positions is
-CH2-CH-CH- may be prepared in the following steps; as
shown in synthesis chart II, reduction of the above
10 tetrapyranyl ether (7) to give thè lactol (8~, reaction of
the resultant lactol (8) with the ylide generated from
(4-carboxybutyl)triphenyl phosphonium bromide to give the
carboxylic acid (16) followed by esterification of (17),
Jones oxidation of the esters (17) to give the compound (18),
15 and removal of the protecting groups.
Using the above tetrapyranyl ether (7) as the
starting material, the compound having the group of
-CH2-CH2 CH2- may be prepared using the same process as
used for preparing PGE2s having the group -CH2-CH-CH- and
20 subjecting the resultant compound (18) to catalytic reduction
to reduce the double bond between the carbon atoms at the 5-
and 6-posi~ions followed by removal of the protecting groups.
Synthesis of 5,6-dehydro-PGE2s having a group
-CH2-C-C- may be carried out by alkylation of the resulting
7 6 5
25 copper enolate generated after 1,4-addition of a mono-
- alkylcopper complex or a dialkylcopper complex of the
following formula:
6~9
- 15 -
Cu~><x CU~<X \
O ~) ~ O O J
to 4R-t-butyldimethylsilyloxy-2-cyclopenten-1-one with 6-
alkoxycarbonyl-l-iodo-2-hexyne or their derivatives.
The ll-B type PGEs can be prepared according to
synt~esis chart III. The 15-keto-PGEs of this invention may
be used as a medicine for animals and human beings and is
usually applied systemically or locally by the method of
oral administration, oral administration by spraying,
intravenous injection ~including instillation), subcutaneous
injection, suppository and the like. Dose is determined
depending on the animal to be treated, the human patient,
age, body weight, symptoms therapeutic effect,
administration route, treating time and the like, but is
preferably 0.001 ~ 500 mg/Kg.
As a solid composition for oral administration,
tablets, powders, granules and the like are included. The
solid composition containing one or mvre ac~ive substances is
. ~ mixed with at least an inactive diluentj e.g. lactose,
: ~mannitol, glucose, hydroxypropyl cellulose,~fine crystalline
: cellulose, starch, polyvinyl pyrrolidone~ magnesium aluminate
metasilicate. The composition may contain in addition to the
inactive diluent, other additives, for example, lubricants
: (e.g. magnesium stearate), disintegrators
... ~ ,, .. ~ .
.
- 16 -
(e.g. cellulose calcium gluconate~, stabilizers (e.g. -,
~ or ~-cyclodextrin, etherated dextrin (e.g. dimethyl~
dimethyl-~-, trimethy]-~- or hydroxypropyl-~-cyclodextrin3,
branched cyclodextrin (e.g. glucosyl- or maltosyl-cyclo-
5 dextrin), formyl cyclodex~rin, sulfur-containing cyclodextrin
or misoprotol). Such cyclodextrins may form an inclusion
compound with 15-keto-PGEs in some cases to increase the
stability of the compounds. The stability may often be
increased by forming a lyposome with a phospholipid. Tablets
lO and pills may be coated with an enteric or gastroenteric
film, e.g. white sugar, gelatin, hydroxypropylcellulose,
hydroxypropylmethylcellulose phthalate and the like, if
necessary, and furthermore they may be covered with two or
more layers. Additionally, the composition may be in the
15 fcrm of capsules made of easily absorbed substances, e.g.
gelatin.
Li~uid compositions for oral administration include
pharmaceutical]y acceptale emulsions, solutions, suspensions,
syrups, elixirs and the like and contain a generally used
20 inactive diluentr e.g. purified water or ethyl alcohol. The
compo~ition may contain additives, e.g. wetting agents and
suspending agents as well as sweeteners, flavors, aromatics
and preservatives.
The compositions for oral administration may contain
25 one or more active substances.
The injection of this invention for non-oral
administration includes sterile aqueous or nonaqueous
~3~
- 17 -
solutions, suspensions, and emulsions. Diluents fo~ the
aqueous solution or suspension contain, for example,
distilled water for injection, physiological saline and
Ringer's solution. Diluents for the nonaqueous solution
S and suspension contain, for example, propylene glycol,
polyethylene glycol, vegetable oils, e.g. olive oil,
alcohols, e-g- ethanol and polysorbates. ~he composition
may contain other additives, e.g. preservatives, wetting
agents, emulsifying agents, dispersing agents and the
like. These are sterilized by filtration through, e.g. a
bacteria-preventing ~ilter, compounding with a sterilizer,
gas sterilization or radiation sterilization. These can be
prepared by producing a sterile solid composition and
dissolving it into sterilized water or a sterilized solvent
for injection before use.
Example 1 (fervescence activity by intracerebro-
ventricular injection)
Wister male rats (8 weeks old) were used as test
animals.
Rats were anesthetized by intraperitoneal injection
with 1.25 g/kg of urethane and a stainless guide cannula was
inserted into the lateral ventricle for administration o~ a
test drug according to Pellegrino's Rat brain atras.
~ Each test drug was dissolved in an artificial
cerebrospinal Eluid and 400 ng of drug in 3~ 1 was injected
through the injection cannula for 15 seconds. The
- ~3~ t9
- 18 -
artificial cerebrospinal fluid alone was injected as a
control. Body temperature was measured continuously at the
rectum. The rise in body temperature is shown as an increase
in degrees (C) from the temperature before administration.
The results are shown in Table 1.
Table 1
Test DrugRise in body temperature
22 +0.7
23 +0.4
24 +0.5
26 +0.1
1 +0.4
2 +0.4
6 +0.8
7 +0.6
8 ~0.4
Control 0
Exam~le 2 (fervescence a~tivity by intravenous
injection)
Test animals were Wister male rats (8 weeks old)
weighing 200 ~ 10 ~. The rats were anesthetized by
intraperitoneal injection of 1.25 g/kg of urethane. Each
test drug was dissolved in ethyl alcohol. The ethyl
alcohol solution was diluted with Ringer's solution at least
50 times just before use and 1 mg~kg of each test drug
- ~9 -
was intravenously administered. Body temperature was
~easured in the same manner as in Example 1.
Ringer's solution containing ethyl alcohol was
administered as a control. The rise in body temperature is
5 shown as an increase in degrees ( C) from the temperature
before administration. The results are shown in Table 2.
Table 2
Test Drug Rise inTest Drug Rise in
Body Tem- Body Tem-
perature (C) perature (C)
. .
1 0 18 + 0.7
2 + 0.9 19 + 1.0
3 0 20 + 1.3
4 t 0.8 21 + 1.1
5 + 0.4 22 0
6 0 23 + 1.0
7 + 0.8 24 + O.S
8 + 0.6 25 + 0.2
9 + 0.7 26 + 0.2
10 + 0.2 27 + 0.3
11 + 1.5 28 + 0.4
12 + 0.9 29 + 1.0
13 + 1.1 30 ~ 1.7
14 + 1~0 31 + 1.0
15 + 0.7 32 + 1.4
16 + 0.8 33 + 0.9
17 + 0.4 34 + 1.0
- 20 -
T~ble 2 (continued)
Test Drug Rise inTest Drug Rise in
Body Tem- Body Tem-
perature (C~ perature (C)
+ 1.0 48 + 0.2
36 + 0.6 49 f 0.2
37 + 0.2 50 + 0,2
38 + 0.3 Sl + 002
39 + 0.9 52 + 0.2
+ 1 . 4 53 f 0 . 5
41 + 0.8 54 + 0.2
42 + 1 . 4 55 + 0 . 2
43 + 0.2 56 + 1.0
44 + 0.2 57 0
+ 2.0 58 + 0.7
46 + 0.6 S9 f 1.2
47 + 0.3 control 0
Test ~
(Figuren~rs oE n.m.r. charts of corresponding
compounds are shown in brackets~after the compound names
respectively.) ~;
5~13,14-dihydro-15-keto-PGEl
2: 13,14-dihydro-1S-keto-PGEl ethyl ester
3: 13,14-dihydro-lS-keto-i2-PGEl
4: 13,14-dihydro-15-keto-~2-PGEl methyl ester
S: 13,14-dihydro-lS-keto-20-ethyl-PGEl methyl ester
16: 13 ,14-dihydro-6,15-diketo-PGEl
3~
- 21 -
7: 13,14-dihydro-6,15-diketo-PGEl methyl ester
8: 13,14-dihydro-6,15-diketo-PGEl ethyl ester
9: (+) 13,14-dihydro 6,15-diketo-PGEl ethyl ester
10: 13,14-dihydro-6,15-diketo-PGEl n~butyl ester
11: 13,14-dihydro~6,15-diketo-16R,S-methyl-PGE
methyl ester
12: 13.14~dihydro-6,15-diketo-16R,S-methyl-PGE
ethyl ester (Fig~3)
13: 13~14-dihydro-6,15-diketo-16,16~dimethyl-PGE
ethyl ester
14: 13,14-dihydro-6,15-diketo-16R,S-fluoro-PGE
ethyl ester,
15: 13,1~-dihydro-6,15-diketo-19-methyl-PGEl methyl
ester
16: 13,14-dihydro-6,15-diketo-19-methyl-PGEl ethyl
ester (Fig.4)
17: 13,14-dihydro-6,15-diketo-11-dehydroxy-llR-
hydroxymethyl-l9-methyl PGEl methyl ester
18: 13,14-dihydro-6,15-diketo-20-methyl-PGEl ethyl
ester
19: 13,14-dihydro-6,15-diketo-11-dehydroxy-llR-
methyl-PGEl methyl ester
20: 13,14-dihydro-6,15-dlketo-11-dehydroxy-llR-
methyl-PGEl ethyl ester (Fig.5)
21: 13,14-dihydro-6,15-diketo-16RjS-fluoro-llR-
dehydroxy-llR-methyl-PGEl ethyl ester (Fig.6)
22: 13,14-dihydro-15-keto-PGE2
~' ' .
~3~
22
23: 13,14-dihydro-lS-keto-PGE2 methyl ester
24: 13,14-dihydro-15-keto-PGE2 ethyl ester (Fig.7)
25: 13,14-dihydro-15-keto-PGE2 n-propyl ester
26: 13,14-dihydro-15-keto-PGE2 n-butyl ester
27: 13,14-dihydro-15-keto-PGE2 benzyl ester
28: 13,14-dihydro-15-keto-PGE2 hydroxyethyl ester
2g: 13,14-dihydro-15-keto-~2-PGE~ methyl ester
(Fig~)
30: 13,14~dihydro-15-keto-3R,S-methyl-PGE2
methylester
31: 13,14-dlhydro-15-keto-3R,S-methyl-PGE2
ethyl ester (Fig.9)
32: 13,14-dihydro-lS-keto-16R,S-methyl-PGE2 methyl
ester
33: 13,14--dihydro-15-keto-16~,S-methyl-PGE2 ethyl
ester (Fig.10)
34: 13,14-dihydro 15-keto-3R,S,16R,S-dimethyl-PGE2
methyl ester
35: 13,14-dihydro-15-keto-16,16-dimethyl-PGE2
methyl ester
36: 13,14-dihydro-15-keto-16,16-dimethyl-PGE2 ethyl
ester
37: 13,14-dihydro-lS-keto-16R,S-hydroxy-PGE2 ethyl
: : ester
:
38: 13,14-dihydro-lS-keto-16R,S-fluoro-PGE2
: (tautomeric isomer between the carbonyl group at the
~: carbon atom at th~;lS-position and the hydroxyl
~: : :
: :
; ~ ~
~3q~
- 23 -
group on the carbon ato~ at the ll-position is
confirmed.)
39: 13,14-dihydro~15-keto-16R,S-fluoro PGE2 methyl
ester
40: 13,14-dihydro-15-keto-16R,S-fluoro-PGE2 ethyl
ester
41: 13,14-dihydro-15-keto-16R,S-fluoro-20-methyl-
PGE2 methyl ester (Fig~ll)
42: 13,14-dihydro-15-keto-16R,S-fluoro-ll-
dehydroxy-llR-methyl-PGE2 ethyl ester (Fig.12)
43: 13,14-dihydro-15-keto~ dehydroxy-llR-methyl-
PGE2 ethyl ester
44: 13,14-dihydro-15-keto-17S-methyl-PGE2 methyl
ester
45: 13,14-dihydro-15-keto-19-methyl-PGE2 methyl
ester
46: 13,14-dihydro-15-keto-19-methyl-PGE2 ethyl
ester
47: 13,14-dihydro-15-keto-20-methoxy-PGE2 methyl
ester
48: 13,14-dihydro-15-keto-20-methoxy-~2-PGE2 methyl
ester
49: 13,14-dihydro-15-keto-3R,S-methyl-20-
methoxy-PGE2 methyl ester
50: 13,14-dihydro-15-keto-16,16-dimethyl-20-
: methoxy-PGE2 methyl ester
51: 13,14-dihydro-15-keto-20-isopropylidene-PGE2
~3~
`
- 24 -
52: 13,14-dihydro-15-keto-20-isopropylidene-PGE2
methyl ester
53: 13,14-dihydro-15-keto-20-ethyl-PGE2 methyl
ester
54: 13,1~ dihydro-15-keto-20-ethyl-PGE2 ethyl ester
55: 13,14-dihydro-15-keto-20-ethyl-11-dehydroxy-
llR-methyl-PGE2 methyl ester
56: 15-keto-16R,S-fluoro-PGE2 methyl ester (Fig.13)
57: PGEl
58: PGE2
59: PGE2 methyl ester
. As shown in Table 2, 15-keto-PGEs obviously show
fervescence activity even by peripheral administration, e.g.
~, intravenous injection.
Example 3
Experiments were carried out in the same manner as in
Example 2 exceptthat the test drugs were administered
intravenously at a dose of S mg/kg. The results are shown
in Table 3.
Table 3
Test Drug Rise in body temperature (C)
25+1.3
: 26~ +0.9
:
fervescence (recovery of body
temperature) activity at hemorrhage load)
: 15 Wister male rats (weight: 200g) were
anesehetized by
- ':
- 25 -
injecting them in the back with 1.5 g/kg of urethane.
~emorrhaging was then induced by withdrawal of blood by
puncturing the heart for a total volume of 3 ml equivalent to
~.5~ of the body weight. After leaving the rats in a room
for 60 minutes, they were intravenously administered with
test solutions prepared by dissolving the test drugs into
Ringer's solution so as to administer to the rat a dose of
0.5 or 1 mg/kg, and the change in the body temperature wa~
observed. Ringerls solution was administered ~s a control.
The results are shown in Table 4 and Figs. ~ and 2.
Table 4 (Fervescence Activity on the Rat Loaded
with Hemorrhaqe)
Test Drug Rise in Body Temperature (C)
_
0.5 m~/kg 1 mg/kg
8 +0.7 +0.8
15 16 +0.4 +0.6
18 +0.4 +0.8
~0.5 +0.8
24 +0.8 ~1.1
Control 0
Fig. 1 shows changes in body temperature before and
after administration of test drug 24, (13,14-dihydro-15-
keto-PGE2 ethyl ester) to a rat loaded with hemorrhage.
Fig. 2 shows cha~ges-~in body temperature before and
after administration of test drug 8, (13,14-dihydro-6,15-
diketo-PGEl ethyl ester~ to a rat loaded with hemorrhage.
- 26 -
In the figures, (1) (2) and (3) correspond to the
results of administration of doses 1 mg/kg, 0.5 mg/kg and
Ringer's solution alone, respectively.
As shown in Table 4 and Figs. 1 and 2, the group of
5 rats administered with the 15-keto-PGEs of this invention are
observed to have dose response fervescence although body
temperature continued to fall in the group administered with
Ringer's solutions.
The compounds of the present invention are useful as
10 drugs for increasing body temperature wherein the lower body
temperature resulted from hypothermia caused by serious
bleeding and surgery. This can be seen from the above
results, as the fervescence activity of the compounds
administered to the animals under conditions of low body
15 temperature (33C) (i.e. those unable to maintain normal body
temperature caused by bleeding and being out of homeostasis) ?
was evident from the rise in body temperature.
Example 5 ~Sedation Activity)
A l9-methyl type compound having strong fervescence
20 activity was compared with PGE2 methyl ester in sedation
activity. A hybrid adult do~ was held on a dog holder and
intravenously administered with a test drug dissolved in
Ringer's solutions at the forefoot.
~ sedatlon activity was judged as follows:
Z5 Sedation Degree 1: Disappearance of body movement
2: Closing of the eyes
3. Leaning on the holding belt
.. .. .
,
- 27 -
Table S
Test Drug Dose Rise in Body Sedation Degree
mg/kg Temperature (C) 1 2 3
.
O.S 0.5 - - -
lS O.S 1.2 - - -
16 O.S 1.1 - - -
S9 0.01 0.4 + ~, +
As obviously shown in the results in Table 5, the test
drugs of this invention show no sedation activity although
lG PGE2 methyl ester shows clear sedation activity.
EXAMPLE 6 (acute toxicity)
Acute toxicity (LD~o) of the test drugs was
estimated on Slc-ddY female mice (5-weeks old) by an
intravenous injection. Results are shown in Table 6.
Table 6
test drug LD50 mg/kg
2 >1000
16 > 300
23 >1000
20~ ~ 24 1000
The lS-keto-PG~s of this invention have fervescense
:: activity and the ester compound shows the activity by
peripheral administration, eOg. intravenous injection or
~:~ oral administration,in the same manner as by
intracerebroventri=ular injection It brings on an ir~rowement
:
- 28 -
in body mechanism, e.g. fervescence activity in an animal in
a condition of shock and being unable to maintain normal body
mechanisms, e.g. body temperature by, for example, bleeding,
namely, out of homeostasis.
Accordingly, the 15-keto-PGEs of this invention is
useful as a drug for recovering from hypothermia caused by
hypothermic operations, e.g. surgical operations, hypothermia
accompanied with a decline of basal metabolism ratio caused
by, for example, thyroid hormone hyposecretion, hypothermia
caused by brain disorders, hypothermia caused by serious
bleeding and consciousness disorders, hypothermia caused by
lowering of the surrounding temperature, hypothermia caused
by heatloss from the surface of the body caused by sweating
and vasodilation, hypothermia and asphyxiation, and is also
useful as a drug for raising the body temperature which has
fallen under shock conditions to recover various body
mechanisms and to improve the homeostasis and maintaining
ability. Additionally, it is useful as a drug for preventing
the above hypothermias.
.
.
~]
~o~ -
o~ ~ o
o ~
~ 3 ~]
o~ ~ T c;l~ o o
ol~ o
.
~,
0 ~ L'~ ~
o~~ ~<o l 0
- ~ ~ n
ol~l 1111~
: ~ O ~ ~
: ; ~
o~ o~
~ ~ olll llllo ~ clll ll~lo -- ~ --
~ ~ ok` k Clll~ o
~;
-- 6Z -- : -
'~ .
~]
~3
~ o~llllo ~
al >
~, ~o]
~r~ ^
o ~
... . .. . ................................... .
~- ?
~ , ,
~ o ? ~o~o]
o] ----~1111 ~ ~ '
/ ~ ~
:
:
~ 0~ ~
~ ~ ,o~o `-
3~o~ ~
Glll<>~l\lo~
1-~
~ ~] ~(] ~ ~
O~ 1~
.
o
< ~
/ >
<~ '!' ""-~
""~_~
-
~: :
~3~:D6~
. ~ . .
~/o
o olll o
~o~ ~] ~]
o~ ~ G O O
' ' / ~
~1
~i O I ~0]
h~ ~ ~ ] o =1ll C
,U~ o~"",~
.C 0~ 110
~ U~ I `,'
- - Z -
""'' '' ' ' ~ , , ~