Note: Descriptions are shown in the official language in which they were submitted.
- I - 1 3 ~ 4 2 9 1
5-1625611+2/=lZFO
Optically active tetrahydro-2-furanone derivative as microbicide
The present invention relates to optically active (aS,l'R)-3-[N-
(methoxyacetyl~-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydro-
furan-2-one, to the preparation thereof, to microbicidal co~po-
sitions which contain this compound as active component, and to theuse of such compositions as microbicides in plant protection.
3-[N-(Methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)7aminotetra-
hydrofuran-2-one is disclosed as a microbicidally active compound in
German Offenlegungsschrift 2 804 299 or in the corresponding
GB patent specification 1 577 702. According to this publication,
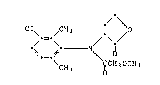
the compound of formula I
/ \
Cl\ /CH3 1* O
' ~ ` ~ `a/ (I),
CH3 ~CH2OCH3
has, as structural feature, a centre of asymmetry at the position
marked by an asterisk. In the form of the racemate, thls compound
can be resolved into the optical antipodes in conventional manner,
the different configurations having microbicidal activity of
different strength.
The publication r~ferred to above gives no indication as to how
great this difference in activity may be, nor does it say which of
the two antipodes actually has the greater micro~icidal activity.
- 2 - 1 31 4 29 1
Furthermore, the above publication makes no reference, within the
broad chemical scope thereof, to further possibilities of isomerism
beyond the furanone ring.
However, the compound of formula I conventionally obtainable as
racemate exists not only in the form of a single pair of enantiomers
by virtue of the asymmetric substitution at the lndicated *C-atom,
but as a mixture of 4 isomers, i.e. of two diastereoisomeric pairs
of enantiomers.
The reason is that the molecule, in addition to containing the
aforementioned centre of asymmetry, has a rotation isomerism
(atropisomerism) contingent on the chiral axis ( ~
and caused by the asymmetric chlorine-substitution of the 2,6-di-
phenyl ring in which rotation is hindered. Racemic 3-[N-(methoxy-
acetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one,
named as compound 30 in German Offenlegungsschrift 2 804 299, is a
1:1 mixture consisting of two diastereoisomeric pairs of enantiomers
which melt at 140~C and 115C respectively and which can be separ-
ated from each other. A suitable method of separation is adsorption
chromatography. Both pairs of enantiomers, as racemates, can be
resolved into the optical antipodes in conventional manner. The
(aS,l'S) and (aR,l'R) enantiomers of 3-[N-methoxacetyl~-N-(3-
chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one can be
isolated from the pair of (aS,l'S~(aR,1'R) enantiomers which melts
at 140C and is obtained as by-product, and the (aR,l'S) and
(aS,l'R)-3 enantiomers of 3-~N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)]aminotetrahydrofuran-2-one can be isolated from the
desired pair of (aR,l'S)(aS,l'R) enantiomers which melts at 11~C,
for example by chromatography on an optically active phase.
The approximate 1:1 composition of the diastereoisomeric pairs of
enantiomers obtainable according to German Offenlegungsschrift
2 8Q4 299 varies from 45 to 55 %.
1 31~291
-- 3 --
It has now been found that the (aS,l'R) enantiomer of 3-~N-(me-
thoxyacetal)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydro-
furan-2-one has a surprisingly strongly enhanced microbicidal
activity in conjunction with an unexpectedly long duration of action
compared with the mixture of isomers of formula I.
Accordingly, the present invention relates also to a compound of
formula I in which the proportion of (aS,l'R) enantiomer is greater
than the 25 % by weight to be theoretically expected of a racemic
mixture of two diastereoisomeric pairs of enantiomers. In 8 narrower
sense, the present invention relates to a compound of formula I in
which the proportion of (aS,l'R) enantiomer is at least 28 % by
weight, preferably at least 40 % by weight and, most preferably, at
least 50 % by weight. Most especially preferred for practical
purposes as plant fungicide is a compound of formula I in which the
proportion of (aS,l'R)enantiomer is at least 70 % by weight, in
particular at least 90 % by weight. The invention further relates to
the pure (aS,l'R) enantiomer of 3-1N-(methoxyacetylj-N~(3-chloro-
2,6-dimethylphenyl)]aminotetrahydrofuran-2-one.
Racemic (aR,1'S)(aS,l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)]aminotetrahydrofuran-2-one also exhibits substantially
increased microbicidal activity and duration of action compared with
the mlxture of isomers of formula I, the extent of said increase in
activity being smaller than that oF the (aS,1'R) enantiomer.
The pair of ~aR,l'S)(aS,l'R) enantiomers in an approximate ratio o~
1:1 of these two components (45:55 to 55:45), is particularly
suitable for use as plant fungicide~
Hence the present invention also relates to the racemic mixture of
(aR,l'S) and (aS,l'R) isomers.
~31~291
-- 4 --
The preparation of the (aS,l'R) enantiomer of 3-[N-(methoxyacetyl)-
N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one can be
effected by a two-step chromatographic separation of the mixture of
isomers of formula I and of (aR,l'S)(aS,l'R)-3-EN-tmethoxyacetyl)-
~-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one obtained
therefrom which melts at 115C.
The first step of the separation of the racemic mixture of two
diastereoisomeric pairs of enantiomers takes place on a stationary
solid phase using a liquid phase (eluant) to giV2 the desired pair
of (aR,l'S)(aS,l'R) enantiomers. For this separation step it is
possible to use all adsorbing materials known to the skilled person,
e.g. silica gel, Al203, or organic polymer resins ~polyamide,
polyacrylamide and the like), as well as agarose, sepharose and, in
particular, modified cellulose. Acetylated or benzoylated cellulose
is especially suitable. The pair of (aR,l'S)(aS,l'R) enantiomers
can then be used as obtained in formulated form as plant fungicide
or can be further resolved.
The second separation step comprises separating the solution of the
racemic mixture of (aR,l'S)- and (aS,l'R)-3-[N-(methoxyacetyl)-N-(3-
chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one into the
individual enantiomers on an optically active phase and isolating
(aS,l'R)-3-lN-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]ami-
notetrahydrofuran-2-one from the solution.
Acylated cellulose such as acetylated or benzoylated cellulose can
be used for example as optically active phase. Examples are tri-
acetyl cellulose and tris(3-methylbenzoyl)cellulose.
In a preferred variant of the above described method of separation,
all four enantiomers of the mixture of isomers obtainable according
to German Offenlegungsschrift 2 ~04 299 can be separated in only one
step instead of two steps. In this variant, the dilute solution of
the mixture to be separated in a 1:1 mixture of hexane/isopropanol
is adsorbed on to modified cellulose as optically active phase and
1314291
eluted in succession with a suitable mobile phase (eluant). The
solution is preferably adsorbed on to acylated cellulose (e.g.
acetylated or benzoylated cellulose), preferably tris(3-methyl-
ben~oyl)cellulose, as stationary phase, and subsequently eluted with
a 1:1 to 9:1 mixture of hexane/isopropanol. The solutions of the
eluted isomers are collected in fractions and the isomers are
isolated as residue after evaporation of the mixture of solvents.
In another preferred variant of the preparatory process, the pair of
(aS,l'S)(aR,l'R) enantiomers which melts at 140C and is obtainable
as by-product after separation of the mixture of isomers can be
racemised once more to the starting mixture. Such racemisation or
isomerisation can be effected for example by heating the pair of
(aS,l'S)(aR,l'R) enantiomers to 140-180C in the melt, preferably
in the presence of a tetraalkylammonium salt, e.g. tetrabutyl-
ammonium bromide, until equilibrium is attained. The resultant
product, which again consists of two pairs of diastereoisomeric
enantiomers, can be recycled to the separation process. The present
invention also relates to this additional reisomerisation process.
The racemisation of (aR,1'S)-3-[~-(methoxyacetyl~-N-(3-chloro-2,6-
dimethylphenyl)]aminotetrahydrofuran-2-one by opening the lactone
ring with alkali and by the subsequent cyclisation under suitable
conditions is also possible. The racemate obtained by this pro-
cedure can also be recycled to the separation process.
The absolute configuration of the enantiomers was determined by
X-ray structural analysis and can be illustrated as follows:
/ \
~CH3 H.
(aS,l'S) enantiomer) ~
=~ C0-CH2-U-CH3
C~ \CH3
- 6 - 1 31 4 29 1
! =o
(aR,l'R) enantiomer)
C~-CH2-O-CH 3
CH3
C~ /CH3 H.,i _ ! o
(aR,l'S) enantiomer) ~
\CH 3 CO - CH 2 - O - CH 3
/CH3 H~-
(aS,l'R) enantiomer) ~
=-\ CO-CH2-O-CH3
C~ CH3
The invention also relates to a process for obtaining (aR,l'R)-
(aS,l'R)-3-[N-(methoxyacetyl~-N-(3-chloro-2,6-dimethylphenyl)]ami-
notetrahydrofuran-2-one, which has also a substantially greater
fungicidal activity than the racemate disclosed in German Offen-
legungsschrift 2 804 2~. This pair of diastereoisomers in a ratio
of 1:1 or approximately 1:1 (45:55 to 55:45) is an especially
suitable plant fungicide, and it likewise constitutes a further
object of this invention.
In the preparatory process of this invention, a combination of a) a
method of separating two isomers of an intermediate and b) a
subsequent chlorination process is used in accordance with the
following scheme:
, 131~2~1
N~ separation / H3 H~ ~
~=~\ \ [e.g. chromatography] =~\ CO-CH2-O-CH3
CH3 CO-CH2-O-CH3 CH3
(racemats) (l'R~ enantiomer
~ - ~ H~-
Cl2 /
b) (l'R) enantiomer ~ (aR,l'R) enantiomer
~ - N\ - =0
C~CH2 I}CH3
Cl CH3
(aS,1'R) enantiomer
The mixture of (aR,l'R) and (aS,l'R) enantiomers resulting from the
chlorination can be used as obtained as fungicide or separated in
turn by chromatography to obtain the (aS,l'R) enantiomer by the
method of the initial separation step a).
Racemic 3-[N-methoxyacetyl)-N-(2,6-dimethylphenl)]aminotetrahydro-
furan-2-one used as starting material is also disclosed in German
Offenlegungsschrift 2 804 299 or GB patent specification 1 577 702.
For the above described chromatographic separation step a) it is
possible to use all adsorbing materials known to the skilled person,
e.g. silica gel, Al203, or organic polymer resins (polyamide,
polyacrylamide and the like), and also agarose, sepharose and, in
particular, modified cellulose. Acetylated or benzoylated cellulose
is especially suitable.
131~2~1
The chlorination of (1'R)-3-N-(methoxyacetyl)-N-(2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one is conveniently carried out in an
inert solvent. Suitable solvents are chlorinated aromatic and
aliphatic hydrocarbons such as chlorobenzene, methylene chloride,
chloroform, carbon tetrachloride and the like. It is preferred to
use a lower alkanecarboxylic acld, e.g. formic acid, acetic acid or
propionic acid. A preferred solvent is formic acid.
The chlorination is preferably carried out in the temperature range
from 0 to 50C, e.g. at room temperature.
It is also useful to carry out the chlorination in the presence of a
Lewis acid such as aluminium chloride, zinc chloride, iron(IlI)
chloride or -tin(IV) chloride. A preferred Lewis acid is iron~III)
chloride. The Lewis acid is used in an amount of 1-8 ~O~ based on the
~l'R) isomer to be chlorinated. The chlorination can also be carried
out without a Lewis acid. The chlorination is carried out under
normal pressure or slightly elevated pressure. Sulfuryl chloride is
used for the chlorination; but chlorine is the preferred chlorina-
ting agent.
Process a) for separating the ~l'R) enantiomer from racemic 3-[N-
(methoxyacetyl)-N-(2,6-dimethylphenyl)]aminotetrahydrofuran-2-one on
a solid phase (adsorption chromatography) and b~ the chlorination to
give a mixture of (aR,l'R)(aS,1'R) isomers (atropisomers) results in
a high yield of an active component that exhibits a fungicidal
activity of the same order of magnitude as the highly active
(aS,l'R)-3-[N-(methoxyacetyl)-N-(2,6-dimethylphenyl)laminotetra-
hydrofuran-2-one.
It has been found that, in particular, (aS,l'R)-3-[N-methoxy-
acetyl)-N-(2,6-dimethylphenyl)~aminotetrahydrofuran-2-one, and also
racemic (aR,l'S)(aS,l'R)-3-lN-(methoxyacetyl)-N-(2,6-dimethyl-
phenyl)~aminotetrahydrofuran-2-one and the mixture of (aR,l'R) and
(aS,1'R) isomers of 3-[N-methoxyacetyl)-N-(2,6-dimethylphenl)~-
aminotetrahydrofuran-2-one are greatly superior in microbicidal
131~2~
activity to the racemate existing in four isomeric forms disclosed
in German Offenlegungsschrift 2 804 299. These two mi~tures of
isomers and the (aS,l'R) enantiomer each have, for practical
purposes, 8 very useful microbicidal spectrum for protecting
cultivated plants without damage being caused to them by undesirable
side-effects. Examples of cultivated plants within the scope of this
invention are typically: cereals, maize, rice, vegetables, sugar
beet, soya beans, ground nuts, fruits trees, ornamentals, and
especially vines, hops, cucumber plants (cucumbers, marrows,
melons), solanacea such as potatoes, tobacco and tomatoes, as well
as banana, cocoa and natural rubber plants.
The (aS,l'R) enantio~er or the aforementioned mixtures of isomers
thereof are able to inhibit or destroy fungi occurring on plants or
parts of plants (fruit, b~ossoms, foliage, stems, tubers, roots) of
these and related crops of useful plants, while at the same time the
parts of plants that grow later are protected from attack by such
fungi. The compounds are effective against the pathogenic fungi
belonging to the following classes of fungi: Ascomycetes (e.g.
Erysiphaceae); Basidiomycetes such as especially rust fungi; Fungi
imperfecti (e.g. Moniliales; and especially against the Oomycetes
belonging to the class of Phycomycetes, e.g. Phytophthora, Perono-
spora, Pseudoperonospora, Pythium or Plasmopara. The isomers also
act systemically. They can further be used as seed dressing agents
for treating seeds (fruit, tubers, grains) and plant cuttings to
protect these from attack by fungal infections as well as from
phytopathogenic fungi that occur in the soil.
Target crops to be protected within the scope of the present
invention comprise e.g. the following species of plants:
cereals (wheat, barley, rye, oats, rice, sorghum and related crops),
beet (carrots, sugar beet and fodder beet), pomes, drupes and soft
fruit (apples, pears, plums, peaches, almonds, cherries, straw-
berries, raspberries and blackberries), leguminous plants (beans,
lentils, peas, soybeans), oil plants (rape, mustard, poppy, olives,
sunflowers, coconut, castor oil plants, cocoa beans, groundnuts),
- lO - 1 31 ~ 2 ~
cucumber plants (cucumber, marrows, melons), fibre plants (cotton,
flax, hemp, jute), citrus fruit (oranges, lsmons, grapefruit,
mandarins), vegetables (spinach, lettuce, asparagus, cabbages,
carrots, onions, tomatoes, potatoes, paprika), lauraceae (avocados,
cinnamon, camphor), or maize, tobacco, nuts, coffee, sugar cane,
tea, vines, chestnuts, hops, bananas, pineapples, grass (e.g. on
golf-courses) and hay. This recitation constitutes no limitation.
The compounds of this invention are normally applied in the form of
compositions and can be applied to the crop area, plant or sub-
strate to be treated, simultaneously or in succession, with further
compounds. These further compounds can be both fertilisers or
micronutrient donors or other substances that influence plant
growth. They can also be selective herbicides, insecticides,
fungicides, bactericides, nematicides, mollusicides or mixtures of
several of these substances if desired together with further
carriers, surfactants or application promoting adjuvants custom-
arily employed in the art of formulation.
Suitable carriers and adjuvants can be solid or liquid and corres-
pond to the substances ordinarily employed in formulation tech-
nology, e.g. natural or regenerated mineral substances, solvents,
dispersants, wetting agents, tackifiers, thickeners, binders or
fertilisers. Especially useful adjuvants are phospholipids.
A preferred method of applying a compound of this invention, or an
agrochemical composition which contains at least one of said
compounds, is foliar application. The number of applications and the
rate of application depend on the risk of infestation by the
corresponding pathogen (species of fungus). However, the compounds
can also penetrate the plant through the roots via the soil (sys-
temic action) by drenching the locus of the plant with a liquid
formulation, or by applying the compounds in solid form to the soil,
e.g. in granular form (soil application). The compounds may also be
applied to seeds (coating) by impregnating the seeds either with a
liquid formulation containing a compound of the invention, or
13142~1
coating them with a solid formulation. In special cases, further
types of application are also possible, e.g. selective treatment of
the plant stems or buds.
The compounds of the invention are used in unmodifled form or,
preferably, together with the adjuvants conventionally employed in
the art of formulation, and are therefore formulated in known manner
to emulsifiable concentrates, coatable pastes, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble
powders, dusts, granulates, and also encapsulations in e.g. polymer
substances. As with the nature of the compositions, the methods of
application, such as spraying, atomising, dusting, scattering,
coating or pouring, are chosen in accordance with the intended
objectives and the prevailing circumstances. Advantageous rates of
application are normally from S0 g to 5 kg of active ingredient
(a.i.) per hectare, preferably from 100 g to 2 kg a.i./ha, most
preferably from 200 g to 600 g a.i./ha.
The formulations, i.e. the compositions, preparations or mixtures
containing a compound (actlve ingredient3 of the invention and,
where appropriate, a solid or liquid adjuvant, are prepared in known
manner, e.g. by homogeneously mixing and/or grinding the active
ingredient with extenders, e.g. solvents, solid carriers and, where
appropriate, surface-active compouDds (surfactants).
Suitable solvents are: aromatic hydrocarbons, preferably the
fractions containing 8 to 12 carbon atoms, e.g. xylene mixtures or
substituted naphthalenes, phthalates such as dibutyl phthalate or
dioctyl phthalate, aliphatic hydrocarbons such as cyclohexane or
paraffins, alcohols and glycols and their ethers and esters, such as
ethanol, ethylene glycol, ethylene glycol monomethyl or monoethyl
ether, ketones such as cyclohexanone, strongly polar solvents such
as N-methyl-2-pyrrolidone, dimethyl sulfoxide or dimethylformamide,
as well as vegetable oils or epoxidised vegetable oils such as
epoxidised coconut oil, sunflower oil or soybean oil; or water.
- 12 - 1 3 1 42 9 ~
The solid carriers used e.g. for dusts and dispersible powders, are
normally natural mineral fillers such as calcite, talcum, kaolin,
montmorillonite or attapulgite. In order to improve the physical
properties it i5 also possible to add highly dispersed silicic acid
or highly dispersed absorbent polymers. Suitable granulated adsorp-
tive carriers are porous types, for example pumice, broken brick,
sepiolite or bentonite; and suitable nonsorbent carriers are
materials such as calcite or sand. In addition, a great number of
pregranulated materials of inorganic or organic nature can be used,
e.g. especially dolomite or pulverised plant residues, e.g. cork
powder or sawdust.
Depending on the nature of active ingredient to be formulated,
suitable surface-active compounds are non-ionic, cationic and/or
anionic surfactsnts having good emulsifying, dispersing and wetting
properties. The term "surfactants" will also be understood as
comprising mixtures of surfactants.
Suitable anionic surfactants can be both water-soluble soaps and
water-soluble synthetic surface-active compounds.
Suitable soaps are the alkali metal salts, alkaline earth metal
salts or unsubstituted or substituted ammonium salts of higher fatty
acids (C1o-C2z)~ e.g. the sodium or potassium salts of oleic or
stearic acid, or of natural fatty acid mixtures which can be
obtalned e.g. from coconut oil or tallow oil. Suitable aurfactants
are also fatty acid methyltaurin salts as well as modified and
unmodified phospholipids.
More frequently, however, so-called synthetic surfactants are used,
especially fatty sulfonates, fatty sulfates, sulfonated benzimid-
azole derivatives or alkylsulfonates~
Non-ionic surfactants are preferably polyglycol ether derivatives of
aliphatic or cycloaliphatic alcohols, or saturated or unsaturated
fatty acids and alkylphenols, said derivatives containing 3 to 30
131~291
- 13 -
glycol ether groups and 8 to 20 carbon atoms ln the (aliphatic)
hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of
the alkylphenols.
~urther suitable non-ionic surfactants are the water-soluble adducts
of polyethylene oxide with polypropylene glycol, ethylenediamino-
propylene glycol and alkylpolypropylene glycol containing 1 to
lO carbon atoms in the alkyl chain, which adducts contain 20 to
250 ethylene glycol ether groups and 10 to lO0 propylene glycol
ether groups. These compounds usually contain l to 5 ethylene glycol
units per propylene glycol unit.
Fatty acid esters of polyoxyethylene sorbitan, e.g. polyoxysthylene
sorbitan trioleate, are also suitable non-ionic surfactants.
Cationic surfactants are preferably quaternary ammonium salts which
contain, as N-substituent, at least one Cg-C22alkyl radical and, as
further substituents, unsubstituted or halogenated alkyl, benzyl or
hydroxy-lower alkyl radicals. The salts are preferably in the form
of halides, methylsulfates or ethylsulfates, e.g. stearyltrimethyl-
ammonium chloride or benzyldi(2-chloroethyl)ethylammonium bromide.
The surfactants customarily employed in formulation technology are
descrlbed, inter alia, in the following publications:
McCutcheon's Detergents and Emulsifiers Annual, MC Publishing Corp.,
Ridgewood, New Jersey, 1979;
Dr. ~elmut Stache "Tensid Handbuch" (Handbook of Surfactants), Carl
Hanser Verlag MunichlVienna 1981.
In the storage sector, preferred additives are those that are safe
for human and animal nutrition.
The fungicidal compositions of this invention normally contain
0.1 to 95 ~0 by weight of the (aS,l'R) enantiomer in a higher
proportion of the four isomers of the compound of formula I than
- 14 - 1 31 ~2~1
that corresponding to the theoretical amount of 25 % by weight,
together with 99.9 % to 5 % by weight of a solid andlor liquid
carrier.
Preferred compositions are those in which the active component
comprises 28 % by weight or more of the (aS,l'R) enantiomer or those
containing in addition an equally large or lesser amount of the
(aR,l'S) enantiomer.
Further preferred compositions are those in which the active
component comprises 40 % by weight or more of the (aS,l'R) enantio-
mer and those containing in addition an equally large or a lesser
amount of the (aR,l'R) enantiomer or an equally large or lesser
amount of the (aR,l'S) enantiomer.
Particularly preferred compositions are those in which the active
component comprises 50 % by weight or more of the (aS,1'R) enantio-
mer and those in which the active component additionally comprises
an amount of the (aR,1'R) enantiomer or of the (aR,1'S) enantiomer
to make up lOO % by weight or also a lesser amount.
Further preferred compositions are those in which the active
component comprises 70 % by weight or more of the (aS,l'R) enantio-
mer, in particular those in which the active component comprises
preponderantly (90 % by weight or more) the (aS,l'R) enantiomer.
The particulars given above thus refer to amounts of the four
isomers of 3-~N-(methoxyacetyl)-N-(2,6-dimethylphenyl)]aminotetra-
hydrofuran-2-one, the (aS,l'R) enanatiomer of which is preferably
the ma~or constituent, followed proportionately by either tke
(aR,l'R) or the (aR,l'S) enantiomer, while each of the remaining
enantiomers may be present in smaller amounts.
The indicated amounts refer exclusively to the compound of formula I
and include small amounts of other compounds which may possibly be
present in the compositions of the invention.
.,,,, ~ ,
- 15 - 1 31 4 291
Whereas commercial products will preferably be formulated as concen-
trates, the end user will normally employ dilute formulations.
The compositions may also contain further auxiliaries such as
stabilisers, antifoams, viscosity regulators, binders, tackifiers as
well as fertilisers or other active ingredients for obtaining
special effects.
Such agrochemical compositions constitute an object of the present
invention.
The following Examples illustrate the invention, without implying
any limitation to what is disclosed therein. Parts and percentages
are by weight.
Preparatory Examples
1.1 Preparation of the pair of (aR,l'S)(aS,l'R) enantiomers of
3-~N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetra-
hydrofuran-2-one, and of the pair of (aS,l'S)~aR,l'R) enantiomers
of 3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetra-
hydrofuran-2-one
70 g of the mixture of isomers of 3-[N-(methoxyacetyl)-N-(3-chloro-
2,6-dimethylphenyl)]aminotetrahydrofuran-2-one obtained according to
German Offenlegungsschrift 2 ao4 299, are separated into the
individual isomers in a flas~ chromatography column (silica gel with
a 1:1 mixture of ethyl acetateldiethyl ether as eluant).
Result:
33.8 g of (aS,l'S)(aR,1'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)]aminotetrahydrofuran-2-one, m.p. 140C,
32.5 g of (aR,l'S)(aS,l'R)-3-[N-(methoxyacetyl)-N-~3-chloro-2,6-di-
methylphenyl)]aminotetrahydrofuran-2-one, m.p. 115C.
- 16 - 1 31 4 29 ~
1.2 Preparation of (aS,l'S)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-
dimethylphenyl)]aminotetrahydrofuran-2-one and (aR,l'R)-3-EN-(meth-
oxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-
2-one enantiomers
1 g of (aS,l'S)(aR,l'R)-3-~N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)3aminotetrahydrofuran-2-one ls separated into the
individual enantiomers in a medium pressure chromatography column
packed with triacetyl cellulose under a pressure of 2 bar with an
eluant consisting of a mixture of ethanol (95 % vol. %) and water
(5 vol. ~O).
Result:
0.215 g of (aR,l'R)-3-1N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one, m.p. 117-120C [~]2D4 in
CHCl3): -64 + 1 (ee >98 %),
0.232 g of (aS,l'S)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one, m.p. llS-117C, E~]2DO (in
CHCl3): -64 + 1 (ee >99 %).
1.3 Preparation of (aR,l'S)-3-[X-(methoxyacetyl)-N-(3-chloro-
2,6-dimethylphenyl)]aminotetrahydrofuran-2-one and (aS,1'R)-3-[~-
(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydro-
furan-2-one enantiomers
0.5 g of (aR,l'S)(aS,l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)~aminotetrahydrofuran-2-one is separated into the
lndividual enantiomers in a medium pressure chromatography column
packed with triacetyl cellulose under a pressure of 2 bar with an
eluant consisting of a mixture of ethanol (95 % vol. %) and water
(5 vol. %).
Result:
0.154 g of (aR,l'S)-3-~N-(methoxyacetyl)-N-(3-chloro-2,6-di-
methylphenyl)]aminotetrahydrofuran-2-one, m.p. 98-100C, [~]D
(in CHCl3): -91.2 + 1 (ee >95 %),
- 17 - 1 31 ~ 29 ~
0.152 g of highly active (aS,l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-
2~6-dimethylphenyl)]aminotetrahydrofuran~2-one~ m.p. 94~96C,
[~32D (in CHCl3): +96 ~ 1 (ee >99 %).
1.4 Prepsration of the individual (aS,l'S), (aR,l'R), (aR,l'S) and
(aS,l'R) isomers of 3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one in one separation step from the
mixture thereof
20 ~l of a l % solution of the mixture of isomers in a 1:1 mixture
of hexane/2-propanol are applied to a HPLC column (0.46 x 25 cm)
packed with tris(3-methylbenzoyl)cellulose. The chromatography is
carried out at a rate of flow of l ml/min. with an eluant consisting
of 60:49 mixture of hexane/2-propanol. The four isomers are separ-
ated under these conditions. The retention times of the individual
isomers are as follows:
isomeTs retention time (min.)
(aR,l'S) 21.7
(aS,l'S) 31.0
(aS,l'R) 42.5
(aR,l'R) 67.7
The isomers are isolated in pure form after evaporation of the
mixture of solvents and after drying the residue. (The physical data
correspond to the values given in the preceding Examples). The
tris(3-methylbenzoyl)cellulose used in the stationary phase is
prepared as follows:
50 ml of heptanol are added to 10 g of tris(3-methylbenzyl)cellulose
in 300 ml of methylene chloride. The resultant solution is then
added dropwise to a solution of 0.7 ~O sodium lauryl sulfate
(Z40 ml), which is stirred at 400 rpm. Methylene chloride is then
removed by evaporation at the same rate of stirring at 40-42C
131~2~
- 18 -
(bath temperature). The residue is isolated by filtration and washed
with water and ethanol. The powdery product is dried at 80C for
20 hours in a vacuum drler. Yield: 9.6 g (96 % of theory). The
spherical particles having a diameter of 10-20 ~m can, if required,
be fractionated by sieving or sedimentation. Further physical
properties of the product:
specific surface area: 57.8 m2/g (according to BET)
heat of fusion ~H : 12.7 Jlg.
The selective preparation of a mixture of (aR,l'R) and (aS,l'R)
isomers of 3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]-
aminotetrahydrofuran-2-one by chlorination of (l'R)-3-[N-(methoxy-
acetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-one
can be effected readily and in high yield (Example 2.1). When
formulated, this mixture can be used as a highly effective plant
microbicide. If desired, however, the (aS,l'R) enantiomer can also
be separated therefrom (Example 2.2).
The (l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]-
aminotetrahydrofuran-2-one to be used as starting material for the
chlorination can be separated from the corresponding racemate in
accordance with the procedure of Example 2Ø
1314291
- 19 -
Preparatory Examples
2.0 Separation of the enantiomer intermediates (l'S)-3-[N-(meth-
oxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]aminotetrahydrofuran-2-
one and (l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one
. . _ _ . . . _ _ .
2 g of 3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]amino-
tetrahydrofuran-2-one are separated into the two enantiomers in a
medium pressure chromatography column packed with triacetyl cellu-
lose under a pressure of 2 bar with an eluant consisting of ethanol
(95 vol. %) and water (5 vol. ~O).
Result:
0.808 g of (l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one, m.p. 63-65C, [~]D (in
CHCl3): +84.5 ~ 1 (ee >99 %),
0.978 g of (l'S)-3-[N-(methoxyacetyl)-~-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one, m.p. 62-64~C, [~]2D0 (in
CHCl3): -80.3 ~ 1 (ee >95 %)
2.1 Preparation of an approximately 1:1 mixture of ~aR,1'R)- and
~aS,1'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethylphenyl)]-
aminotetrahydrofuran-2-one
0.222 g of (1'R)-3-[N-(methoxyacetyl)-N-(3-chloro-2,6-dimethyl-
phenyl)]aminotetrahydrofuran-2-one of formula I is dissolved in
30 ml of formic acid. Then 0.06 g of chlorine are bubbled into the
solution at room temperature in the presence of a trace of iron(III)
chloride. The solution is stirred for about 1 hour and the solvent
is removed by evaporation under reduced pressure. The residue is
dissolved in ethyl acetate and the solution is extracted with water.
The extract is dried over sodium sulfate, filtered, and the filtrate
- 20 - 1 31 ~ 29 ~
is concentrated by evaporation. The dried mixture melts at 79-82C
and consists of about 50 ~/O of each of the two isomers, i.e. the
ratio of the isomers varies from 45:55 to 55:45.
2.2 Preparation of pure (aS,l'R)-3-[N-(methoxyacetyl)-N-(3-chloro-
2,6-dimethylphenyl)]aminotetrahydrofuran-2-ona
.. .. . . .. _
The residue obtained from the chlorination in Example 2 is dissolved
in ethyl acetate. The solution is extracted with water and the
aqueous extract is dried over sodium sulfate, filtered, and the
filtrate is concentrated by evaporation. The crude mixture is
separated in a flash column packed with silica gel and eluted with a
1:3 mixture of ethyl acetate/hexane.
Result:
0.070 g of (aR,1'R)-3-EN-(methoxyacetyl)-N-~3-chloro-2,6-dime~hyl-
phenyl)3aminotetrahydrofuran-2-one, [~]D (in CHCl3): +63.8 + 2,
0.075 g of (aS,l'R)-3-~N-~methoxyacetyl)-N-~3-chloro-2,6-dimethyl-
phenyl)~aminotetrahydrofuran-2-one, E~D (in CHCl3): +88 + 3.
Formulation Examples for compounds of formula I, including isomers
and mixtures thereof which can be prepared in accordance with
Examples 1.1 to 1.4 and 2.1 and 2.2.
.. . .. _ . .
EPercentages of ~aS,1'R) isomer in the compound of formula I
indicated in brackets]
1. Emulsifiable concentrates a) b) c)
a compound of formula I 25 % 40 % 50 %
[70 % of (aS,l'R)~
calclum dodecylbenzenesulfonate 5 % 8 % 6 %
castor oil polyethylene glycol ether
(36 moles of ethylene oxide~ 5 %
tributylphenol polyethylene glycol ether
(30 moles of ethylene oxide) - 12 % 4 %
- 21 - 1 31 4291
cyclohexanone - 15 % 20 %
xylene mixture 65 % 25 % 20 %
Emulsions of any required concentration can be produced from such
concentrates by dilution with water.
2. Solutions a) b) c) d)
a compound of formula I 80 % lO % 5 % 95 %
[40 % of (aS,l'R)]
ethylene glycol monomethyl ether 20 % - - -
polyethylene glycol (mol.wt. 400) - 70 % - -
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oil - - 1 % 5 %
petroleum distillate (boiling range
160-190C) - - 94 %
These solutions are suitable for application in the form oE micro-
drops.
3. Granulates a) b)
a compound of formula I 5 % 10 %
180 % of (aS,l'R)]
kaolin 94 %
highly dispersed siliclc acid1 %
attapulgite - 90 %
The active ingredient is dissolved in methylene chloride, the
solution is sprayed onto the carrier, and the solvent is subsequent-
ly evaporated off in vacuo.
4. Dusts a) b)
a compound of formula I 2 % 5 %
[50 % of (aS,l'R)]
highly dispersed silicic acidl % 5 %
talcum 97 %
kaolin - 90 %
131~291
- 22 -
Ready-for-use dusts are obtained by intimately mixing the carriers
with the active lngredient.
5. Wettable powders a) b) c)
a compound of formula I 25 % 50 % 75 %
[g2 % of (aS,l'R)]
sodium lignosulfonate 5 % 5 %
sodium lauryl sulfate 3 % - 5 %
sodium diisobutylnaphthalenesulfonate - 6 % 10 %
octylphenol polyethylene glycol ether
(7-8 moles of ethylene oxide) - 2 %
highly dispersed silicic acid 5 % 10 % 10 %
kaolin 62 % 27 ~0
The active ingredient is thoroughly mixed with the adjuvants and the
mixture is thoroughly ground in a suitable mill, affording wettable
powders which can be diluted with water to give suspensions of the
desired concentration.
6. Emulsifiable concentrate
a compound of formula I E28 % of (aS,l'R)3 10 %
octylphenol polyethlene glycol ether
(4-5 moles of ethylene oxide) 3 %
calcium dodecylbenzenesulfonate 3 %
castor oil polyglycol ether
(35 moles of ethylene oxide) 4 %
cyclohexanone 30 %
xylene mixture 50 %
Emulsions of any required concentration can be obtained from this
concentrate by dilution wlth water.
?. Dusts a) b~
a compound of formula I [97 % of (aS,l'R)] 5 % 8 %
talcum 95 %
kaolin - 92 %
131~2~
- 23 -
Ready-for-use dusts are obtained by mixing the active ingredient
with the carrier, and grinding the mixture in a suitable mill.
8. Extruder granulate
a compound of fo}mula I [85 % of (aS,l'R)] 10 ~o
sodium lignosulfonate 2 %
carboxymethylcellulose 1 %
kaolin 87 %
The active ingredient is mixed and ground with the adjuvants, and
the mixture is subsequently moistened with water. The mixture is
extruded and then dried in a stream of air.
9. Coated granulate
a compound of formula I [90 % of (aS,1'R)] 3 %
polyethylene glycol (mol.wt. 200)3 %
kaolin 94 %
The finely ground active ingredient is uniformly applied, in a
mixer, to the kaolin moistened with polyethlene glycol. ~on-dusty
coated granulates are obtained in this manner.
10. Suspension concentrate
a compound of formula I [55 % of (aS,1'R)] 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
(15 moles of ethylene oxide) ~ %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
37 Y0 aqueous formaldehyde solution0.2 %
silicone oil in the form of a 75 %
aqueous emulsion 0.8 %
water 32 %
- 24 - 1 31 ~ 2~
The finely ground active ingredient is intimately mixed with the
adjuvants, giving a suspension concentrate from which suspensions of
any desired concentration can be obtained by dilution with water.
3. Biological ~xamples
3.1 Pythium ultimum on Beta vulgaris (sugar beet, cv. Kleinwanzleben
Monogerm~ and Pythium ultimum on Zea mays (maize, cv. Sweet Corn)
Test subject: soil fungus: protective local soil application.
Test method: Mycellum of Pythium ultimum is mixed with soil (500 ml
of mycelium suspension per 10 litares of soil) and 250 ml plastic
dishes are filled with the fungus/soil mixture. After incubation for
4 days at 10C, 10 seeds of the test plant (maize or sugar beet) are
sown in each dish. On the next day, 50 ml of a spray solution of
each test compound-prepared from a 25 % wettable powder and water
are poured in concentrations of 20, 6, 2, 0.6, 0.2, 0.6 and 0.02 ppm
on to the dishes. After an incubation phase of 7 days at 22C, the
activity of the test compounds is assessed by counting the number of
emerged plants in accordance with the following rating:
microbicidal activity in % rating
> 95
80-95 3
50-80 6
0-50 ~ (= no activity)
The results are reported in Table 1.
2s- 131~291
Table 1
Isomer of the Rating ** at a concentration of
compound of active ingredient (ppm)
formula I
(active ingredient) 20 o 20.6 0.2 0.06
1. mixture of maize1 1 2 6 9 9
isomers * beet1 1 1 2 8 8
2. racemate maize1 3 8 9 9 9
(aS,l'S)(aR,l'R) beet 2 2 8 9
3. racemate maize 1 2 3 5 8 9
(aR,l'S)(aS,l'R) beet 1 1 1 1 5 8
* According to German Offenlegungsschrift 2 0804 299 consisting of
4S % of the pair of (aR,l'S)(aS,l'R) diastereoisomers and 55 % of
the pair of (aS,l'S)~aR,l'R) diastareolsomers of the compound of
formula I.
** Rating as average of 3 parallel tests.
The racemate 3) of this invention exhibits fungicidal activity in
the concentration range down to 0.6-0.2 ppm.
3.2 Action against ~hytophthora infestans on tomato plants
a) Residual-protective action
After a cultivation period of 3 weeks, tomato plants are sprayed
with a spray mixture (0.02 % a.i.) prepared from a wettable powder
formulation of the test compound. After 24 hours the tomato plants
are infected with a sporangia suspension of the fungus. Evaluation
of fungus attack is made after the plants have been incubated for
5 days at 90-100 % relative humidity and 20C.
- 26 - 1 31 ~291
b) Systemic activity
After a cultivation period of 3 weeks, tomato plants are sprayed
with a spray mixture (0.002 % a.i., based on the volume of the soil)
prepared from a wettable powder formulation of the test compound.
Care is taken that the spray mixture does not come into contact with
the parts of the plants above the soil. After 48 ho~rs the treated
plants are infected with a sporangia suspension of the fungus.
Evaluation of fungus attack is made after the infected plants have
been incubated for 5 days at 90-100 % relative humidity and 20C.
The results were evaluated in accordance with the rating described
in Example 3.1 and the concentration necessary to achieve the
rating 1-2 is based in each case on the concentration of the most
active (aS,l'R) enantiomer. The concentration factors (f) obtained
in this manner are listed in Table 2.
3.3 Plasmopara viticola on vines (vine seedlings, cv. Gutedel
(Chasselas), in 3 pots, each with 1 plant per test compound
a) Residual protective foliar application
Five-week-old vine seedlings are sprayed with a spray mixture
prepared from a formulation of the test compound and infected 1 day
later with a sporangia suspension (200,000 sporangiaslml) of
P. viticola. The infected seedlings are incubated at 20C in a
greenhouse. A 14 hour incubation phase at 100 % relative humidity is
followed by incubation for 4 days at 75-85 % and, finally, to induce
sporulation, by incubation for one further night at 100 % humidity.
Evaluation of fungus attack i8 made after this 6 day incubation
period (rating as in Example 3.1).
- 27 - 1 31 4 291
b) Systemic activity
Five-week-old vine seedlings are sprayed with a spray mixture
prepared from a formulation of the test compound. Three days later
the plants are infected with a sporangia suspension (200,000
sporangias/ml) of P. viticola. The test is carried out as described
in a).
The concentration factors calculated in accordance with Example 3.2
are listed in Table 3.
3.4 Systemic action against Pythium debaryanum on Beta vulgaris
The fungus is cultivated on carrot chip nutrient solution and added
to a mixture of soil and sand. The infected soil is filled into
flower pots in which sugar beet seeds are then sown. Immediately
after sowing, an aqueous suspension of the test compound formulated
as wettable powder is poured on to the soil (20 ppm a.i., based on
the volume of the soil). The pots are then stood for 3 weeks in a
greenhouse at c. 20~C. The soil is kept uniformly moist during this
time by gentle spraying.
The test is evaluated by counting the number of emerged sugar beet
plants as well as the number of healthy and sick plants.
The concentration factors calculated in accordance with Example 3.2
are indicated in Table 2.
3.5 Systemic action against Pythium debaryanum on Zea mais
The fungus is cultivated on carrot chip nutrient solution and added
to a mixture of soil and sand. The infected soil is filled into
flower pots in which maize seeds are then sown. Immediately after
sowing, an aqueous suspension of the test compound formulated as
wettable powder is poured on to the soil (20 ppm a.i., based on the
- 28 - 131~291
volume of the soil). The pots are then stood for 3 weeks in a
greenhouse at c. 20C. The soil is kept uniformly moist during this
time by gentle spraying.
The test is evaluated by counting the number of emerged sugar beet
plants as well as the number of healthy and sick plants.
The concentration factors calculated in accordance with Example 3.2
are indicated in Table 2.
Table 2
. . ~
Isomer of the Concentration factors (f) of
compound of the tests
formula I 3.2 3.3 3.4 3.5
(actlve ingredient) a b a b
aS,l'R enantiomer 1 <1 <1 1 1 l
mixture of isomers* 3.3 l <1 3.3 3.3 lO
aS,1'S enantiomer 3.3 3.3 ~3 lU lO 3.3
aR,l'R enantiomer 33 3.3 >3 lOO lO 33
aR,l'S enantiomer 33 3.3 >3 3.3 33 10
It is evident from Table 2 that the (aS,l'R) enantiomer of this
invention has on average a 3-30 times better activity than the other
three enantiomers and the racemate (mixture of isomers according to
German Offenlegungsschrift 2 804 299).
3.6 Inhibition of fungus growth in vitro (agar incorporation test)
0.1 ml of a 0.75 % solution of each test compound in acetone is
diluted with 15 ml of water under sterile conditions. 1 ml of each
stock solution or of the sterile dilution series prepared therefrom
by addltion of water is incorporated at 50C in 19 ml of nutrient
medium in petri dishes of 9 cm diameter. The concentrations of each
test compound are 50, 5, 0.5, 0.05 and 0.005 ppm per dish. Two hours
131~291
- 29 -
later these dishes and the control dishes without test compound are
inoculated with Phytophthora capsici in the form of agar discs
(0.6 mm 0, the side overgrown with fungus face down). The inocul-
ated cultures are kept at 60-70 % relative humidity and at 18C in
the dark until evaluation is made. After 4-6 days, when the control
petri dish is two-thirds overgrown with fungus, the test series is
evaluated by plotting the concentration of test compound loga-
rithmically against the radial growth (of the control test in %) on
graph paper. To compare the individual test compounds, that con-
centration of each test compound is determined at which growth is
50 % of the control test (ELso value). The ELso values are reported
in Table 3:
Table 3
Isomer of compound of ELso
formula I at ppm
(active ingredlent) a.i./dish
. .. _ . _
aS,1'R enantiomer 0.011
(aR,1'R) and ~aS,1'R) 0.011
diastereoisomers
mixture of isomers* 0.064
aR,l'S enantiomer 0.26
aS,1'S enantiomer 0.62
aR,l'R enantiomer 2.7
Test 3.6 shows that an equally pronounced activity ls achieved with
an approximately 1:1 mixture of the highly active (aS,1'R) enantio-
mer with the (aR,1'R) enantiomer.
3.7 Example for determining the long-term activity as soil
fungicide
Each of the test compounds formulated as wettable powder is stirred
in 100 ml of water and mixed with 20 litres of soil. The concen-
tration of the test compounds is based on dry soil.
~31'~2~1
- 30 -
Tobacco plants (Hocks variety) grown in sterile soil are planted in
the 5- to 6-leaf stage in soil which contains fungicide (in 5 litre
pots). Two days later the furrows on both sides of the plant are
artificially infected by inoculation of altogether 2 x 20 ml of a
spore suspension (containing 10,000 sporangias/ml) of Phytophthoraa
nicotianae.
Evaluation is made at regular intervals with ratings (R) on a scale
from 1-100 on the basis of withering of the leaves, damage to the
stalk and extent of damage. ~here protection is complete, R = 0.
The plant is regarded as being protected until the first typical
signs of disease appear. The test lasts 100 days during which time
the tobacco plant ripens.
The long-term activity of the individual test compounds is compared
with the aid of factors. This is done by determining the duration of
action in days in respect of the indi~idual test compounds employed
in different concentrations (ppm), at which action the protect-
ion (R) corresponds to that of the mixture of isomers* (defined in
Table 1) at a concentration of 0.25 ppm after 50 days. The com-
parison factor (f) is calculated in accordance with the formula:
0.25 duration (days)
f = _ ,
conc. (ppm) 50
The factors so calculated for the individual test compounds are
summarised in Table 4, which shows the pronounced long-term action
of the (aS,l'R) enantiomer and the (aR,l'S) and (aS,l'R) pair of
enantiomers compared with the other three enantiomers and with two
mixtures.
- 31 - ~ 91
Table 4
Isomer of the compound of Factor of
formula I the long-
(active ingredient) lasting
---~ action
aR,1'R 0.025
(aS,1'S)(aR,l'R) 0.17
aR,l'S 0.17
aS,l'S 0.25
mixture of isomers*
(aR,l'S)(aS,l'R) ~of the 2
invention]
aS,l'R [of the invention] 4
3.8 Action against Phytophthora infestans on tomatoes
a) Residual-protective action
b) Systemic action
The test method corresponds to that described in Example 3.2.
3.9 Action against Plasmopara viticola on vines
a) Residual-protective action
b) Systemic action
The test method corresponds to that described in Example 3.3.
The pair of (aR,l'S)(aS,l'R) enantiomers and the ~aR,l'R)(aS,l'R)
diastereoisomers were tested side by side in both indications and
their activity was compared with the most active (aS,1'R) enantio-
mers, which was tested in parallel. Table 5 shows, on the lines of
Table 2, at a how much higher rate of concentration each of the
~ 32 - ~ 13~429~
diastereoisomers had to be used (concentration factor f) to achieve
the same complete activity (95 %; rating 1) as the (aS,1'R) enant-
iomer.
Table 5
. . .~
Isomers of the compound of Concentration factor f)
formula I (active ingredient) Ex. 3.8 Ex. 3.9
a) b) a) b)
. ..
aS,l'R enantiomer 1 1
pair oE (aR,l'S)(aS,l'R) 3.3 3.3 1 3.3
enantiomers
.. ___ . __
(aR,l'R)(aS,l'R) 1 10 ~.3
diastereoisomers . _
In contrast to the results of Table 2, which show the (aS,l'R)
enantiomer to be an outstanding active component compared with the
other enantiomers and with the racemic compound (mixture of
isomers*) of the prior art, Table 5 shows a surprisingly similar
degree of activity of the pair of enantiomers, although the active
(aS,l'R) enantlomer is only one of the two constituents of each
pair. This result indicates an evident interaction of the enantio-
mers which is able to enhance the biological activlty.