Note: Descriptions are shown in the official language in which they were submitted.
~31~52
- 1 - O.z. OOS0/39235
Preparation of quinolines
The present invention relates to a process for
the preparation of quinolines by reacting an aniline with
an ~,B-unsaturated aldehyde or ketone in sulfuric acid
in the presence of a catalytic amount of iodine at elevated
temperatures.
It is known ~hat quinolines can be prepared by
the Skraup method by reacting an aniline with glycerol
or an ~,~-unsaturated aldehyde or ~,B-unsaturated ke-
tone in the presence of an oxidizing agent, such as nitro-
benzene, arsenic pentoxide, iron(III) oxide or picric
acid, in concentrated sulfuric acid (A. Weissberger and
E.C. Taylor, Heterocyclic Compounds, Vol~ 32, I, pages
100-117). The yields obtained are moderate. Furthermore,
the use of these oxidi~ing agents causes considerable
pollution of ~astewater, so that this procedure is unsuit-
able for an industrial process.
GB-A-549 502, moreover, discloses the use of
iodine as an oxidizing agent in ~he reaction of an anil-
ine ~ith glycerol in concentrated sulfuric acid. Inthis case too, the yields obtained are only ~oderate.
Another disadvantage is that a large excess of glycerol
is required here.
It is an object of the present invention to pro-
vide a process which can be carried out on a large indus-
trial scale and gives quinolines in high yields.
~ e have found that this object is achieved by a
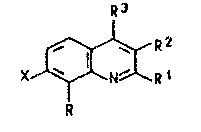
process for the preparation of quinolines of the formula
R3
XJ~XR 1
where R is C1-Cs-alkyl, R1, R2 and R3 are each hydro-
gen or C1-Cs-alkyl and X is hydrogen or h,3logen, with
the proviso that R may further~ore be hydrogen if X is
1~4~52
- 2 - o.Z. 0050/39235
hydrogen, by reacting an aniline of the formuta
X~NHz ( I I )
R
with an ~ unsaturated aldehyde or ketone of the for-
mula
1 2
R i--CH=C--i--R3 t I 11 )
in sulfuric acid in the presence of a catalytic amount
of iodine at elevated temperatures, wherein the reaction
is carried out in about 70-85X strength by weight sulfuric
acid.
The reaction can be carried out as follo~s:
The aldehyde or ketone III ;s added to a solution, pre-
heated to 100-140C~ of aniline II in sulfuric acid and
a catalytic amount of iodine in the course of from oO to
90 minutes, the temperature of the reaction mixture as
far as possible being prevented from falling belQw 100C.
The temperature is about 100-150C, prefer-
ably 115-130C. After the end of the addition, the reac-
tion solution is neutralized by adding an aqueous alkali,
for example sodium hydroxid~ solution, at from 70 to 100C,
the desired product separating from the aqueous phase in
the form of an organic phase. ~orking up is then carried
out in a conventional manner, for example by ex~raction.
The concentration of the sulfuric acid may vary
from about 70 to about 85% by ~eight. Advantageously,
the sulfuric acid used has a concentrat;on of about 7û-80X
by weight.
The starting materials are advantageously used in
a molar ratio of aniline II to aldehyde or ketone III of
from about 1 : 1 to 1 : 1.5, preferably from about 1 : 1.1
~.; .,~ .
.~,
~ 31~5~2
- 3 - O.Z~ 0050/39235
to 1 : 1.3. The molar amount of sulfuric acid is from 2 to
8, preferably from 2.5 to 6, moles per mole of aniline II.
The catalytic amounts of iodine required in the
reaction can be added to the reaction mixture ir the form
of inorganic iodine compounds, for example ele~ental
iodine, hydriodic acid or a metal iodide, such as calcium
iodide or sodium iodide, in solicl or liquid form, for
example in the form of an aqueous solution. The amount
of iodine used is from 0.1 to 2, preferably from 0.2 to
1.5, mol %, based on aniline II.
It is surprising that the novel process gives
the quinolines I in high yield and purity, since under
the drastic reaction conditions side reactions of the
unsaturated starting compounds III with iodine were to
be expected (Houben-Weyl, Methoden der org. Chemie,
Vol. 5/4, pages 530 and 535).
The process according to the invention can be very
advantageously carried out if glycerol is used instead of
the starting compounds III, the starting compound III
twhere R1, R2 and R3 are each hydrogen) being formed in
situ from a glycerol. ~here glycerol is used, the novel
process can be carried out in the same way as when alde-
hydes or ketones III are employed. In this case, however,
it is extremely advantageous if, after the addition of the
glycerol, the mixture is stirred at about 100-150C, advan-
tageously 130-145C, the resulting water of reaction being
distilled off si~ultaneously. It is advisable to distill
off not less than 60 - 70~ of the ~ater of reaction formed.
It is sufficient to continue stirring for a few hours, for
example from 2 to 4 hours. In this procedure, ~ith an ex-
cess of glycerol of 1.2, preferably 1.1, moles per mole of
aniline II, the quinoline I is obtainable in high yield.
~ t was not to be expected that the reaction would
give quinolines in h;gh yield and purity starting from
a s~all molar excess of glycerol and using dilute sul-
furic acid, since G~-A-549 50Z discloses that, despite
a large excess of glycerol, carrying out the reaction in
13145~2
- 4 - O.Z. ~050/39235
dilute sulfuric acid results in very low yields. More-
over, the measure of distilling off water of reaction,
in which more than 90~ of the iodine used leaves the
reaction mixture, would likewise have been expected to
lead to a reduction in the yield or extension of the
reaction times.
Examples of starting compounds II are aniline,
Z-methylaniline, 2-ethylaniline, 2-propylaniline, 2-iso-
propylaniline, 2-butylaniline, Z-pentylaniline, 2-tert-
butylaniline, 3-chloro-2-methylaniline, 3-chloro-2-ethyl-
aniline, 3-chloro-2-propylaniline, 3-chloro-2-isopropyl-
aniline, 3-chloro-2-butylaniline, 3-fluoro 2-methylanil-
ine, 3-fluoro-2-ethylaniline, 3-fluoro-2-propylaniline,
3-bromo-2-methylaniline and 3-bromo-2-ethylaniline.
Examples of suitable starting compounds III, in
addition to glycerol, are acrolein, methacrolein, croton-
aldehyde, penten-2-al, hexen-2-al, methyl vinyl ketone,
ethyl vinyl ketone and propyl vinyl ketone.
The alkyl substituents R, R1, R2 and R3 in the
formulae I, II and III may be straight-chain or branched
and are, for example, methyl, ethyl, n-propyl, isopropyl,
n-butyl, sec-butyl and tert-butyl. Suitable halogen sub-
stituents R are chlorine, bromine and fluorine, preferably
chlorine.
The quinolines of the formula I are used as inter-
mediates for herbicidal active ingredients (DE-A-3 108 873
and DE-A-3 233 089).
EXAMPLE 1
7-chloro-8-methylquinoline
a) 72~8 9 (1030 moles) of acrolein are added to a
solution of 6Z5 9 of 80~ strength sulfuric acid (5.0
moles), 141.5 9 (1.0 mole) of 3-chloro-2-methylaniline
and 2 9 (0.013 mole) of sodium iodide in the course of
60 minutes at from 115 to 120C. Stirring is continued
for 10 minutes, after which the pH is brought to 7-8 with
25~ strength sodium hydroxide solution and extraction is
carried out with 200 ml of toluene at from 70 to 90C.
13145~2
- 5 - O.Z. 3050/39235
Evaporating down the toluene extract gives 164.6 9 (yield
86.1%) of 7-chloro-3-methylquinoline having a purity of
~2.9% according to gas chromatography.
b) 101.3 9 (1.1 moles) of g~ycerol are added to a
S solution of 550 9 of 80% strength sulfuric acid (4.5
moles), 141.5 9 (1.0 mole) of 3-chloro-2-methylaniline
and 1 9 (0.007 mole) of sodium iodide in the course of
90 minutes at 140C. Thereafter, stirring is carried
out for 2 hours at from 140 to 145C, 60 ml of water be-
ing distilled off simultaneously. The reaction solution
is then brought to pH 7-8 ~ith 25X strength sodium hydrox-
ide solution and extracted with 200 ml of toluene at from
70 to 90C. Evaporating down the toluene extract gives
180.0 9 (yield 95.9%) of a product which, according to
gas chromatography, contains 94.6% of 7-chloro-8-methyl-
quinoline and 0.25% of 3-chloro-2-methylaniline.
Distillation of the crude product gives 166 9
(yield 92.8~) of 7-chloro-8-methylquinoline having a
purity of 99.2X; bp1: 96-98C; mp.: 40-42C.
EXAMPLE Z
7-chloro-4,8-dimethylquinoline
91 9 ~1.30 moles) of methyl vinyl ketone are ad-
ded to a solution of 840 9 of 70% strength sulfuric acid
t6.0 moles), 141.5 9 (1 mole) of 3-chloro-2-methylaniline
and 4 9 tO.027 mole) of sodium iodide in the course of
60 minutes at from 115 to 125C. Stirring is carried out
for 2 hours, 35 described in Example 1a), after ~hich the
mixture is ~orked up. 179 9 of crude product are ob-
tained. Distillation of crude product gives 167.2 g
(yield 86.8%) of 7-chloro-4,3-dimethylquinoline having
a purity of 99.5%; mp.: 51C.
EXAMPLE 3
4-methylquinoline
91 9 (1.30 moles) of methyl vinyl ketone are ad-
ded to a solution of 840 g of 70% strength sulfuric acid
(6.0 moles), 93 9 (t mole) of aniline and 4 9 (0.027 mole)
of sodium iodide in the course of 60 minutes at from 115
~ 1 3 ~ 2
- 6 - O.Z. 0050/39235
to 125C. Stirring is carried out for 10 minutes, as des-
cribed in E~ample 1a), after which the mixture is worked
up. 146.4 9 of a brown oil are obtained. Distillation
of the crude product gives 107.3 9 (yield 75%) of 4-methyl-
S quinoline having a purity of 99.3%; bp1: 80C.
EXAMPLE 4
7-chloro-Z,8-dimethylquinoline
91 9 (1.30 moles) crotonaldehyde are added to a
solution of 840 9 of 70~ strength sulfuric acid (6.0
moles), 141.5 9 (1 mole) of 3-chloro-2-methylaniline and
3 9 (0.02 mole) of sodium iodide in the course of 60
minutes at 110C.
After working up has been carried out as described
in Example 1a), 190.2 g of 7-chloro-2,8-dimethylquinoline
are obtained. Distillation of the crude product gives
146.8 9 (yield 75.7%) of 7-chloro-2,8-dimethylquinoline
having a purity of 98.8~ according to gas chromatography;
bp1: 102C.
EXAMPLE 5
7-chloro-3,8-dimethylquinoline
91 9 (1.30 moles) of methacrolein are added to
a solution of 840 9 of 70X strength sulfuric acid (6.0
moles), 141.5 9 (1 mole) of 3-chloro-2-methylaniline and
4 9 (0.027 mole) of sodium iodide in the course of 60
minutes at 12QCO
After ~lorking up has been carried out as des-
cribed in Exa~ple 1a), 198.4 9 of 7-chloro-3,8-dimethyl-
quinoline are obtained. Distillation of the crude pro-
duct gives 180.7 9 ~yield 90.7X) of 7-chloro-3,8-dimethyl-
quinoline having a purity of 96.1% according to gaschromatography; mp. 75-78C.