Note: Descriptions are shown in the official language in which they were submitted.
DESCRIPTION
The invention relates to an active substance-containing plaster for
the controlled administration of active substances to the skin, which
has a back and a skin side, wlth a back layer, an active substance
reservoir subdivided substantially perpendicularly to the ækin contact
surface of the plaster and having one or more active substances, &
contact adhe~i~e device on the skin side and optionally a cover layer
detachable prior to the application of the plaster, it being posslble
to reduce the size of ~he active substance release surface of the
plaster by a predetermined amount by removing part of the active
substance reservoir, as well as the use thereoP and a process for
the controlled administration of active substances to the skin.
The invention more partlcularly relates to those plaster used as trans-dermal therapeutic systems for the controlled administration of medical
active substances or also cosmetically actiYe substances to the human
or animal skin. A therapeutic system is a medicament or active
substance-containing means or administration form, which delivers
one or more active 6ubstances in continuous manner, at a predetermined
rate and over a given time interval to a predetermined application
point. These systems are therapeutic precision instruments, whose
construction requires extraordinary measures in order to ensure
continuous active substance release.
Plaster-like therapeutic systems have already been developed for the
most varied uses and, apart from a t opical effect, a systemic effect
can also be obtained. The multiplicity of active substances applicable
in this way and their different chemical, physical and pharmacological
characteristlcs make it impo6sible to cover all therapeutic problems
with a single system.
Numerous therapeutic systems for the administration of medical active
substances to the skin are known, a summary bein8 e.g. provided in
Klaus Heilmann "Therapeutische Systeme", Ferdinand Enke Verlag~
~.~
131 ~2
Stuttgart, 1~77. The prior art systems were not able to provlde a
completely satisfactory action in all cases.
In the conventional structure of a transdermal plaster-like ~herapeutic
system there is an active substance reservoir, which contains the
active substance in solid, liquid or dissolved form and a pressure-
sensitive adhesion layer (contact adhesive), through which the system
is closely linked with the skinO It is important that a whole-area
contact is ensured be~ween the active substsnce re:Lease ~urfaces to
the skin throughout the application oE the system, in order to make
sure of the active substance release kinetics. This can be achieved
not only through an uninterrupted adheslon layer, but also by rest-
ricted adhesion areas in the skin contact layer of ~he plaster.
Although the hitherto known plaster-like therapeutic system permit
a uniform or continuously decreasing application of an active 6ubstance
over a predetermined timet they do not permit specific active substance
release kinetics, such as a plsnned reduction of the active substance
release after a predetermined time, or a graduated active substance
release quantit~ per unit of ti~e.
This messure is e.g. necessary if the active substance dose has to
be reduced in a planned manner dllring the application period for ther-
apeutic reasons. The same problem arises if, during the application
of a combination product, one active substance in the combi~ation
must be interrupted after a predetermined time. In such cases, it
is in principle possible to solve the problem by using two transdermal
plasters, ~hereof one is removed at the given time. However, this
leads to problems for patients, particularly if they are aged. There-
fore it is easy to forge~ to appl~ the second plaster. In a combin-
ation of two plasters wi~h different active substance release surfaces,
there is also a risk of choo6ing the incorrect combina~ion (two
identical plasters), or the removal of the wrong plaster. The same
complications occur if plasters with different active substances have;
to be combined and there is in this case also the proble~ of double
~ 3 ~
-- 3 --
storage. Thus, the use of two plasters leads ~o numerous disadvantages
and the aYoidance thereof forms an objective of th~e lnvention.
The proposal of combinlng two plasters, whereby part thereof can be
detached with the aid of a predetermined breaking line, admittedly
improves compliance, but leads to handling problems. Thus, the part
to be detached can only be handled by raising from the skin and in
undesired manner at least a part of the plaster still remaining on
th~ skin i9 raised snd must be firmly held during detachment. Thus,
pressure is exerted on the sensitlvs transdermal s~stem, which can
negatively influence the active substance release rate.
US Patent 4 297 995 describeæ a manipulatable active sub~tance plaster,in which the active substance reservoir i9 subdivided, but i5 in~e-
grated into a single plaster. I'he active substance reservoir parts
are arranged in concentric rings about a disk-like central portion.
The overall construction of the plaster with a mechanical fixing of
the reservoir to the back layer onl~ makes it possible to change the
active substance release surface prior to the applicatio~ of the
plaster, i.e. prior to plaster application the doctor/patient can
choose which dose is to be administered, but for changing the active
substance release during plaster application, the plaster must be
rsised from the skin9 whilst interrupting therapy and this can also
lead to damage and contamination to the plaster support surface.
As yet no satisfactory solution has been found for the associated
problems of repositioning and the permanent refixing of the system
to the skin.
Therefore the problem of the present invention is to provide an
improved therapeutic transdermal system, which makes it possible to
realize more complicated changes to the active substance release than
ha~e hltherto been possible.
This problem is solved by a plaster of the aforementioned type, which is
characterized in that at least part of the active substance reservoir
~3~ 2
-- 4 --
cnn be detachedl whilst leaving one or more parts of said reservoir
on the skin and the part of the acti~e substance reservoir left behind
on the skin has a better adhesion to the skin than to the back layer.
Due to the fact that9 according to ~he inYentiOn, the act-lve substancereservo-lr is partly detachable whereby the part of the ac~ive substance
reservoir which is not to be detached has a greater adhesion to the
skln than to the back layer, after removing a predetermined plaster
part with the active subst&nce reservoir part adherlng thercto, there
is left behind a predetermlned acti~e substance reservoir part on
the skin, which can e.g. be alone removed following the desi~ed appli-
cation perlod. Advantageou61y the active substance reservoir of the
inventive plaster is in two parts.
In the case of given therapies with varying or marked concentration-
fluctuating active substance administrations, lt can also be advAn-
tageous for the ac~ive substance reservoir to be in three parts.
The active substAnce release surfAces of the actlve substance reservcir
parts can be geometrically identical or different. The actire sub-
stance release surface~ can be ~uxtaposed, or one active substance
reservoir part can completely surround one or more other active sub-
stance reservoir parts, considered in a flat or surfsce-based manner.
The subdivision of the partial surfaces i~ dependent on the therapeutic
requirements. Thus, e.g. one active substance reservoir part can
circularly surround one or more other active substance reservoir parts.
.
The release surface - size ratio of one active substance reservoir
part to another is preferably in the range between approximately
1:1 and 1:10. The same active substance or the same active substance
combination can be present ln all the active sub6tance reservoir parts.
Particularly preferred products sre plasters with the following active
substances, b~t it is also possible to process random other trans-
dermally administrsble active substance combinations known to the
medical Expert: asthma/bronchodilators, such as e.g. clenbuterol,
proctaterol and salbutamol and vasodilatorsl such as e.~. bencyclane
and clnnarizlne.
~316~6~
Thus, with the aid of the inventive plaster, in such cases it i8
possible to reduce the admlnistration dose in planned and controlled
manne} durlng application.
However, the active subs~ance reservoir parts can also contain diff-
erent active substances or different active substance co~binations,
80 that during application it is possible to interrupt or stop one
active substance or ac~ive substance combination. Example~ for di~f~
erent active substances in the active substance reservoir parts are:
oestrogen/gestagen (contraceptives), dexamethasone/prednisolone (in
the case of inflammatory, rheumatic muscle and jOillt diseases), nitro-
glycerin/ ~-blockers (for cardiac diseases)9 phenytoin/phenobarbital/
caffeine (for epilepsy) and amitriptyline/chlordiazepoxlde (psycho-
pharmaceuticals).
.
All suitable active substances belong to the groups having either
a topical or systemic sction. In at least one active substance reser-
voir part there csn be different active substance/active substance
combinations as compared with the other sctiYe substance reservoir
part or parts.
The preferred use of the inventive plasters is in local and systemic,
transdermal acti~e substance administration in humaa or animal medicine
or in cosmetics.
The invention is described in greater detail hereinafter relative
to non-l~mitative embodiments and with reference to the attached
drswings, which are not true to scale and whereln show:
ig. 1 a cross-section through a first embodiment of an inventive
plaster with a two-part active substance reservolr.
ig. 2 a plan view of the skin side of a second embodiment of the
inventive plaster without a protective layer.
ig. 3 a cross-section along line I-I of fig. 2.
~ 3~ 62
-- 6 --
ig. 4 a plsn vlew of the skin side of a third inventive embodiment
without protectlve layer
Fig. S a cross-sectiQn through the e~bodiment Df flg. 4 along line
II-II on a larger scale.
Fig. 6 a plan view of the skin side of a four~h embodiment of the
invention without a protective la~er.
Fig. 7 a ~ross-section through the e~bodiment of fig. 6 along line
III~III.
Fig. 8 a cross~sec~lon through a flfth embodiment of the invention.
Fig. 9 a cross-section through a sixth embodlment of the invention.
ig. 10 a plan view of the skin ~ide of another embodiment of an
inventive plaster ~ith a three-part active substance reser-
voir and without a protective layer.
Fig. 11 a larger scale cross-sec~ion along line IV-IV of fig. 10.
The preferred embodi~ents of the invention shown in the individual
drawingR will now be described. The active substance reservoir part
left last on the skin is referred to as the first active substance
reservoir part, whilst the second and third active substance reservoir
~parts are those which are detached with the back lsyer.
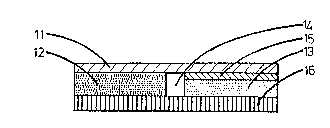
Fig. 1 shows a preferred emb~diment of an inventive, optionally circ-
ular or angular plaster with 8 two part, contact ~dhesive active
substance reser~oir ln cross-section. The second active substance
reservoir pzrt 12 directly adheres to the back layer 11 and i8 separ-
ated from the first contact adhesi~e active subs~ance reservoir par~
13 br a reserroir separatlng layer 14, which can be constructed as
a gap or space between the active substance reservoir parts, or can
be fill~d by an inert s~parating material. Active substance reservoir
part 13 sdheres to the back layer 11 by ~eans of a peel-off layer
15, which brings about a gradual adhesion of the two active substance
reservoir parts 12,13 to the back layer. The peel-off layer 15 can
e.g. be a polymer or ~etal film or foil, a textlle fabric or a la~inate
~ 3 ~
thereof and following the removal of the back layer 11 with the second
active substance reservoir part 12 forms a protective layer for the
first active substance reservoir part 13 left on the skin in order
to protect the latter. The adhesion of the first active substance
reservoir part 13 to the ækin must be greater than the adhesion between
the peel-of layer 15 end the back layer 11. The protective layer
16 is removed prior to the application oE the plaster. By the use
of the same dotting system for the surfaces representing the açtive
substance reservolr parts 12,13 ln fig. 1, it is ~ade clear that in
both active substance reservoir parts 12,13 there is the same active
subst~nce or active substance combination, so that with the plaster
according to fig. 1 it is possible to brin8 about a gradual decrease
of the ~ctive substance release to the skin.
.
Fig, 2 is a plan view of the skin side o a further inventive angular
plaster wlth a two-part, non-contact adhesive active substance reser-
voir, from which the protective layer has already been removed. Thus,
on the skin side; the plaster has contact adhesive la~ers 21,22, which
are separated from one another by the inert reservoir separating layer
23, which can once again be a simple gap or an inert materlal.
Fig. 3 is a cros6-section through the embodiment shown in fig. 2 along
line I-I thereof. The non-adhesive active substance reservoir parts
24,25 are fixed at the back by a contact adhesive layer 27 to the
back layer 28, the first active substance reservoir part 24 adhering
to the back layer by means of a peel-off layer 26 loca~ed between
the first eontact adhesive layer 27 and the first active substance
reservoir part 24. Peel-off layer 26 is designed in such a way that
its adhesion to the first active substance reservoir part 24 is greater
than to the contact adhesive layer 27. Thus, on detaching the back
layer 28 and the second active substance reservoir part 25 fixed by
means of the contact adhesive layer 27 to the back layer 28, peel-
off layer 26 together with the first active substance reservoir part
24 remains on the skin and assumes for said remaining part 24 the
function of a protective layer protecting the reservoir material from
~3~6~
-- 8 --
contamination and damage from the outside or also against the escape
of ¢.g. volatile active 6ubstance components.
The skin side contact adhesive layers 21,22 on the non-adhesive active
substanc~ reser~oir parts 24,25 are so sd~usted that the adheslon
of the con~act adhesive layer 22 of the first active substance reser-
voir part 24 to the skin i8 grea~er than the adhesion between peel-
off layer 26 and contact adhesive layer 27. The differently repreæ-
ented surfaces in the drawing are intended to show that there are
different active substances or active substance combinations in both
active substance reservoir parts 24,25.
Fi8. 4 æhows another preferred embodiment of an inventive plaster
in plan view on the skin side thereof. The oval plaster has a two-
part active substance reservoir and a contact adhesive lsyer 41 respon-
sible for the fi~ing of the active substance reservoir parts 42,43
to the back layer 45 also forms the fastening to the skin of the
therapeutic system.
Fig. 5 shows a lsrger scale cross-section along line II-II of fig.
4. The contac~ adhesive active substance reservoir parts 42,43, which
are separated from one another by an inert reservoir separating layer
46, in this case in the form of a gap, are surrounded in bag-like
manner by the back layer 45 carrying a contact adhesive layer 41.
~ere again a separating layer 44 ensures that the adhesion of the
first active substance reservoir part 42 to the skin is greater than
the adhesion between the peel-off layer 44 and the contact adhesi~e
layer 41 and also ensures that the first active substance reservoir
part 42 left on the skin after detaching the second active substance
reservoir part 43 and the back layer 45 is protected by a layer.
The two actlve substance reservoir parts 42,43 contain different active
substances or actlve substance combinatlons, as is ~ade clear by the
arrangement of the surfaceæ.
Fig. 6 shows another inventive plaster with a two-part active substance
~ 3 ~ 2
.
re~erroir, in which one active substance reservoir part 61 concentric-
811y surrounds the other actlve substance reservoir part 637 the
representntion being from the skin side and with the protective layer
removed. The contact adhesive, first circular active substance reser-
voir 63 is separated from the circular second act:ive substance reser-
voir 61 by an inert reserroir ~eparating layer 62, whlch can also
be in the orm of a gap. This embodiment has the advantage that on
reducing the active substance release surface b~ removing part of
the plas~er with ~he second active substance reservoir p~rt 61 and
together ~ith the back layer 65, it is possible to freel~ choose the
plas~er removal direction.
Fig. 7 shows a cross-section through the embodiment of fig. 6 repres-
ented along line III-III thereof. The circular, second sctive sub-
stance reservoir part 61 is directly adjacent to the back layer 65,
whilst the peel-off layer 64 is arranged between the clrcular, first
active substance reservoir part 63 and the back layer 65. The adhe~ion
of the first sctive substance reservoir par~ 63 to the skin and that
between the peel-off layer 64 a~d the back layer S5 is greater than
the adhesion between the peel-off layer 64 and active subs~ance reser-
voir part 63, so that on removing the back layer 65 the ~econd acti~e
subs~ance reservoir part 6I is removed, whilst leaving the first actlve
substance reservoir part 63 on the skin and which is now covered by
the peel-of la~er 64. The inert reservoir separating layer 62
separates active substance reservoir parts 61,63. In this embodiment,
the active substance is the same in both active substance reservoir
parts 61,63.
Fig. 8 shows snother preferred embodimen~ of the inventlon in cross-
section, in which the two parts o~ a non-adhesive active substance
reservoir are once again cons~ructed as a circular disk 82 with 8
surroundi~g ring 81. The~ are separated from one another by an inert
reservoir separating layer 83. Back layer 88 is covered by a contact
adhesive layer 87, whlch is directly in contact wlth the second active
substance reservoir part 81.
~ 3 ~ 2
-- 10 -
.
Between the contact adhesive la~er 87 and the first actlve substance
reservoir part 82 is provided an inert peel-off layer 86~ whieh is
so designed that its adhesion to the contact adhesive la~er 87 iB
le~s than the adhesion between the contact adhesive layer 85 and the
skin. The remaining adhesion values on the boundary layers of said
plaster part must naturally be above the adhesion value to the skin.
The skin side contact adhesive layers 84,85 of the active substance
reservoir parts 81,82 ensure contact with the skin and prior to the
application of the inventive plaster are advantageousl~ covered by
a protective layer 89.
In this embodLment, both active substance reservoir parts 81,82 have
diferent active substances or active substance combination~.
Fig. 9 shows a cross-sectiorl through another embodiment of the in~en-
tion, in which the back la~er 95 6urrounds the coneentric arrangement
of the contact adheslve active substance reservoir parts 91,92 to
the skin side. For fixlng the overall plsster and the active ~ubstance
reser~sir parts to the skin, the whole surface of back layer 95 is
covered wlth a co~tact adhesive layer 93. To the latter adheres the
second active substance reservoir part 91 in a direct manner~ whilst
the first active substance reservoir part 92 is fixed via a peel-off
layer 94. The adhesion of peel-off la~er g4 to the active substance
reservoir part 92 is above and the adhesion of layer 94 to contact
adhesive layer 93 is below the adhesion between the first active sub-
stance reservoir part 92 and the skin. This permits a selective
removal of back layer 95 together with the second active substance
reservoir part 91. Through a corresponding change to the structure,
it is also possible to bring about a selective removal of the first
actlve s~bstance reservoir part 92. Reference numeral 96 designates
he inert reservoir Reparating layer or the gap between the active
substance reser~oir parts 91,92. Up to application, the overall
arrangement i6 covered by a detachable protective layer 97. The acti~e
substances or active substance combinations are different in both
reservoir parts 91,92 in this embodiment.
~31~62
~ 11 --
Another preferred embodiment of the invention wi~h a three-part active
substance reservoir is shown in fig. 10. The latter is a plan view
of the skin side of nn inventive plaster withollt the protective layer.
A firs~, circular, con~act adhesive active substance reservolr part
105 is surrounded by a second, circular, contact adhesive active sub-
stance reservoir part 103 and is separated therefrom by an inert
reservoir separating layer 104. The third contact adhesive sctive
substance reservoir part 101 surrounds the second active substance
reservoir part 103 in concentric ring form. Bet~een the second and
third active substance reservoir parts 101,103 ls provided a further
inert reservoir separating layer 102. Fig. 11 is a larger scale cross-
section along line IY-IV.
Active substance reservoir part 101 adheres directly to back layer
108. A first peel-off layer 106 connected to back layer 108 covers
the active substance reservoir parts 103,105. The adhesion thereof
to the back layer 108 is less than the adhesion of the active-substance
reserroir parts 103,105 to the ækin, so that active substance reservoir
parts 103,105 remain on the skin on removing the back layer 108 wi~h
active substance reservoir part 101. Thus, the first peel-off layer
106 then orms a protective, new layer for the rema~ning parts of
the plaster. The contact adhesive active substance reservoir part
103 adheres directly to the first peel-off layer 106 and can be re~oved
together with the latter in a further removal step, whilst leaving
behind on the skin the first active substance reservoir part 105,
this being made possible by the second peel-off layer 107 between
the first actire substance reservoir part 105 and the flrst peel-off
layer 106. The adhesion of the second peel-off layer 107 to the first
layer 106 is less than the adhesion of the first active substance
reservoir part 105 to the skin. The inert reservoir separating layers
102,104, which can also be formed by gaps, are located between active
substance reservoir parts 101,103 or 102,105. The chosen ex~mple
shows for active substance reservoir parts 103,105 the same actire
substance or active substance combination, indicated by the identical
dotting in flg. 11~ whilst the active substance reservoir part 101
~ 3 ~
12 -
hss a different actlYe substance or active substance comblnation.
The drawings which merely lllustrate the invention in an e~emplified
manner, are not lntended to restrict the invention ei~her as regards
geometrical shape, the association of speciflc indi~idual components,
or aæ regards the size of tlle active substance release surfsces.
As is well known to the Expert in this field, al.L these quantities
can be adapted to the therapeutic requirements and obviously account
must be taken oE rational production. The part of the active substance
reservoir part to be detached can also have a peel-off layerD which
then has a greater adhesion to the back layer than the peel-off layer
of the reservoir part remaining on the skin. This construction is
advantageous if the active substance reservoir parts are to have the
same thickness. The contact adhesive layers, particularl~ those on
the skin side, can have contact adhesive-free areas for improved active
substance permeability, or can be constructed solel~ as individual
contact adhesive surfaces, e.g. can be embedded in the active substance
reservoir material. The release of the active substances fro~ the
active substance reservoir parts can optionally be regulated b~ control
membranes, which are embedded in per se known manner in th~ reservoir
mass in one or more active substance reservoir parts, or can be located
between the active substance reservoir and the skin side contact
adhesive layer, or within the skin side contact adhesi~e layer.
:
For the construction of an active substance reservoir, as used accord-
ing to the invention, it is possible to use the standard measures
and materials. The basic materials csn be conetituted by low and
high molecular weight, natsral and/or synthetic substances, whose
choice is a function of the characteristics of the active substances
to be administered and the therapeutic requirements. Apart from the
basic material, the active substance reservoir can also contain further
suitable additives known or obvious to the ~xpert on the basis of
his knowledge, such as e.g. solubilizers, softeners, plastlcizers,
tackifiers, stabilizers, fillers and enhancers. The composition in
the reservoir parts can be the same or different, which is once again
dependent on the active substances to be administered and the desired
~ 3 ~ 2
- 13 -
release rates or kinetics. In the case of an identical reservoir
oomposition and only one active substance to be adminlstered, it can
be approprlate to have a graduated concentration setting of the actlve
substance in the different reservoir parts. Sultable active substances
for the inventive plasters are all those which alone or with adjuvants
are able to mi8rate into the skin.
The back layer covering the actire substance reservoir on the skln
remote side can be permeable or impermeable. It must be flexible
and, as a result of the mechanical stabilization of the plaster, serves
to remove part of the a~tive substance reservoir. If components of
the reservoir or the incorporated active substances are volat~le,
then the back layer must be impermeable to these substances. It can
be in one or ~ulti-layer form. Suitable materials for the production
thereof are e.8. polymeric substances, such as polyethylene, polyprop-
ylene, polyesters and polyamldes. Further materlals can be metal
foils, such as e.g. aluminium foil, either alone or coated with a
polymeric substrate. Permeable back layers are e.g. textile fabrics,
such as non-woven fabrics and the like, but also porous polymer
materials. The back layer can optionally carry a marking for indic-
ating the optimum plaster remoYal direction. Optionally peel-off
layers are provided between the active substance reservoir parts ln
place of a gap. They can be constituted by the same materials as
described hereinbefore for the separating layer between the active
substance reservoir parts and the back layer. The protective layer
detachable prior to application and which covers the skin side contact
adhesive surfaces can be made from the same materlal as used for making
the back layer, provided that they can be made detachable, e.g. by
applylng a silicone layer. Other detachable protective layers, as
known to the Expert in the field of plasters and in particular plaster-
like therapeutic systems are e~g. polytetrafluoroethylene, treated
paper, cellophsne, polyvinylchloride, etc. To facilltate detach-
ability, the protective layer can be provided with removal aids in
per se known manner. The protective layer can also be larger than
the plaster, e.g. if several plasters are arranged on an uninterrupted
1 3 ~
.
- 14 -
web of the pro~ective layer material.
Contact adhesive layers used for the inventive plasters can be cons-
tituted by all physiologically unob~ectiollable contact adhesives which
are inert to active substances and other active subs~snce reservoir
componen~s, e.g. can be based on rubber, rubber-llke, synthetic homo-
co- or block polymers, polyacrylstes and their copolrmers, poly-
urethanes and silicones.
The inventive plaster can be produced by all known plaster technology
methods. This is demonstrated in the following production e~ample
for a plaster with a two-part actiYe substance reserYoir.
Production Example.
~, .
In a roller application process to a film or foil given a repelling
~inish b7 siliconizing and which serves as an intermediate co~ering,
is spplied an active substance-containing reservoir material solution
and which, after drying at 65C, forms a contact adhesl~e layer of
55 g/m2 and having the following composition:
~, .
1. Acrylate copolymer I 68.86 parts by weight
2. Acrylate copolymer II 10.39 parts by weight
3. POE-(10)-oleyl slcohol 5.20 parts by weight
4. Active substance 15.56 parts by weight
Acrylste copolymer I i9 Durotac 280-2516 of National Starch & Chemicsl
B.V., Netherlands; Acrylate copolymer II i9 Eudragit E100 of Rohm
Pharma, Germany; POE-(10)-oleyl alcohol is Bri; 97 of Atlas Chemical
Industries, GB.
~nto one half of the actlve substance-contsining contact adhesive
layer is applied a non-siliconized polyester film (~hickneæ~ 0.036
mm~ and to the other hal~ the non-siliconized slde of a one-sided,
siliconized polyester film (thickness 0.036 mm). After removing the
1 3 ~ 2
- 15 -
intermediate covering ring-like active ~ubstance reservoir parts ars
punched out of the first half and are applied Wit}l the contact adhesive
side to a slliconized, aluminium vapour-deposited polyester film (prot-
ective layer). From the second half are punched disk-like actlve
substance reservoir parts with a slightly smatler diame~er than the
internal diameter o~ the rings into which they sre centrally inserted
with the contsct adhesive side directed towards the protective layer.
The open side of the thus obtained union is covered by a polyester-
based, contact adhesively finished 0.015 mm thick back layer (Durotac
280-2516, 30 g/m2 dry), the outwardly directed side being adhesive-
free. The distance between ~he actlve substance reservoir units is
approximately 14 mm, so that the back layer i9 in contact with the
protective layer between the units. The thus obtained laminate is
then supplied to a production process, in which by circular punching
at a distance of approximately 7 mm from the outer edge of the rings,
all-round closed disk-like plasters are obtained, one or more of which
are packed in sealed bags.