Note: Descriptions are shown in the official language in which they were submitted.
` ~3~2~8
H~ECHST AKTIENGESELLSCHAFT Dr.SW/gm HOE 87/F 346
Description
A ~ethod for the aeration, ~ithou~ exit gases, o~ ~er-
~entation ~edia
The invention relates to a me~hod for the aeration, ~ith-
out exit gases, of fermentation media ~i~h oxygen or
oxygen-containing gases, the oxygen being transported by
means of fluid carrier media into the fermentation medium,
and there entering the fermentation med;um via the liquidl
liquid phase boundary, and subsequently the fluid carrier
med;um which has been depleted in oxygen and enriched in
carbon dioxide being again enriched with oxygen, without
emission of exit gas streams, with the rarbon dioxide
~hich is displaced thereby being desorbed and removed.
The cultivation of live cells, especially in large amounts,
is nowadays an essential constituent of the production of,
for example, antiviral substances such as interferon, or
of biologically active substances such as hormones. What
are called hybridoma cells - a fusion product of antibody-
producing cells with myeloma cells - are cultivated in
large amounts especially for the preparation of monoclonal
antibodies which have the ability ~o recogni~e very parti-
cular epitopes of a protein (of a macromolecule) and to
bind thereto.
In conventional laboratory methods for the cultivation of
cells, the latter are suspended in a medium in a Petri
dish. Oxygen and carbon dioxide diffuse through the sur-
face of the med;um into the cells. The depth of the cul-
ture medium influences the ra~e of diffusion of oxygen and
carbon dioxide to the cells. As the depth of the medium
increases, the ratio of the surface to the volume of the
medium decreases. At a particular time the concentrations
of oxygen and carbon dioxide in the air over the dish, and/
or the rate of dissolution in the medium, are insufficient
~ 3 ~
to supply the total volume of the medium with sufficient
gas and to satis~y the requirements of the growing cells,
i.e. ~he available oxygen becomes the factor limiting
growth. Accordingly, the state of the art teaches that
the depth of the medium ought to be in the region of mm
for static cultures. In general, the upper limit of cell
density for cultures of this type is about 105 cells/ml
of culture medium when the air ouer the culture medium
is used to supply oxygen.
When methods for the cultivation of cells are carried out
on the industrial scale it is difficult to make available
sufficient amounts of oxygen to satisfy the needs of the
cells~ Gases are frequently bubbled directly through the
medium in order to supply the cells with the required gas
concentrations. ~owever, direct bubbling through is un-
suitable especially for the cultivation of sensitive cells.
These cells are physically damaged when a gas is bubbled
through at a sufficient rate to mainta;n appropriate gas
concentrations in the medium, or by mechanical stirrers
because of the high shear stress. In addition, protein
constituents of the medium, uhich are normally necessary
in all cell cultures, form a foam, ~hich may trap the cells,
on the surface of the medium. The cells entrapped in the
foam rapidly die It is possible to add antifoam agents
to the medium but these are occasionally toxic for the
cells in the culture and, in addition, can be separated
from the product during working-up only with difficulty.
An elegant method of cultivating even sensitive cells on
the industrial scale and, at the same time, ensuring the
necessary supply of oxygen is the introduction of oxygen
into the culture medium by means of a flùid carrier medium.
It is known that, for example, fluor;nated hydrocarbons
are able to dissolve oxygen physically about 20 times
better than can water. Thus~ if such fluorinated hydro-
carbons are saturated with oxygen (or air) they can idealLybe used as a fluid carrier medium for transporting oxygen
into a culture medium. The oxygen then migrates across
-- ~3~
-- 3 --
the liquid/liquid phase boundary from the fluorinated
hydrocarbon ;nto the aqueous culture medium. The oxygen-
depleted fluor;nated hyclrocarbon can then, because it is
immiscible with water, easily be removed from the culture
medium, enriched with oxygen again and returned to the
culture medium. Methods of this type are described~ for
example, in the American Patent US 3,850,753. Th;s des-
cribes aerobic fermentation with simul~aneous aeration,
stirring and/or shaking in a system composed of water and
inert solvents immiscible with water, such as perfluoro-
C1-Czo-alkanes. S. ~ang, ~iotechnol. Lett. Vol. 7
(1985) pages 81-86 is concerned with the aerob;c fermen-
tation of E. coLi ~PP01 in the presence of perfluoromethyl-
decalin. The organic solvent is introduced through a
nozzle, and continuously removed from the system, in a
downward direction. No foam format;on occurs~ The use of
a polylysine-stabilized perfluor;nated hydrocarbon emul-
sion (FC-70, a tr;(C1s-perfluoroalkyl)am;ne, FC-77, a mix-
ture of C8F18 and cyclic C8F16O), which is utilized
as a microcarrier for the cultivation of adherent cells or
is employed as a blood substitute, is described in Science,
Vol. 219, pages 1448-1449 (1983). European Patent Appli-
cation EP 0,16~,813 describes a method for the cultivation
of animal or plant cells whose oxygen requirement during
fermentation is covered by an oxygen-saturated fluorinated
hydrocarbon solution. One of the two phases - ferment-
ation medium or fluid oxygen-carrier medium - can be oper-
ated continuously.
Despite all ~he advantages associated with the industrial
fermentation of live cells, there is one aspect which
should not be forgotten and which appears especially on
fermentation of cells manipulated by genetic engineering:
the exit air which is produced by such fermenters and which
may also contain small amounts of cellular mater;al. Ex-
treme care must be taken, especially on fermentation ofmicroorganisms wh;ch have been modif;ed by genetic engin-
; eering, to ensure that the exit air no longer contains
~ cellular material. The industrial elaboration reguired
~i2~
for this is at present great tsafety fermentation).
The object of the invention was to provide a fermenta~ic,nmethod in which both the supply of oxygen to the fermen-
tation medium is ensured in an optimum manner and, at the
same time, the requirements of safety fermentation (absence
of exit gas) are met in an economic manner.
This object is achieved by a method for introducing oxygen
into fermentation media with the aid of fluid carrier media
by transport of the oxygen into the fermentation Medium
across the liquid/Liquid phase boundary, where the oxygen
- which is removed by the fermentation medium in the oxygen-
depleted carrier medium is replenished in an aeration
device ~ithout emission of exit gas streams, and the carbon
dioxide which is formed cluring the fermentation and which
is likewise dissolved in the carr;er medium is partially
or completely desorbed and removed.
Fermentation media are defined as the generally customary,
usually aqueous, culture media which, besides the suspen-
ded cells, contain the nutrient med;um with suitable sour-
ces of carbon and nitrogen. E~ample~ of cells ~hich canbe cultivated are animal, plant or microbial cells, which
; can also be immobilized or encapsulated. Flu;d carrier
media are defined as those liquids which, at the least,
a) are only slightly ~iscible or, preferably, are com-
pletely immiscible with water
b) are able to bind oxygen reversibly better than can
~ater
c) are inert to the cells used,
and, furthermore, preferably also
d) have a higher specific gravity than the culture medium
(water)
2~
-- 5
e) at the same time are also able to bind carbon dioxide
reversib~y better than can waterO
Examples of suitable fluid carrier media are perfluorinated
straight-chain or branched C1-C2û-alkanes, perfluorin-
S atecl Cs-C14-cycloalkanes, perfluorinated tetrahydro~
furan, perfluorinated tetrahydropyran, perfluorinated
adamantane and the perfluors-Cs-C14-cycloalkanes, per
fluorotetrahydrofurans, perfluorotetrahydropyrans and
perfluoroadamantanes substituted by C1-Cs-perfluoroalkyl,
as well as perfLuoro-C1-C20-alkoxy compounds and per-
fluorinated polyethers such as C10F222 ( ~ HOSTINERT
130), C13F2gO3 (HOSTINERT 175), C16F3404 (HOSTINERT
216) and Cz2F4606 (HOSTINERT 272, manufactured by
Hoechst AG).
Examples which may be mentioned are:
perfluorinated derivatives of heptane, octane, nonane, tri-
butylamine, N-methylmorpholine, 1- methyldecalin, deca-
lin, naphthalene, methyldecalin, methylnaphthalene, 2-
butyl-, 2-pentyl-, Z-hexyl- and 2-heptylfuran, and 2-butyl-,
Z-pentyl-, 2-hexyl or Z-heptyltetrahydrofuran.
However, also suitable are silicones such as dimethyl- or
phenylmethylsilicones, polyvinylpyrrolidones or those sub-
stances which are not toxic to the cells and can be used
as blood substitutes (see DMW No. 35, volume 105, Stuttgart,
Aug. 29, 1980~ pages 1197-1198 and J. Fluorine Chemistry~
9 t1977), 137-146).
Exchange of gases between the fer0entation media and the
oxygen-carrier medium takes place across the liquid/
liquid phase boundary. It has been demonstrated in this
connection that the oxygen-transfer rate is considerably
higher than in the case ~f a gas/liquid phase transfer
such as takes place, for example, when oxygen tair) is
bubbled through the fermentation medium. The release of
oxygen to the fermentation medium takes place simultaneouslY
~L3~2~
,, ,
-- 6
with uptake of carbon dioxide by the carrier medium be-
rause, usually, carbon dioxide is also more ~physically)
soluble in the oxygen-carr;er medium than in the aqueous
culture broth.
The best procedure for the method according to the inven-
tion is such that, for example, the fluid carrier medium
is treated with oxygen or air or an oxygen/air mixture in
a bubble column. The fluid carrier medium ~hich has been
saturated ~ith gas in this way is now contac~ed with the
culture medium, when the exchange of gases described above
takes place. This can be carried out in such a way, for
example, that the medium which has a higher specific gra-
vity is allowed to drip in a down~ard direction through a
column of liquid medium with a lower specific gravity, or
else the medium which has a lower specific gravity is
allowed to rise in an upward direction through the medium
which has a higher specific gravity. In both cases the
phases separate again so that the oxygen-depleted and
carbon dioxide-enriched fluid carrier medium can be drawn
off and passed to the bubble reactor, where the physically
dissolved carbon dioxide is driven off by the excess oxy-
gen (air) bubbling through, and the fluid carrier medium
is again enriched with oxygen. It is essential to the
invention that the carbon dioxide which is driven off is
dra~n o~f together with excess oxygen (air3 from the gas
chamber of the bubble column, and the carbon dioxide is
completely or partially chemically or physically desorbed~
This can be carried out, for example, by scrubbing with an
alkali metal hydroxide solution, ~hen solid alkali metal
carbonate is formed. The residual gas (~ainLy composed of
oxygen) is, according to the invention, returned to the
bubble column. This completes the gas circulation, and
no exit gas is able to escape. An advantage of this method
is that it is necessary tv replenish only the oxygen actu-
ally consumed by the culture medium. Another advantage ofthe method is that it is possible, by means of a bypass
around the carbon dioxide scrubber, to control the carbon
dioxide concentration in the gas and thus in the liquid
;
~3~25~
., .
-- 7
carrier medium and thus, in turn, in the culture medium,
which makes it possible to control the pH accurately~
A further advantage of the method according to the inven-
tion is that both the fermentation solution and the fluid
carrier medium can be operated continuously, i.e. it is
possible during the fermentation process both for the
aqueous culture medium to be continuously rene~ed and -
where appropriate with a stationary ~ermentation medium -
for the fluid carrier medium to be continuously regener-
1û ated. The relevant phase - the fermentation medium and/or
the fluid carrier medium - is accordingly designated the
"continuous phase". In the case where the fluid carrier
medium is dripped through a column of the liquid fermen-
tation mediu~ (irrespective of whether the fluid carrier
medium now has a lower or higher specific gravity than the
fermentation medium), it suffices merely to introduce the
oxygen-carrier medium in order to bring about adequate mix-
ing of the fermentation medium. However, it is also pos-
sible, where appropriate, to mix the fermentation medium
by the ~se of suitable conducting devic~s in the fermenter
or by an external cycling (c;rculation).
A preferred embodiment of the method can be carried out
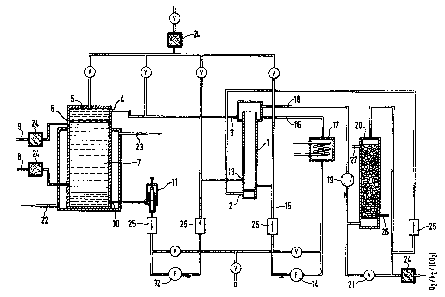
with the device depicted in F;gure 1. A fluid oxygen-
carrier medium, which has a higher specific gravity ~han
waterO for example a fluorinated hydrocarbon, ;s treated
in a bubble column ~1), via a supply line (2) with a gas
mixture containing mainly oxygen. The oxysen-enriched
fluorinated hydrocarbon is taken off via a discharge line
t3) and passed via an opening (4) into a fermenter t5)
whose temperature can be controlled. The oxygen-enriched
fluorinated hydrocarbon is dripped through a perforated
plate (6) (diameter of the perforations bet~een 0.5 and
~ mm) into the aqueous culture medium (7) which can, where
appropriate, be operated continuously via the supply (8)
and discharge (9) lines. ~hile the fluorinated hydro-
carbon, which has a higher speci~ic gravity, is dripping
do~n ;t releases oxygen to the aqueous culture medium and,
~2~6~
. .
-- 8 --
at the same time, is enriched ~ith carbon dioxide from the
culture medium. The fluorinated hydrocarbon which has
been depleted/enriched in this way is taken off via an
opening (10) and returned via a leveling loop t11) (which
controls the level of liquid fluorinated hydrocarbon in
the fermenter) and a pump (12) through the opening (13) to
the bubble column. A circula~ing pump (14) provides~ in
conjunction with the suppLy and discharge lines (15) and
(16), for thorough mixing of the fluorinated hydrocarbon
in the bubble column. The heat exchanger (17) included in
the circulation adjusts the temperature of the fluid car-
rier medium to correspond to that of the fermentation.
The excess pressure of oxygen, which continuously prevails
in the bubble column, drives out the carbon dioxide which
is present physically dissolved in the recycled, oxygen-
depleted fluorinated hydrocarbon. At the same time, the
fluorinated hydrocarbon is again enriched with oxygen.
The exit gas, which mainly contains carbon diox;de and
excess oxygen (air), is taken off v;a a line (18) and
passed by means of a bLower (19) ts a carbon dioxide
scrubber (20) which is filled, for example, with sodium
hydroxide soLution.
The carbon dioxide is precipitated here as a carbonate, and
the gas which now mainly contains only oxygen (air) is
returned v;a the supply line (2) to the bubble column. A
def;ned carbon dioxide concentrat;on in the system can be
provided for by a bypass (21) around the carbon dioxide
scrubber, by ~hich means it is possible to control the pH
in the fermenter.
The sterile system is charged with the fluid carrier med-
ium via the connector (a) with simultaneous opening of the
valves (v). The valves tv) are closed during the fermen-
tation. The temperature of the fermenter (5) can be con-
trolled via the supply and discharge lines (22) and ~Z3).
((24) = sterile filter, (25) - flo~ meter, (26), ~27) =
supply and discharge lines, respectively, for charging/
emptying the carbon d;oxide scrubber).
-` ~L3~2~
_ 9
Ex~ples
The apparatus depicted in Figure 1 was used for aerobic
fermentations with per~luorinated hydrocarbons as oxygen
carriers~
5 Fermenter tS) : diameter 220 mm
height 220 mm
Perforated disk (6) : material: stainless steel
diameter of perforations 1 mm
Gas flow through the bubble colu~n: 100 - 500 l/h
Oxygen carrier:
Hostinert 216
Molecular mass: 902 g/mol; boiling point: 216C; surface
tension (25C)~ 15.4 mN/m; dynamic viscosity: 8.78 mPa s
(20C); mlO2/100 ml: 51.4 (25C); density: 1.839 kg/l
(20C).
Hostinert 175
Molecular mass: 736 g/mol; boiling point: 175C; surface
tension ~25C) 14~6 mN/m; dynamic viscosity: 4~39 mPa-s;
(2ûC~ mlO2/100 ml: 57 (25C); density: 1.816 kg/l
(20C)
Hostinert circulation flou I: (16)-(14)-(15) ~see Figure I]
180 l/h
Hostinert circulation flow II: (4)-(6)-t1û) [see Figure I]
20-150 l/h.
A) GeneraL infor~ation on the ~ethod
The sterilized system and the reactor (see Figure 1) are
charged with sterile fluid carrier medium in accordance
with the setting on the leveling looP, the temperature is
controlled, and aeration is carried out in the bubble
column with nitrogen/oxygen mixtures (0 to 100% 2, 100
to 0X Nz) and with addition of carbon dioxide. The reac-
tor is subsequently charged with 10 l of nutrient solution,
the temperature is controlled, and homogeneous mixing and
~` ~3~ 2~
-- 10 --
oxygen enrichment are carried ou~ via the flow of Hostinert
(~t-6-10). The fermenter is inoculated with the preculture
and, during the fermentation, parameters such as pH, and
concentrations of substrate and product are monitored and
controlled. The dissolved oxygen content is controlled
via the flow of Hostinert (4-6-10). The carbon dioxide
formed during the fermentation can be entirely or partially
removed in the scrubber ~20), and the consumed oxygen can
be replenished.
Ex~mple 1
Fermentation of Escherichia coli K 12
Nutrient medium: 0.8 % meat peptones (trypsin digest)
û.8 % casein peptone (trypsin digest)
0.3 % yeast extract
0.5 % sod;um chloride
1 ~ glucose
Temperature: 37C; pH: 7.0
The fermentation was carried out in the manner described
in section A. The fluid carrier medium used was Hostinert
216, which is saturated with air in the bubble column.
The fermentation medium is inoculated with 100 ml of pre-
culture, and the oxygen supply is ensured by introducing
Hostinert 216 (60 l/h). The Hostinert flow is increased
up to 150 l per hour as appropriate for the growth of the
cells in the exponential phase of growth, with the satur-
ation of the disperse phase being carried out with a nitro-
gen/oxygen mixture.
A generation time of 30 minutes is achieved in the expo-
nential phase of growth, with a dry mass content of 0.32
g/l at the end of the exponential phase.
~3~L2~68
1 1 -
Exa~ple 2
Fermen~ation of bakers' yeast (Saccharomyces cerevisiae)
Synthetic medium containing 1 % glucose and
2.48 g/l (NH4)2HP04
0.4 g/l MgS04 . 7 H20
0.2 g/l KCl
0.12 g/l CaCl2 . 2 H20
20 mg/l NaCl
20 mg/l m-inositol
1.2 mg/l Ca pantothenate
0.8 mg/l H3~03
û.4 mg~l ZnS04 . 7 H20
0.4 mg/l MnS04 . 7 H20
O.Z mg/l FeCl3 . 6 ~l2
0.2 mg/l Na2Moo4 . 2 ~l2
0.12 mg/l NaI
0.04 mg/l CuS04 . 5 H20
0.02 mg/l biot;n.
The temperature of the fermentation solution and the
~ Host;nert 216 is controlled at 30C, and the PH ;s
maintained constant at S.O during the fermentation by addi-
tion of 1 normal sodium hydroxide solution~ ~fter inocu-
lation with 100 ml of preculture (24 hours old, synthetic
medium containing 1 X glucose), the Hostinert flow through
the reactor is adjusted from ~ l/h to 120 l/h, and the
fermentation is operated continuously u;th a dilution rate
of D = 1 h 1 (inflow rate f = 1 l/h, connectors t8) and
(9)). Once equilibrium has been reached the biomass
amounts to 4.5 g/l.
Exa~p~e 3
Hybridoma cells are microencapsulated as in Example 11 of
German Patent Application P 37 060 10.4, to which express
reference is made at this pointJ
~3~ 2~
- 12 -
The reactor is charged with Dulbecco's medium, the tempera-
ture is controlled at 37C, and the fermentation solu-
tion is enriched with oxygen using ~ Hostinert 175 which
is saturated with a nitrogen/oxygen t1:1) mixture contain-
ing 5 ~ carbon dioxide. The fermentat;on medium is inocu-
Lated with 10 ml of capsules (capsule diameter 500 ~m;
104 capsules) per liter, and the medium is changed dis-
continuously during the fermentation.
Day Cell count per mlug of antibody per ml
reactor volumereactor volume
_ __ _
3 2 x 105 8
4* 4 x 105 20
9.6 x 105 30
8* 7 x 105 S0
1510 1.7 x 106 110
13 1.û4 x 106 90
* The medium was changed on these days.
The antibody content (mouse IgG) in X by weight was deter-
mined using an enzyme immunoassay (Behring ~erke AG,
Marburg).