Note: Descriptions are shown in the official language in which they were submitted.
1 3 ~ 029~
PROCESS AND APPARATUS FOR TREATMENT
0~ LIQUIDs, ~ CUDARLS DESALINIZATION
OF A~UEOUS SOLUTIONS
The invention relates to a process and an apparatus
for treatment of liquids, particularly demineralization of
aqueous solutions, by which the solution and the ions dissolved
in it are subjected in succession to different absorption and
desorption procedures and thereafter the liquid is obtained as
the treated product.
It is commonly known that liquids, particularly
aqueous solutions, such as water of all kinds for industrial
uses, have to be practically free of salts or their ionic
dissociation components. To serve this purpose, various
commonly known processes are used for the treatment of such
liquids, whereby such dissolved salts are removed therefrom.
Furthermore, it is known that the soiuble ion
components of the soluble salts originating from a liquid and
absorbed into an ion-exchange layer can be displaced by means
of low currents coming from the electrode chambers. The
e'ectric resistance of the system caused by those displacing
ion streams is kept low, and therefore the necessary changes in
the electric poten~ial even in cases of very high
demineralization effects results in that only a small amount of
residual salt concentration remains. But it has been proven
. , .
;,;
1 3 1 02q~
that in cases of demineralization effects of such a kind the
degree of current efficiency, i.e., means the transformation of
electric equivalents into chemical ones, becomes constantly
lower because a relatively high displacing i.on current is
needed or the displacement of even smaller quantities of ionic
salt components.
Furthermore, processes are known, according to which
the ionic components of the salts dissolved in a liquid are led
a 6~1 o~
directly to ~he electrodes after absorption in~miXed
ion~exchange resins by an electric field wherein a partial
stream of the liquid being demineralized is used for rinsing
the electrodes. The disadvantages of this procedure are, most
of all, that volatile ion components, especially chlorides, are
discharged at the anode, and extremely corrosive and toxic
chlorine gas is liberated. There is even more chlorine
liberated, as higher amounts of salts are removed.
Furthermore, it is known that the ionic components
diffusing out of the mixed bed can be absorbed within
additional exchange masses, being arranged between the mixed
bed and the electrodes, and being diffused in parallel by
rinsing streams. At the same time, ~he electrodes, i.e., the
anodes and the cathodes, are washed by a third liquid stream
originating from the same supply pipe. This requires that not
only three waste water streams of a suitable quantity
, i, . . . ...
1 3 1 ~29~
with the corresponding heat loss, must be taken into account,
but ~hat this large number of waste water streams must be
arranged and adjusted to the whole mode of proc~dure.
A further disadvantage is that the migration of the
ions away from the electrodes is hindered by the exchange
masses being arranged between the mixed bed and the electrodes
because these exchange masses func~ion, with respect to the
moving ions, as oppositely charged screening barriers. This
has the effect of increasing the electrical resistance and
consequently the consumption of electrical enersy.
A further disadvantage of this known process of
desorption is that the absorption layer of mixed anion- and
cation-e~change masses has a high electric resistance because
the path of each single sort of ions is constantly blocked by
op~ositely-charged ions, therefore blocking exchange sites
during the desorption and movemer.t of the ions in the electric
field. All of the disadvantages of these processes become
aggravated the higher the amounts of salt admitted which have
to be removed.
It has now been discovered that the consumptlon of
energy is at its lowest level when the demineralization
process, working according to the displacement procedure, is
applied to a liquid having a high salt content. In other
words, the raw liquid, e.g., untreated water, is admitted,
--3--
1 31 3?9~
27543~2
and the residual salt content remains relakively high. Therefore,
only a partial demineralization is carried out. The procedures
according to the present method of desorption, have shown that an
optimal consumption of energy ls achieved only when a simultaneous
demineralization takes place down to a very low residual salt
content.
Here the invention undertakes the task of demineralizing
aqueous solutions without the use of regenerating chemicals,
thereby reducing a high degree of salt content of a solution by
means of ion displacement, and eliminating almost totally the
remaining residual salt content by means of desorption Imigration
of ions) with unblocked absorption layers, and keeping down the
energy consumption of both demineralization steps to an optimal
low level. This is undertaken by using only a single waste water
stream and coupling the two partial steps of the demineral1zation
process by an arrangement of barrier layers, and absorption media
as well as electrode chambers, thereby giving rise to a complete
demineralization process o~ which the electric resistance, and
thus also its total consumption of energy, is especially low.
There is herein also provided a device for the practice of the
process over a wide extent of variable through-puts, which is
practicable and reliable ln operatlon, and capable of maintaining
a constant output flow over a long period of time.
According to the invention, this function is technically
solved in that the fluld to be demineralized is submitted to a
pre-treatment for a partial demineralization by means of ion
--D2--
, '
1 3 1 029~
27543-2
displacement and ~o a post-treatment for a total demineralization
by means of ion desorption. Both treatments are connected into
one process.
Thus, according to one aspect, the invention provides a
process for demineraliziny an aqueous liquid containing salts,
comprising:
successively passing the liquid through alternatlng
separate layers of at least one means ~or exchanging cations and
at least one means for exchanging anions to obtain a substantially
demineralized liquid, said means for exchanging cations containing
labile hydrogen ions and said means ~or exchanging anions
containing labile hydroxyl ions;
applying electric energy be~ween an anode and a cathode
across the dlreotion of the flow of the liquid and through said
layered means, whereby catlons and anions disassociated from the
salts are transferred in opposite directions out of said layers
and toward the cathode and anode, respectively; and
flushing said cationæ and said anions.
According to another aspect, the invention provides a
process ~or de~ineralizing an aqueous llquid contalning salts,
comprising a displacement stage and a desorption stage;
said displacement stage comprislng:
alternatingly passing ~he liquld through at least one
means or exchanging cations and at least one means ~or exchanglng
anlons to obtain a partially demineralized li~uid, said cation-
exchange means containlng labile hydrogen ions and said anion-
1 3~ ~298
27S43-2
exchange means containing labile hydroxyl ions, whereby cations
and anions disassociated ~rom the salt3 are exchanged for the
labile hydrogen ions and ~he labile hydroxyl lons, respectively;
applying electrlc energy between a first anode and a
first cathode across the direction of flow of the liquld, whereby
hydrogen ions and hydroxyl ions migrating in opposite d~rections
are exchanged for the cations and anions, respectively, in the
means for exchanging ions, ~hereby restor:ing sald labile hydrogen
and hydroxyl ions to said ion-exchange means and allowing for the
displaced cations and anions ~o migrate in opposite direc~ions;
and
passing at least one first flushing solutlon across the
direction of the electric current capable of receiving the cations
migrating from said means for exchanging cations and the anlons
- mlgrating from said ~eans for exchanging anions, whereby salts are
formed and discharged with said first flushing solution; and
said desorptlon stage comprising:
successively passing the partially demineralized liquid
~rom said displacement stage through alternating separate layers
of at least one cation-exchange means and at least one anion-
exchange means to ohtain a substantially demineralized liquld;
applying electric energy between a second anode and a
second cathode across the direction of ~low of the liquid and
through said layered means, whereby residual catlons and anions
disassociated ~rom the salts are transferred ln opposite
-5a-
,~i~,;
1 3 1 ~29~
27543-2
directions ou~ of said layers and to~ard the cathode and the
anode, respectlvely; and
flushlng said catlons and said anions.
According to still another aspec~, the invention
provides an apparatus for demineralizing an aqueous liquid
contalning salts, comprisiny:
at least one anode compartment ~ith an anode therein;
at least one cathode compartment with a cathode therein;
at least one desorption compartment positloned between
sald anode and said cathode compartments; sald desorption
compartment containing alternating separate layers of at least one
cation-exchange means and at least one anion-exchange means, each
said lon-exchange means belng ad~acent to both said cathode and
said anod~ compartments;
~eans for introduaing and removing said aqueous liquld
~:: from said layered desorpt.ton comp`artment, wherein the liquid being
removed ls substantially demineralized;
me~ans for ~roviding selective permeation of anions :into
;said anlon compartment, said means separa~ing said layered
: desorption compartment ~ro~ said anode compartment and means for
:
providin:g selective permeation of cations into said catlon
: : :: :
compartment, said means~separating said desorption compartment
from said cathode compartment;
`
~ means for applyin~ an electrlc poten~al between said
~, ::
: ~ : anode and said cathode and across said layered desorption
5b-
D~
1 3 ~ Q2q8
275~3-2
compartment, whereby cations and anions disassociatad from the
sal~s ln said layered desorption compartment are transferred in
opposite directions ou~ of said layered desorption compartment and
toward the cathode and the anode, respectively; and
means for flushlny said cations and said anions.
According to yet another aspect, the invention provides
an apparatus for demineralizlng an aqueous liquid conta:Lning salts
which includes a displaceman~ stage and a desorption stage;
said displace~ent stage comprising:
at least one first anode compartment with an anode
therein;
at least one first cathode compartment with a cathode
therein;
at least one cation-exahange compartment containing a
cation-exchange means thereln;
at least one anion-exchangc com.partment containing an
anion-exchange means therein;
at least one first flushing compar~ment positioned
between said cation-exchange compartment and said anion-exchange
compartment, all of said foregoing compartments separated $ro~
:each other by means for providing selective permeation of ions;
means for introducing and removing the aqueous liquid
from each of said cation-exchange and anion-exchange co~partments,
wherein said liquid is partlally demineralized;
means for flushing said cations and said anions from
-5c-
' ~ ',;
1 3 ~ 02~
27543-~
said flushlng compartment; and
means for applying an electric potential between said
first anode and said first cathode and ac:ross said cation-exchange
means, said anion-exchange means, and said flushing compartment,
whereby cations and anions disassociated :Erom the salts migrate in
opposite direc~ions toward said first cathode and said first
anode, respectively; and
said desorption stage comprisin~,
at least one second anode compartment with an anode
therein;
at least one second cathode compartment with a cathode
therein;
at least one desorption compartment positioned between
said second anode and said second cathode compartments; said
desorption compartment containing alternating separate layers of
at least one cation-exchange means and at leas~ one anion-exchange
means, each said means being ad~acent to said sacond cathode and
said second anode compartments;
means for introducing said partially demineralized
liquid from sald displacement stage into said layered desorption
compartment and means for removing said liquid from said layered
desorption compartment, said outflo~ing liquid being substantially
demineralized;
means for provlding selective permeation of anions into
said second anode compartment, said means separatlng said layered
; -5~-
..
; ;,;
1 3 1 029~
27543-2
desorptio~ compartment from said second anode compartment and
means for providing selective permeation o~ cations into said
second cathode compartment, said means separating said layered
desorptlon compartment from said second cathode compartment,
means for applying an elec~ric pa,tential between said
second anode and said second cathode across said layered
desorption compartment, wherein residual anions and cations
disassociated from ~he salts in said layered desorption
compartment are transferred in opposite directions out of sald
layered compartment and toward said second anode and said second
cathode, respectlvely; and
means for flushing said cations and said anions from
said desorption stage.
~ ithin the area of the process of abæorption of the
ionlzable ~al~ components dissolved in the liquid, different ions
are produced in the electrode chambers separated from this liquid.
These ions are each fused in a stream with the fluld passing
through ~he respective absorption process. The diffusion or
movement of the ion streams by means of absorption processes are
influenced by an electric field in the form of an electrlc
potential. On their way to their respective processes of
a~sorption ~displacement procesæ), these ion streams pass boundary
layers~ which layers screen o~f these processes and the ion-
exchange masses involved therein from the respective eIectrode
chamber~ and cause within these masses an ion displacement such
-5e-
1 3 1 02q8
27543-2
that they expel the ions from the respective process of absorption
out of the liquid phase and ~he ion-exchange phase in a stream
with the direction of the liquid stream, and into another boundary~
layer. Of these, one boundary layer separates the cation-
absorption zone and the other boundary layer separates the anion- -
absorption zone, from the hrine zone commcn to the processes of
absorption. ~uch process conslsts of a cation- and an anion-
exchange phase. These ions migrating from the li~uid phase and
the ~on~exchange phase penetrate the boundary layer which
separates the respective brine zone from the re~pective process of
~ 20 :
:~ :
:::
:~ :
: ~: :
; -5f- :
::
1 3 1 02q~
27543-2
absorption. However, after having reached the brine zone these
ions are kept back in the brine zone by a boundary layer, which
layer screens off ~he process of absorption from differently-
charyed ions, whereby the different ions produced in the electrode
chambers and ~he streams formed by them are influenced by means of
the electric potential. Only one portion of the ionizable salt
components of the fluid being demineralized is removed into the
brine zone.
The fluid which is to be further demineralized i5 led to
a desorption zone in which it is again submitted to a special
process of desorption. This desorption zone is comprised of
alternating layers of cation- and anion-exchange masses in which
the res~dual components of the ionizable salts in the liquid being
treated are absorbed.
2~
-6-
, ,:',
~3~2q8
These are desorbed by means of an electric field in the form of
a potential which is produced be~ween the respective electrodes
contained in the electrode chambers. The anode chamber with
the respective anodetused as one of the electrode chambers for
the production of the displacement ion streams forms, together
with the desorption zone, a brine zone. This anode chamber is
separated from the brine zone by a cation-permeable
anion-blocking membrane. The brine zone is separated from the
desorption ~one by an anion-permeable cation-blocking
membrane. At the same time, a further ion stream, in addition
to the displacement ion stream, is produced at the anode in the
anode chamber and ~iffused into the brine zone adjacent to the
desorption zone se?arated from the cation-permeable membrane.
A cathode chamber with a cathode serves as the second electrode
chamber, and is separated from this desorption zone by a
cation-permeable anion-blocking membrane. A rinsing liquid
flows in sequence first through the brine zone adjacent to the
desorption zone, then through the cathode chamber adjacent to
the desorption zone and then through the central ~rine zone
common to the p~ocesses of absorption and desorption
(displacement zones) of the pre-treatment.
Thus, the ionic salt components originating from the
li~uid being trea~ed are washed out by desorption from the
region of the post-treatment, and by ion di~placement out of
, ~,.,
1 31 ~2~8
the region of the pre-treatment in the form of a single common
brine.
To practice this process, a device is used consisting
of at least one vessel with displacement chambers as well as a
desorption chamber arranged there.in for a liquid being treated
in these chambers. These chambers are equipped with pipes for
the supply and the discharge of the liquid, as well as for the
supply and the discharge of a brine to be removed from this
device.
According to the invention, this device is
characterized in that the displacement chambers and the
desorption chamber for the liquid to be treated are formed by
ion-exchange membranes as boundary layers. The displacement
chambers are filled with an ion-exchange mass each, and ~he
desorption chamber is filled with at least two layers of
exchange masses. Each ion-exchange membrane is permeable to
certain ions, but impermeable to oppositely-charged ions as
well as to the liquid. Cathode chambers are provided for the
cathodes ne.~t to the displacement chambers and to the
desorption chamber.
An anode chamber is provided for an anode inserted
nex~ to the displacement chambers as well as the desorption
chamber. One of the cathode chambers is adjacent to a
displacement chamber filled with an anion-exchange mass and the
--8--
a~:
1, ~
1 3 1 02~8
other cathode chamber is adjacent to a desorption chamber
filled with layers of cation- and anion-exchange masses. On
one side, the anode chamber is adjacent to the displacement
chamber being filled with a cation-exchange mass. To the other
side, the anode chamber forms with the desorption chamber a
brine chamber.
These chambers are connected with each other in a
selecti~e ion-permeable way by ion-exchange membranes designed
as selective barrier layers. Cation-permeable ion-exchange
membranes are provided for the displacement chamber containing
the cation-exchange mass, and anion-permeable ion-exchange
membranes are provided for the displacement chamber containing
t~e anion-exchange mass. A cation-permeable ion-exchange
membrane and an anion-permeable ion-exchange membrane are
provided to separate the desorption chamber from the cathode
and the brine chambers, respectively. The anode chamber is
formed on both sides by cation-permeable ion-eYchange
membranes. The brine chamber adjacent to the desorption
chamber is connected to the cathode chamber adjacent to the
desorp~ion chamber by at least one pipe. This cathode chamber
is connected by at least one pipe to the brine chamber provided
between the cation and anion displacement chambers.
Through absorptive and desorptive displacement, a
large portion of the ionic salts in the liquid are transferred
into a brine chamber being demineralized by ion streams
_g _
, ......
,
,; . ,
1 3 1 ~298
27543-2
originating from the electrodes and mlgrating within the electric
field. By desorption, the residual ionic components of the fluid
being demineralized in the desorption chamber filled with layers
of cation- and anion-exchange masses are transferred into an
adjacent brine chamber and a cathode chamb~er by means of a
potential.
In addition, the present invention also provides a
number of advantages in Cerms of energy and cost with respect to
process engineerlng and apparatus designing. These advantages are
in particular:
a) The introduction of the liquid containing salts which
has to be demineralized into the dlsplacement chambers and the
migration of ion streams from the electrodes in the electrode
chambers into the li~uid being treated result in a low electric
resistance for the migrating ionic ealt components. Therefore, an
especially low electric potential suffices for the transport of
the larges~ amount of the salts. The efficiency of the current,
or the transformation of an electric equivalent into a chemical
one, remains high because the a~ount of the displacement ion
streams is adjusted to only attain partial demineralization. The
advantage resulting from these two measures is that the
: : consumption of energy, for which the electric potential and the
intensity of the current are
-10-
1 3 1 ~2~8
important factors (as is already known), to eliminate the
largest amount of salts from the li~uid being demineralized
(e.g. 9S%) remains especially low.
b) The introduction of the liquid containing the
residual amount of salts into a desorption cham~er causes (by.
means of an electric po~ential) an almost complete removal of
the residual content of salts and therefore produces a liquid
having a high degree of purity.
c) An advantage of the process according to the
invention is that by layering cation- and anion-exchange masses
in the.desorp~ion chamber, both the cationic and the anionic
salt components of the liquid being demineralized can move
without being hindered even at a low electric potential.
Hence, the result is a very low consumption of energy even in
the case of an extremetly satisfactory purification effect.
d) A further advantage of the invention is that the
concentrated amounts of brine originating.from the desorption
chamber are transferred to the brine chamber between the
displacement chambers and there they lower the electric
resistance, thereby lowering the energy consumption for the
~e.-.oval of the largest portion of salts from the liquid bein~
demineralized.
1 3 1 0298
~75~3-2
A special advantage of the process according to the
invention is the passage of a single brine stream to receive both
the residual amount of sal~s originating from the desorptlon
chamber and the main amount of salts originatlng from the
displacement chambers. This advantage is effective in many
respects .
The amount of waste water is very low in relation to the
amount of sal~ elimlna~ed or the amount of product obtained. As
a result, the amount of waste water to the drainage and the
consumption of raw water, (e.g., in the case of water treatment)
are lower than in other known processes.
Since the amount of waste water is small, the loss of
heat of the whole system is low with respect to the recovery of
heat from the waste water. The use of a single waste water stream
simplifies the control and adiustment of the salt discharge of the
whole system and its operation, and the construction of the
apparatus becomes considérably less expensive.
f) Through the unhindered movement of the ions miyrating
from the anode into the brine chamber adjacent to the desorption
~; chamber and the direct movement of the desorbing cationic salt
components out of the desorption chamber and into the adjacent~
cathode chamberr the electric resistance is greatly lowered at the
stage of desorption, as is the
-12-
....
~ i,
,;. ;,
1 3 1 0298
- necessary driving electric potential and finally the
consump~ion of energy~
g) A fur~her advantage of the process according to
the invention is represented by the arrangement of a brine
chamber between a desorption chamber and an anode chamber,
thereby preventing the discharge of volatile anionic salt
oc~r ~nCe,
~- components at the anode, and therefore the ~e~E~ of
corrosive and toxic effects.
h) An advantage, especially for the construction of
the apparatus, is that both the cross stream of the
displacement ions produced for the demineralization in the
cationic displacement chamber, and the potential for
eliminating the residual content of salts are produced by one
and the same anode. This leads to a clear cost reduction for
the production of the device of ~he invention.
; Further advantageous developments of the invention in
o65e,~ve-~
regard to the production and the specific.device can be
particularly in view of the following Figures. In the
drawings, some examples o the design of the invention are
ill~strated schematically without the scope of the invention
being restricted thereto. These are:
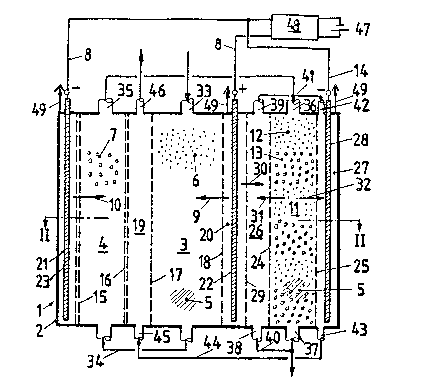
Fig. 1 shows a longitudinal mid-section through a
device according to the invention in plane I - I in Fig. 2.
Shown are two displacement chambers with a brine chamber lying
-13-
~ .J
1 3 1 029~
therebetween, a desorption chamber with its corresponding brine
chamber as well as a joint anode chamber adjacent to the
displacement chamber and to the desoxption chamber and a
ca~hode chamber adjacent to each, arranged in one vessel.
Fig. 2 shows a cross-section through the device in
plane II - II in Fig. 1.
The device 1 according to the invention is built in
accordance with the description of Figs. 1 and 2. It consists
of a vessel 2 for displacement chambers 3 and 4 provided
therein. In the latter chambers, processes of absorption and
desorption occur between a liguid being treated, ion-exchange
masses 6, 7 and ion streams 9, 10 coming from the electrodes
8. In addition, the process of desorption of the liquid being
treated takes place in the ion-exchange masses 12, 13 in a
potential ~ield formed between electrode 14 and one of the
electrodes 8.
The displacement chambers 3 and 4, of which at least
one displacement chamber 3 is provided in vessel 2 for the flow
of cations 9, and a further displacement chamber 4 is provided
for the f low of the anions lO, are each filled with an
ion-exchange mass 6, 7 and a fluid 5 being treated is
successively passed there through. The displacement chambers
3, 4, which are bordered by boundary layers in the form of
ion-exchange membranes 15 to 18, are in an arrangement of one
-14-
1 3 1 02q8
displacement chambex each, (e.g., chamber 3 for cations ~, and
chamber 4, for anions 10) between a joint brine chamber 19 and
the respective electrode chamber 20, 21. More specifica~ly,
the displacement chamber 3 for the cations 9 borders the
electrode chamber 20 for the anode 22, and the displacement
chamber 4 for the anions 10 borders the electrode chamber 21 of
the cathode 23. The ion-exchange membranes 15 to 18 between
these chambers 3/ 4 and ~0, 21 are designed in such a way, that
they each let through the ion streams 9, ].0 into the respecti~e
displacement chambers 3, 4, and therefrom into the brine.
chamber 19. These membranes, howe~er, block the passage of a
liquid 5 as well as the passage of oppositely-charged ior~. In
this arrangement, the hydrogen ions produced by the anoa~,~2 in
the anode chamber 20 are transported into the displacement~;
chamber 3 for the cations 9. The hydroxyl ions produced-by the
cathode 23 in the cathode chamber 21 are transported to the ion
stream 10 in the displacement chamber 4 for anions.
The desorption chamber 11, at least one being arranged
in vessel 2 is filled with ion-exchange masses 12, 13 in the
form of layers, through which laye~s the fluid 5 ~o be treated
is successively passed. The desorption chamjer 11, whic~ is
bordered on both sides by boundary layers in the form of`
ion-exchange membranes 24, 25, is arranged between a brine
chamber 26 and a cathode chamber 27 for the cathode 28. The
1 3 1 029~
anode chamber 20 for the anode 22 is joined to the brine
chamber 26 by a boundary layer in the form of an ion-excahnge
membrane 29. The ion-exchange membranes 24, 25, 29 between the
chambers 20, 26, 11 and 27 are designed in such a way that they
let each pass ion streams 30, 31 respectively into the brine -
chamber 26 and the ion stream 32 into the cathode chamber 27,
but block the passage of liquids (e.g., liquid s)~ as well 2S
the passage of oppositely-charged ions. In this arrangement
the cations 30 are the hydrogen ions produced at the anode 22
in the anode chamber 20, the anions migrating from the
desorption chamber ll into the brine chamber 26 are depicted as
the ion stream 31. The cations migrating ~rom the desorption
chamber ll into the cathode chamber 27 are depicted as the ion
stream 32.
The device l is conceived in a way that a supply pipe
33 for the liquid 5 being treated enters into the displacement
chamber 3 for the cations 9 te.g., at the.top). This chamber
is connected with a discharge pipe attached to said
displacement chamber at the bottom, and a connection 20 by a
boundary layer in form of an ion-exchange membrane 29. The ion
pipe 34 is connected, e.g., at the bottom, to the displacement
chamber 4 for anions lo. At the top end of this displacement
chamber 4 or anions 10 a discharge pipe 35 is attached,
~hrough which the liquid 5 being treated in the displacement
.
-16-
1 3 ~ 02~3
chambers 3, 4 is passed (e.g., the supply pipe 36 arranged on
top into the desorption chamber 11) or further treatment. At
the bottom end of this desorption chamber 11 is attached a
product pipe 37 through which the ~reated liquid 5 can be
collected. The brine chamber 26 is separated by ion-exchange
membranes 24, 29 from the desorption chamber ll on one side,
and from the anode chamber 20 on the other. This chamber 26 is
equipped with a discharge pipe 39 and a supply pipe 38 which is
attached to the product pipe 37 for the treated liquid for the
purpose of introducing a transporting fluid into brine chamber
26 by means of a connecting pipe 40. The discharge pipe 39 is
attached to the supply pipe 42 of the cathode chamber 27 by
means of the connection pipe 41 allowing for the transfer of
the transporting liquid discharged from the brine chamber 26
into the cathode chamber 27.
At the bottom end of the cathode chamber 27, there is
a drainage pipe 43 which is attached by means of a connecting
pipe 44 to the suppIy 45 of the brine chamber lS for the
purpose of introducing the transporting liquid discharged from
~e cathode chamber 27. The brine chamber l9 being separa~ed
at the sides by ~h~ ion-e~change membranes 16, 17 bound on one
side by the displacement chambers 3 for cations 9, and on the
o~he~ side by the displacement chamber 4 for anions lO. The
brine chamber l9 is equipped with a discharge pipe 46 which
-17-
~s
..~,,
.,, ;. ..
1 3 ~ 029~
is connected with a brine discharge no~ shown in the drawing.
The elec~rodes 22, 23, 28 entering the electrode
chambers 20, 21, 27 are connected to an electric power supply
47 of a direct current source by the co~nection of at least one
control and measurement system 48. Electrolytic gases formed
at the electrodes 22, 23, 28 are let o ~ of the device 1 by a
degassifier 49.
The treatment of liquid 5, or the demineralization of
the raw water, is carried out by a pre- and post-treatment
according to the process described in the invention. Each of
the treatments takes place in several steps. In the anode
chamber 20 of the device according to Figs. 1 and Fig. 2,
dilute sulfuric acid, which is a non-consuming medium, allows
the formation of hydrogen ions at the anode 22. This
corresponds to a direct current voltage applied at the
electrodes 22, 23 and at the electrodes 22, 28 and to the
respective electrical resistance between the electrodes.
Electric currents flow from the ampere meter
(ammeters) of a control and measuring circuit 48 through the
.luid 5 which are applied, between the electrodes 22, 23 of the
pre-treatment and between the electrodes 22, 28 in the
post-treatmen~. A hydrogen ion stream 9 coming from the anode
chamber 20, eyuivalent to the electric current between the
electrodes 22, 23, moves within the displacement chamber 3 in
-18-
, .
. .
~ 3 ~ 029~
the direction of the brine chamber 19. In doing so, it crosses
the stream of the liquid s and the ionic salt components
contained in the liquid 5 being from top to bottom through the
e~change mass 6 of the displacement cha~ber 3. The processes
of absorption and desorption occur after ba:Lances are attained
between the concentration of the ions in the aqueous phase and
in the exchange phase. The movement of all ions in the liquid
stream occurs vertically, and the electric potential acts
horizontally as the driving difference. In the course of this
movement, the cationic salt components of the liquid 5 being
treated are removed by the hydrogen ions of the ion stream 9 in
the direction of the brine cell 19, and are the new partners of
the anionic salt components of the fluid 5 being treated. The
quantity of hydrogen ion stream 9 is regulated by varying the
electric potential delivered to the electrodes 22, 23 in such a
way that not all (preferably only 90 to 95% of all) cationic
salt components are removed. This maintains a surplus of
hydrogen ions 9 as well as an equivalent low amount of electric
current to be spent between the electrodes 22, 23, and which
maintains a high degree of current efficiency. The same effect
is achieved if the quantity of flow of the fluid 5 being
treated is varied instead of the electric potential of the
electrodes 22, 23.
--19--
1 31 02~a
The liquid 5 leavirg the displacement chamber 3 is
freed to a large ex~ent of its cationic salt components and is
introduced into the displacement chamber 4 through the
connecting pipe 34 where it is crossed by the ion stream 10.
This ion stream 10 consists of hydroxyl ions which are formed
at the ca~hode 23 in the cathode chamber 21 filled with dilute
soda lye, and which is quantatively eguivalent to the electric
current between the electrodes 22, 23, and move in the
displacement chamber 4 into the direction of the brine chamber
19. Processes of absorption and desorption are also started in
the displacement chamber 4 which are analogous to the
processes in the displacement chamber 3. These processes start
in chamber 4 after balances are attained between the
concentrations of the ions in the agueous phase and in the
anion-exchange phase 7. The movement of the ions in the liquid
stream S occurs vertically as well, and the electric potential
acts horizontally as a driving difference.
In the course of these processes, the anionic salt
components of the liquid S being treated are removed by the
hydroxyl ions of the ion stream 10 in the direction of the
brine cell 19. The hydroxyl ions and the hydrogen ions which
migrated into the liquid 5 in the displacemènt chamber.3, form
water. The hydroxyl ion stream 10 is equivalent in amount to
the electric current between the electrodes 22, 23, and as in
the case of the cationic displacement results in the removal o
-20-
1 3 1 0298
only 90 to 95% of all anionic salt components from the liquid
5~ This results in a high degree of curren~ efficiency.
As the hydrogen ions 9 move more rapidly than the
hydroxyl ions 10 their concentrations, and thus their effect on
the displacement of the cationic 5alt components, are lower.
According to the invention, this fact is compensated for by a
longer residence time, i.e., by a thicker displacement chamber
3, compared to the anionic displacement chamber 4, as shown in
Fig. l and Fig. 2.
It has further been proved tha~ the blocking effect of
the ion-exchange membranes is not complete. In practice there
is permeability of counter ions of opposite charge, and up to
approximately 2% of the ion concentration to be blocked passes
through. This leads to the undesired migration of cations into
the displacement chamber 4. According to the invention,
therefore, this anionic displacement chamber 4 of the
pre-treatment stage is bordered by thicker or double membranes
15, 16 as is shown in Fig. l and Fig. 2.
The liquid 5 is demineralized to the largest extent in
~he displacement cha~'oer 4 and is then led into the desorption
chamber ll through the discharge pipe 35 and through the supply
pipe 36 for the post-treatment. In accordance to a direct
current voltage applied at the electrodes 22, 28 and in
accordance to the elsctriG resistance between these electrodes,
-21-
1 3 1 0298
an electric current and an equivalent ion s~ream 30 consisting
of hydrogen ions are formed at the anode 22. The stream of
hydrogen ions flows through the cation-permeable membrane 29
coming from the anode chamber 20, and passes into the brine
chamber 26. Simultaneously, the residual anionic salt
components of the li~uid 5 migra~e through the anion-exchange
layers 13 through the exchange layers 12, 13 having very good
electric conducti~ity, and then through the anion-permeable
membrane 24 in~o the brine chamber 26. The residual cationic
salt components migrate through the ca~ion-exchange layers 12
having a good electric conductivity as well, and then through
the cation-permeable membrane 25 into the cathode chamber 27.
The electric potential difference at the electrodes
22, 28 acts as the driving difference for the desorption and
movement of both types of ions. During the flow of the liquid
5 being treated through the exchange layers 12, 13 arranged in
several layers the processes described reoccur in each exchange
layer. The residual cationic salt components are only desorbed
by the cation exchange masses 12, and the anionic components
a.e only desorbed by the anion-e~change masses 13, in an
alternatin~ manner. This results In an unblocked path of low
electric resistance becoming available for ion movement and
displacement.
~ i
131029~
The treated liquid 5 leaves the device 1 as a
so-called product practica~y free of salts through the
discharge pipe 37 of the desorption chamber 11. A partial
stream of the treated liquid 5 is conducted through pipe 40 and
through the supply 38 into the brine chamber 26 (and flows
through this chamber from bottom to top) for the purpose of
brine washing. The residual anionic salt components being
desorbed out of the liquid 5 being treated flow through the
desorption chamber 11, are combined with the hydrogen ions 30
of the brine chamber 26 (balancing them electrically), are
washed,out through the discharge 39 and are then directed
through the connecting pipe 41 towards the cathode chamber 27.
The hydroxyl ions formed at the cathode 28 by a direct current
voltage being appIied in a well known manner form water with
the hydrogen ions 30 which are brought in with the rinsing
liguid from the brine chamber 26 into the cathode chamber ~7.
At the same time, the residual anionic sa,lt components being
brought in by the same rinsing liquid are electrically
compensated by the cationic stream 32 consisting of residual
cationic salt components coming from the desorption chamber 11
ar.d diffusing in through the cation-permeable membrane 25. The
brine being formed in this way in the cathode chamber 27 is
introduced into the brine chamber 19 for brine rinsing by means
of a discharge pipe 43 o the cathode chamber 27, through the
connecting pipe 44 and into
-23-
.
1 31 Q2~8
the supply pipe ~5. Therefore, the brine is more efficiently
used as a rinsing liquid. In brine chamber 19, the rinsing
liquid already containing the residual salt content from the
liquid 5 of the post-treatment flows from bottom to top, and in
so doing it absorbs the anionic and cationic salt components -
which are drawn out of the displacemen~ chaMbers 3, 4 and are
permeated into the brine chamber 19, further enriching its salt
content. The brine, now enriched to a high degree, now leaves
the device 1 through the discharge pipe 46 of the brine chamber
19, and arrives into a brine discharge not shown in the
drawings. Material balance calculations according to the
process of demineralization of the invention have shown that
the reuse of the rinsing liquid results in an important
increase of industrial efficiency.
-24-
.; ,,