Note: Descriptions are shown in the official language in which they were submitted.
~3~)~335~
BACKGROUND OF ~HE INVENTION
Field of the Invention
This invention relates in general to certain new and
useful improvements in an aerosol for use in the reduction of
tobacco smoking, and more particularly to an aerosol which uses
a food acid in non-toxic amounts, but sufficient in content and
having proper particle size to simulate sensations in the
respiratory tract which would normally be caused by tobacco
smoke.
.
Brief Description of the Prior Art
In recent years, with the recognition of the harmful
effects of tobacco smoking, there have been numerous campaigns
and programs by governmental agencies and various health groups
and other interested organizations to dis.seminate information
about the adverse health effects resulting from tobacco
smoking. Moreover, and as a result of this recognition of the
harmful effects, there have been many programs directed to
attempts in reducing smoking incidence.
i ~
` '' ' 1' 2.~
- .
-- .
.
' ' ~ '~ ,' ' :
.
~3q~
The present successes in achieving reduction in the
incidence of smoking have been relatively poor with presently
known technigues. The present state of the art involves both
behavioral approaches and pharmacological approaches.
Approximately 80% or more of the tobacco smokers who initially
quit smoking after using some behavioral or pharmacological
approach to singly reduce smoking incidence, generally relapse
and return to the habit of smoking at their former rate of
smoking within about a one year's period of time.
one of the most successful approaches to date in reducing
the incidence of smoking relies upon nicotine containing chewing
gum which is designed to reduce smoking withdrawal symptoms.
The reported success rate, while still relatively low is
approximately twice that of the other methods which have
heretofore been employed. The use of the nicotine gum suffers
from several problems including not only the bad taste and
destruction of dental appliances, but the gastrointestinal upset
which results there~rom and which also reduces compliance. In
addition, it has been found that the nicotine containing gum
does not satisfy the craving that most smokers experience for
the distinct sensations in the throat and chest elicited by
nicotine in the smoke. Over the course of many years of tobacco
smoking, these particular sensations have become an important
part of and associated with the habit of smokers and give rise
to tobacco smoke dependency in most o~ the tobacco smokers.
..:
,
~3~
The circulatory ef~ects o~ nicotine aerosol inhalations
have been studied as set *orth in the October 7, 1967 edition of
the Lancet, pages 754-755. In this case, large doses of
nicotine aerosols were applied to selected individuals in order
to determine the effects on the individuals. Further, circadian
blood nicotine concentrations have been studied as a result of
cigarette smoking, as reported in Clinical Pharmacology and
Therapeutics, December 1982, in an article by Neal L. Benowitz,
M.D., pages 758-764.
A citric acid aerosol spray has been used for assessing
the degree of airway anesthesia on the magnitude of added
inspiratory load in the respiratory tract of individuals, as
reported in "The Effects Of Airway Anesthesia on Magnitude
Estimation of Added Inspiratory Resistive and Elastic Loads" by
N. X. Burki et al, the American Review of ~espiratory Diseases,
127, 2-4, 1983. In this case, the ade~uacy of airway
anesthesia was assessed by absence of any cough in response to
inhalation of a nebulized 20~ solution of citric acid.
HerPtofore, there has not been any attempt to use a food
acid aerosol spray, as for example, a citric acid aexosol spray,
in order to aid in the reduction o~ incidence of tobacco
smoking.
.
- . . ' ': : ' . '
~3~3Sl
OBJECTS OF THE INVENTION
It is, therefore, one of the primary obj~cts of the
present invention to provide a method of aiding in the reduction
of the incidence of tobacco smoking by orally applying an
aerosol containing a selected amount of a food acid which is
capable of simulating the sensations in the respiratory tract
normally caused by tobacco smoke.
It is another object of the present invention to provide a
method of the type stated in which an aerosol is periodically
applied to the oral cavity of an individual to thereby simulate
the sensations in the oral cavity and in the respiratory tract
caused by tobacco smoke and thereby replace the need for tobacco
smoke by an individùal.
It is a furthar object of the present invention to provide
a food acid containing aerosol which has a selected particle
size and also a selected amount of a food acid contained therein
to simulate the conditions and sensations normally obtained by
the inhalation of tobacco smoke.
It is also an ob~ect o~ the present invention to provide a
method of aiding in the reduction o~ incidence of tobacco
smoking by use of an aerosol which contains a combination of a
food acid and tobacco smoke having a proper particle size.
It is another salient object of the.present invention to
provide an aerosol for inhalation to aid in smoking incidence
reduction and where the aerosol contains a liquid carrier with a
food acid therein present in non-toxic amounts and capable of
simulating the sensations in the respiratory tract caused by
- -
- :13~l335~
nicotine in tobacco smoke.
With the above and other objects in view, my invention
resides in the novel features o~ form, construction, arrangement
and combination of steps and compositions and apparatus as
hereinafter described.
.
,, , : .
.
~36~3~i~
BRIEF SUMMARY OF THE DISCI,OSURE
This inveintion relates in broad aspect to a method of
aiding in the reduction of incidence of tobacco smoking. In a
preferred embodiment, the method utilizes an aerosol which
relies upon application of the aerosol to the oral cavity and
respiratory tract of an individual to correspond to the
perceived need for tobacco smoke o~ that individual.
The method of the invention comprises administering an
aerosol to the oral cavity of the individual where the aerosol
contains particles of a food acid, such as citric acid, and
which is present in non-toxic amounts and capable of being
inhaled. The particles are of a proper size and have the food
acid sufficient in ~content therein to simulate the sensations in
the respiratory tract normally caused by tobacco smoke. In this
way, the oral and respiratory tract sensations simulate those
which would be created by tobacco smoke to thereby replace the
need for tobacco smoke of an individual.
In one embodiment, the aerosol is a liquid spray which
contains the food acid in a relatively inert liguid carrier and
where the food acid is present in an amount of about 8% to about
35% by weight in the li~uid carrier. More specifically, the
liquid carrier is water and also, in one of the more preferred
embodiments, the food acid is citric acid. In a more preferred
embodiment, the food acid, such as citric acid, is present in
the liquid carrier in an amount of about 15% by weight to about
25% by weight.
The food acid is preferably selected from the class
' ~ I
-: ,
~L3~
.
consisting of citric acid, ascorbic acid, adipic acid, tartaric
acid and mixtures of the foregoing.
The aerosol may adopt the form of a liquid aerosol so as
to constitut~ an aerosol spray. However, the aerosol may be in
the form of fine particles such as dust-sized particles. The
term "particles" refers to either or both liquid or solid
portions of the aerosol and the term "droplets" is often used to
refer to the particles in liquid form.
~ he aerosol has particles in a proper size so as to
migrate from the oral cavity into the respiratory tract. Thus,
and in a preferred embodiment, the aerosol has droplets of a
size between 1 micron to about 15 microns in diameter. More
preferably, the aèrosol has droplets of a size ranging between
about 5 microns to about 10 microns in diameter.
The present invention also relates to an aerosol
composition which is used for inhalation by an individual to
aid in smoking incidence reduction. The aerosol comprises the
food acid mixed in that liquid carrier in the amount specified
above and preferably containing particle sizes as mentioned
above.
In another embodiment of the invention, it has been found
that the food acid can be mixed with actual tobacco smoke so
that the food acid can migrate to the respiratory tract and the
tobacco smoke will generally remain in the oral cavity. In this
way, many of the health problems which result ~rom inhalation of
tobacco smoke can be reduced, if not eliminated.
In this latter embodiment of the invention, the food acid
g
~3~i33~
is preferably present with particles haviny a first particle
size within the range o~ about l to about 12 microns. The
tohacco smoke is adsorbed on a particulate saccharide which has
a second particle size, as for example, from about 12 microns to
about 15 microns. In this way, the particle sizes are
controlled such that the tobacco smoke will remain in the oral
cavity and the *ood acid, such as citric acid, will migrate to
the upper and lower respiratory tract.
In a further embodiment of the invention, it has been
found that the aerosol can be comprised of saccharide particles
which are finely divided and actual tobacco smoke adsorbed
thereon. These particles are also sized so that they can
migrate to the rèspiratory tract. In this way, many of the
health problems w~ich result from inhalation of large quantities
of tobacco smoke can be reduced, if not eliminated.
In still another embodiment o the invention, it has been
found to be effective to use the food acid aerosol of the
present invention in combination with transdermally applied
nicotine. The nicotine may be applied periodically by means of
a patch placed on the users skin. In this way, nicotine levels
can be increased in the blood to satisfy the perceived
psychological demand for nicotine and the oral sensations
normally obtained with tobacco smoke can.be satisified by the
food acid aerosol of the present invention.
It has also been found to be effective to add an
emulsifier, as for example, lecithin to the food acid aerosol
spray of the invention to act as an emulsifier therein. The use
- ~3~3~1
of the fatty acid emulsi~ier enables reduction of surface
tension and thereby enables the production of droplets of
smaller particle size.
This invention possesses many other advantages and has
other purposes which will be made more clearly apparent from a
consideration of the forms in which it may be embodied. They
will now be described in detail ~or purposes of illustrating the
general principles of the invention, but it is to be understood
that such detailed descriptions are not to be taken in a
limiting sense.
,~
'~ :
':
,
, 11
:.:
.`~ :
.. ~
.`~' ~ ~ , 1 1.
`'` : ,
,
:
. ;:
3083S~
BRIEF DESCRIPTION OF THE DRAWINGS
Having thus descrihed the invention in general terms,
reference will now be made to the accompanying drawings in
which
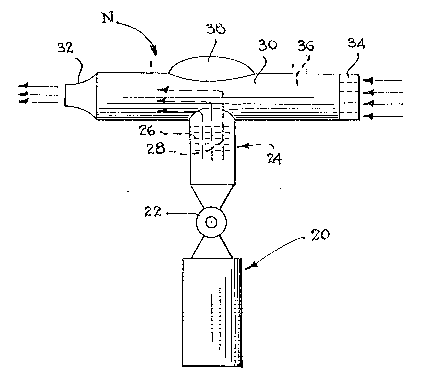
FIGURE 1 is a ~chematic side elevational view of a
nebulizer which may be used for the oral administration of the
food acid containing aerosol;
FIGURE 2 is a graph showing the mean similarity of a food
acid aerosol of the present invention compared to a user's own
brand, as well as a comparison to air inhalation and a low tar
and nicotine cigarette:
FIGURE 3 is a graph showing the mean liking (affection
toward) a food acid aerosol of the present invention compared to
~ 11
à user's own pre~erred cigarette brand, as well as a comparison
to air inhalation and a low tar and nicotine cigarette;
FIGURE 4 is a graph showing the mean strength of a food
acid aerosol of the present invention compared to the user's own
preferred cigarette brand, as well as a comparison to air
inhalation and a low tar and nicotine cigarette;
FIGURE 5 is a graph showing the mean harshness of a food
acid aerosol of the present invention comared to a user's own
preferred cigarette brand, as well as a comparision to air
inhalation and a low tar and nicotine cigarette;
FIGURE 6 is a graph showing the reported satisfaction
after several puffs of a food acid aerosol of the present
invention compared to a user's own cigarette brand preference,
as well as a comparision to air inhalation and a low tar and
- ~3~
nicotine cigarette; and
FIGURE 7 is a graph showing the mean reported cigarette
craving after several puffs of a food acid aerosol of the
present invention compared to a user's own cigarette brand
preference, as well as a comparison to air inhalation and ~ low
tar and nicotine cigarette.
`,' . Il
`: `
~`
:
.
:
: : : 13
'` '
:~ : :::
~`~':: ; , :
~ I
: `::: `
:,
:
~3~)~35~
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The present invention provides an aerosol and a method o~
using a food acid containing aerosol. In a preferred
embodiment, as hereinafter described, the aerosol is liquid
based and is in the form of a spray which may be dispensed from
a nebulizer N, as shown in ~igure 1. The nebulizer generally
comprises a container isiuch as a bottle 20 containing a solution
of a food acid dissolved in a liquid carrier.
Located at the upper end of the container 20 is a valve
22, which may be in the form of a push-button valve or one with
a rotatable valve core capable o~ being manually manipulated by
a user, such that the food acid containing carrier can be
dispensed from thè container upon opening of the valve 22.
Located above the valve is a housing 24 having a plurality
o~ internally located baffles 26. Each of these baffles 26 would
have apertures 28 contained therein which control the size o~
the vehicle droplets. The upper end of the housing 24 is
connected to and in fluid communication with an inhalation tube
which has a reduced end 32 for introduction into a user's
mouth. The opposite end of the tube 30 may have a screen 34
over the open end thereof in order to strain and remove any
foreign particles in an entering air stream.
The tube 30 has an internal chamber 36 which is designed
to receive a charge of the aerosol spray. The tube 30 may also
be provided with an enlarged hump 38 as shown in order to
increase the overall size thereof and to insure that the content
of the food ac~d containing liquid carrier (which may be in
14
! ~ I
'~
'
,
35~
.
vapor form) within the tube is sufficient to constitute one
full inhalation with a desired amount of the food acid.
The bottle 20 is preferably a pressurized bottle
containing an inert gas under pressure. Various inert gases,
such as Freon, or the like, which are normally found in
containers of this type, may be employed.
The screen 34 may take the form of a resistive member
which somewhat restricts the flow of air therethrough. In this
way, the screen can act as a cigarette filter which creates a
draw resistance, much in the same manner as one experiences when
drawing air through a cigarette. For that matter, a
conventional cigarette filter could be fitted upon the
right-hand end of the tube 30.
! The aerosol spray generally contains any food acid which
is capable of being inhaled and which is capabble of providing
the sensations in the oral cavity and respiratory tract ~imilar
to those caused by nicotine in tobacco smoke. Thus, and for
this purpose, the food acid must be present in the aerosol in an
amount sufficient to simulate those sensations created by
tobacco smoke, and thereby avoid the need of tobacco smoke to
create such sensations. The food acid is present in a
relatively minor amount in an inert liquid carrier and generally
in an amount of 8% to about 35% by weight in the liquid
carrier. More preferablyj the food acid is present in an amount
of about 15~ to about 25% by weight in the liquid carrier.
The liquid carrier may be any of those liguid carriers
normally employed in aerosol containers and in addition, the
I
~ , . . .
~3t[1 ~
liquid carriers which are employed may be the s~me as those used
for inhalers, as for example, inhalerG in brochial dilators. It
is important for the liquid carrier to be relatively inert so
that it does not react with the body or with the food acid. one
of the primary liquid carriers which may be employed is water.
However, various low molecular weight alcohols such as ethanol,
etc. could be used. In addition, glycerol, propylene glycol,
etc. are effective carriers for the food acid.
one o~ the most pre~erred food acids which may be employed
is citric acid since it creates sensations in the respiratory
tract most closely approaching those created by normal tobacco
smoXe. However, essentially any ~ood acid which is capable of
being inhaled and which creates some sensation similar to that
created by tobac~o smoke, may be employed when it does not
provide any unpleasantness or adverse side effects. For
example, other food acids which have been found to be effective
in the present invention include ascorbic acid, adipic, tartaric
acid and mixtures of the foregoing.
As indicated previously, the food acid preferably is
present in an amount of about 15% by weight to about 25% by
weight, although it can range from about ~% by weight to about
35% by weight in the liquid carrier. The amount of the food
acid will vary depending upon the overall effects desired and
the particular food acid employed.
It is also possible to add one or more surfactants to the
food acid containing liquid carrier in order to break up the
droplets into smaller size. The lecithin and other surfactants
, ' ' '~ '
' ' '
~3~ 5~
are essentially a mlxture o~ ~atty acids which have been added
to foods to act as emulsi~iers or surfactants. These
surfactants operate to split particles apart so as to reduce
surface tension and thereby enable the generation of smaller
particles. Lecithin is one excellent surfactant which can be
used inasmuch as it is highly compatable with body tissue~
Other surfactants which may be considered for use include
sorbitan trioleate, cetylpyridinum, etc. When a surfactant is
employed, it is preferably added in an amount of about 0.5% to
about 1~ by weight.
In normal tobacco smoke, approximately 0.1 milligrams of
nicotine is obtained in each puff of a medium strength
cigarette. This quantity of nicotine is known to satisfy the
smoking and related tracheal sensations. However, by using the
aerosol spray containing a food acid in the specified amounts
and by controlling droplet sizes, as hereinafter described, it
is possible to obtain the same effect. This is due to the fact
that the size of the particles, such as the liquid droplets,
determines the region of the respiratory tract to which the food
acid would penetrate. For example, by using particles within a
range from about 1 micron to about 5 microns, the food acid
containing particles will penetrate to the lower respiratory
regions for stimulation of those regions. Larger particles,
e.g. droplets, as for example, 5 microns to about 10 microns
would not penetrate very deeply in the respiratory tract and
thus would stimulate the higher respiratory tract regions.
Accordingly, by controlling the particle size, it is possib`le to
17
,
~3~33~i~
stimulate ~hat portion of ~he respira~ory track ~rom which the
smoker receives th0 greatest sensation.
In many cases, the tohacco smoker may desire to obtain
some taste normally provided by cigarette smoke. While the
citric acid and other food acids can simulate the respiratory
tract sensations, they cannot necessarily simulate the taste
provided by tobacco smoke. Accordingly, by incorporating a
relatively small amount of tobacco smoke into the liquid
carrier, it is therefore possible to provide both the stimulated
sensations created by tobacco smoke as well as the taste created
by tobacco smoke.
It is possible to incorporate tobacco smoke directly in
the aerosol of the present invention. In many cases, the
tobacco smoke does not effectively incorporate in the liquid
carrier. Even when the tobacco smoke is dissolved in a liquid~
it provides a rather unpalatable taste. Thus, for this
purpose, the tobacco smoke can be captured on various solid
particulate saccharides, such as starches. In this case, the
tobacco smoke contacts the starch where it can effectively
adhere to the surface of the starch. A jet of air directed
against the starch thereafter generates a fine dust mixture of
the starch containing the smoke particles. This is highly
effective in that the smoke particles on the starch base will
have a size up to about 15 microns which is sufficiently large
so as to remain in the oral cavity and which also allows the
smaller sized citric acid particles to migrate to the
respiratory tract. In this case the citric acid is present in
18
- !
~3~)l33~1
.
solid particulate form with R par~icle ~ize generally not
exceeding about 12 microns. It is also possible to use an
aerosol containing the finely divided particulate starch with
the tobacco smoke adsorbed thereon in absence of the food acid.
Some of the effective starches which can be used in
accordance with the present invention are corn starch, lactose,
which is generally a filler used in pharamaceutical products,
flour, and other polysaccharides which are safe for inhalation.
In this latter embodiment of the invention~ the aerosol
composition will be a solid particulate mattPr composition.
Thus, the nicotine particles on the starch base will be in
admixture with ~olid food acid particles. However, since the
particles are dùst sized particles and are effectively
fluidized, they can be nebulized and ejected from the nebulizer
much in the same manner as a liquid spray.
As indicated previously, it is possible to control the
area of the respiratory tract to which the food acid and the
cigarette smoke will migrate by control of particle size. As
an effective example, when using incorporated tobacco smoke, it
is possible to produce a first mixture having particles within
the range of about 1 micron to about 5 microns which contain the
food acid. These particles will therefore penetrate to the
lower respiratory regions. As a second mixture, it is possible
to use a carrier for the cigarette smoke with smoke particles in
the size of about 12 microns to about 15 microns. This portion
of the aerosol would therefore remain in the oral cavity. The
two components could therefore be mixed and introduced into the
19
~ ' .
:
:~L3~1835;:1L
nebulizer. However, lt is possible to use particle sizes within
a range of about l micron to about 15 microns in accordance with
the present invention.
In the event that the particles of food acid and the
particles of starch tend to agglomerate, the food acid particles
can be held in one compartment and the smoke containing starch
particles can be held in another compartment. In this way the
two sources of particles can be mixed when in a fluidized form
as for example in a tube containing an air stream.
The amount of citric acid or other food acid can be varied
in order to acco~modate smokers who are accustomed to relatively
mild tobacco smoke versus relatively strong tobacco smoke.
Furthermore, ~he baffle system in the nebulizer can be adjusted
to reduce the droplet size for penetration control. In
addition, and as previously described, droplet size can be
controlled by the addition of an emulsifier so that different
sensations in different regions of the respiratory tract can be
obtained.
The dis-association of the local tracheal stimulation
produced by nicotine from its systemic physiological effects
also is believed to allow for an effective extinction training
procedure. Thus, the prior conditioning of the smoker in which
smoke elicited throat stimuli were paired with the desired
pharmacologic e~fects of nicotine in each puff can be
reversed. The aerosol spray can be presented at different
times of the day and may be employed in accordance with the
smokers normal smoking pattern. Under normal conditioning
- 20
I
, .
'
: 130~335i~
theory, it is believed that the use of the food acid can hasten
the extinction of the tracheal desire. A presentation of
tracheal stimulation without an effective dose of nlcotine
reduces the association with the nicotine's desired effects.
It is also possible to use a transdermal application of
nicotine to the individual in addition to the use of the aerosol
in accordance with the present invention. For example, that
transdermal patch described and claimed in U.S. Patent No.
4,715,387 dated December 29, 1987 by ~rs. Jed E. Rose, et al.,
may be employed. In that case, the nicotine containing dermally
applicable patch was applied to the skin of a user such that the
nicotine in the patch was allowed to transdermally migrate into
the blood stream of an individual at a rate sufficient to
correspond to the nicotine level in the blood achieved by tobacco
smoking. In this way, the nicotine can be introduced into the
user's blood stream to satisfy the learned brain response to
nicotine and the aerosol spray can be used to satisfy the oral
and tracheal demands for nicotine without the otherwise harmful
side effects of nicotine.
,~.
- :L3[)~3~i1
- 22 -
The invention is illustrated by, but not limited to the
following examples.
EXAMPLE 1
An aerosol solution was prepared by mixing one hundred grams
of citric acid in about seven hundred grams of water by weight in
order to provide a 15% citric acid solution. This solution was
thereupon introduced into a nebulizer having a lower container
section and allowed to settle for about ten minutes. The
nebulizer contained a tube of about one centimeter diameter and
about two meters in length and which tube is made of plastic
marketed under the trade mark "Teflon". An inlet of the Teflon
tube had a cigarette filter attached to enable users to puff on
the opposite end and feel a draw resistance comparable to that of
a cigarette.
EXAMPLE 2
The citric acid aerosol solution of Example 1 in the
nebulizer of Example 1 was used to test the acceptability of a
citric acid aerosol in fifteen individual cigarette smokers. The
smokers selected all smoked the equivalent of about a pack a day
of cigarettes. Each of the smokers made blind ratings of the
following types from puffing the following substances: (1) the
citric acid aerosol mixture, (2) air, (3) a low tar and nicotine
cigarette, which in this case was Carlton filter hard pack
cigarette, and (4) their own cigarette brand preference.
Each subject received four puffs of each of the above
identified substances in random sequence. The aerosol was
gL3~1~35i~
allowed to settle ~ive to ten 6econds before each inhalation in
order to remove droplets larger than approximately ten microns.
Puffs of smoke were delivered by drawing a volume of 35 cubic
centimeters of 6moke ~rom the cigarettes with a syringe and
injecting the smoke into the Teflon tube prior to inhalation.
To encourage subjects to focus on the tracheo-bronchial
sensations associated with each test, and to minimize potential
bias, each of the subjects were blindfolded. Further, the
nostrils of each of the subjects were occluded with nose plugs
and their mouths were numbed by rinsing with a topical
anesthetic, which in this case was 2% lidocaine.
Each of the subjects rated their liking (pleasure
associated) with each puff as well as the strength of the puff,
the harshness and the similarity to their own customary brand of
cigaretke. The results of these tests are more fully
illustrated in Figures 2-5 of the drawings. These results,
generally indicate that the citric acid aerosol was rated
significantly more similar to the subjects own preferred
cigarette brands than air or another low tar and nicotine
cigarett~.
Figure 2 illustrates that the mean similarity of the
citric acid was rated significantly higher to the subjects own
preferred cigarette brand than air which had a probability value
(P) of less than 0.001 or the low tar and nicotine cigarette
which had a probability value of less than 0.05. By reference
to Figure 3, it can be observed that the subjects tested liked
the citric acid aerosol much better than air havlng a
23
:; :
.
3S~
probability value of less than 0.01, or the low tar nicotine
cigarette with a probability value of less than 0.05. By
re2erence to Figurei 4, it can be observed that the citric acid
aerosol was significantly stronger than air or the low tar
nicotine cigarette. By reference to ~igure 5, it can be
observed that the citric acid aerosol is significantly harsher
than the air or comparable low tar and nicotine cigarette, with
values comparable to the subjects own brand of cigarette..
Based on these tests, it was concluded that the citric
acid aerosol simulated the tracheo-brochial sensations
associated with cigarette smoking and provided an acceptable
substitute for many, if not most of the tested cigarette
smokers.
~, .
EXAMPLE 3
The same subjects used in Example 2 were provided with the
citric acid aerosol prepared in accordance with Example 1 in
order to determine whether the citric acid aerosol inhalations
would diminish craving for cigarettes. In this case, each of
the subjects were given a series of ten puffs of each of four
substances which were (1) air, (2) the citric acid aerosol
spray, (3) a low tar and nicotine cigarette and (4) the users
customary preferred brand of cigarette. The same procedure was
used, as reported in Example 2, to deliver the puffs of the
substances to the subjects. Each of the subjects reported their
craving and satisfaction after each set of puffs.
The mean reported satisfaction of the subjects after ten
~4
~ I I
`
` ' : ` ' ` :
3~
puffs of the four substances showed that the citric acid aerosol
was rated as significantly more satisfying than air with a
probability value of less than 0.005 as shown in Figure 6. The
citric acid aerosol was also rated as reducing craving at a much
greater rate than the air puffs, as shown in Figure 7 with a
probability value of less than 0.05.
Thus there has been described a unique and novel method
and apparatus and composition which enables the effective
reduction in the incidence of tobacco ~moXing and khe attendant
reduction, if not elimination, of dependency on tobacco smoking
without relapse and return to the dependency. It should be
understood that many changes, modifications, variations and
other uses and applications will become apparent to those
Il
`skilled ~n ~he art after considering this specification.
Therefore, any and all such changes, modifications, variations
and other uses and applications which may become apparent to
those skilled in the art after considering this specification
are deemed to be covered by the invention.
.
.
, ' .
,
., ' ' .