Note: Descriptions are shown in the official language in which they were submitted.
~31~7
~LD OF TtlE INVENTIQ~l
The invention relates to an improvernent in a process for recovering natural gas liquids (NGL's)
from a moisture-containing natural gas stream. More particularly, the invention relates to a
process wherein
,~:
, .:-' :
~ .
:
........
r.: '
':"` . ,
2 ~3~ ~4~
the wet natural gas is first dehydrated to increase the water dewpoint depression thereof, then
chilled by a refrigerant to condense out the heavy hydrocarbon fractions (the NGL's). The
resultant NGL stream is treated in a fractionator whereby a liquified fractionation stream and a
gaseous stream are contacted ~n a SCAU (Super Cooled Absorption Unit). The liquified
fractionator stream is used to absorb propane and heavier components which are then further
treated in the same fractionator resulting in increased quantities of NGL produced.
BACKGRC)WND OF THE INVENTION
Heretofore, processes have been developed and applied commercially ~o recover heavy
hydrocarbon fractions contained in raw natural gas. These fractions are commonly referred to
as "naturai gas liquids" or "NGL's".
The application of these processes has grown as uses for the NGL's have developed. For
example, NGL's have been used in Canada for blending with viscous heavy oil to render the latter
transportable through a pipeline.
The prior art processes so developed have involved:
1) Dehydrating the raw natural gas; and
2) then cooling the dried gas to condense the NGL's into separable liquid form.
One known type of process involves contacting the water wet gas with a liquid desiccant, such
as glycol, and then chilling the dried gas in a chiller with a refrigerant such as propane. The
water-rich glycol is regenerated by reboiling. The propane is cooled by a single stage mechanical
compression operation. The chilling step is conducted at a temperature between about 0F and
-1 5F. This process has, for example, been practised in a plant at Rowley in Alberta and yields
a recovery of about 12 Imperial barrels of NGL's per 1 million cubic feet of 0.65 specific gravity
natural gas processed.
X'
:
. .
.
- .
13~8~
It is also known from United States Patent 4,005,997 that improved dehydration can be achieved
by the process disclosed therein. According to this patented process, the glycol dehydration
process previously mentioned is modified by removing residual water in the reboiled glycol using
an azeotropic distillation step employing an azeotropic agent such as isooctane.
The foregoing patented process is known in the industry as the ~RIZO"~ process. The process
has been employed commercially on water wet natural gas but, to the best of applicant's
knowledge, only to dehydrate raw natural gas so that it is better transpor~able in a pipeline.
Although there has been a demand for some years now for a process that would recover more
NGL's at comparable cost to the existing commercially applied processes, to this applicant's
knowledge no such process has been forthcoming, with the exception of the process applied for
under my copending Canadian Patent Application No. 550,020-6, file~ October 22, 1987.
It is therefore the object of the present invention to provide a process yielding a higher percentage
recovery of NGL's, contained in a wet, raw, natural gas stream, than has heretofore been the
case.
SUMMARy QF T~1E INVEN~ION
In accordance with the present invention, there is provided an improvement to the above
mentioned natural gas liquid recovery process described in Canadian Application No. 550,020-6.
The improved process involves the combination of:
- dehydrating the wet natural gas stream using the known DRIZO process process, to
reduce the water content of the natural gas to less than about 10 ppm, preferably 1 ppm;
- cooling a refrigerant by means of compound mechanical compression to a temperature
in the range of -40F to -50F and chilling the dried natural gas with the cooled refrigerant
to condense NGL's from the natural gas;
,
. ..
,
,. .
' ' : ~
.:
~.
4 1316~
- cooling the chilled dried natural gas further by expanding the gas through a Joule
Thomson valve to a temperature of about -~0F to -70F; and
- using a SCAU (Super Cooled Absorption Unit) to extract additional heavier components
of a gas phase into a liquid. The feed streams to the SCAU, being the chilled gas stream
from the chiller which has been further chilled by a Joule Thomson valve and theoverheads from the fractionator which has been further chilled by a Joule Thomson valve.
The present invention, therefore, resides in a process for treating a moisture-containing raw
natural gas stream to recover natural gas liquids comprising:
contacting the raw natural gas stream in extractive relationship with lean glycol containing less
than 250 ppm of water, to reduce the water content of the natural gas stream to less than 10 ppm
and to produce water-rich glycol;
regenerating the lean glycol by reboiling the water-rich glycol and contacting the reboiled glycoi
with an azeotropic agent to remove residual water, whereby lean glycol containing less than 250
ppm of water is obtained for recycling to the extractive step;
chilling the dried natural gas stream to a temperature which is less than about -40F by passing
it in heat exchange with a liquid refrigerant which is at a temperature less than about -45F, to
produce condensed natural gas liquids, dried condensed natural gas, and a vapourized
refrigerant;
mechanically compressing the vapourized refrigerant in at least two stages to liquify and cool the
refrigerant to a temperature less than about -45F, whereby cold liquified refrigeran~ is obtained
for recycling to the chilling step;
X
'
..~ .
;~
~ 3 ~
expanding the partially condensed hydrocarbon gas leaving the chiller in a Joule Thomson valve
to reduce the pressure and lower the temperature further to about -70F;
separating the thus cooied, partially condensed, dried gas stream, in a low temperature separator,
into condensed natural gas liquids and a first oYerhead hydrocarbon gas stream;
fractionating the condensed natural gas liquids in a fractionation column, to provide a second
overhead gas stream and natural gas liquids;
cooling said second overhead gas stream substantially to liquefaction;
followed by counter-currently contacting the substantially liquified stream and the first overheab
gas stream in a Super Cooled Absorption Unit (SCAU) to flash off the lighter hydrocarbon
fractions and absorb the propane and heavier hydrocarbon fractions from said overhead gas
stream into the liquified stream; and recovering the condensed liquid and vapourized stream
therefrom.
By combining a dehydration process that yields a very dry natural gas stream, a refrigeration
process that cools the gas, expanding the gas to reduce the temperature of the gas further and
using a SCAU at very low temperatures to recover additional heavy ends from a gas stream, it
is possible to increase the recovery of propane and heavier hydrocarbons in the NGL's in the
order of ten percent, when compared with recovery from the process described in Canadian
Patent Application No. 550,020-6.
DESCRlpTlC~I OF THE D~AWIN13$
Figure 1 is a block diagram showing the combination of circuits making up the present invention;
Figure 2 is a schematic flow diagram showing the natural gas dehydration and desiccant
regeneration circuit; and
r ~
1 3~6~
Figure 3 is a schematic flow diagram showing the circuit utilized in the process of the present
invention.
DESCRlpTlON OF THE PREFERRED EMBODIMENT
In my copending Canadian patent application, No. 550,020-6, I describe the treatment of the wet
natural gas in the natural gas dehydration circuit and the further chilling of the dried gas in a dried
gas/chilling NGL Condensation Circuit.
X
.. .
....
7 ~3~6~47
In this earlier process, the chilled gas leaving the chiller is introduced into a low temperature
separator vessel. In the low temperature separator vessel, the liquid fractions, i.e. NGL's, settled
to the base and are withdrawn therefrom. The gas stream frorn the low temperature separator
vessel is withdrawn at the top therefrom and sold as sales gas.
The improvement to the above invention will now be described with respect to a simulated
process which has been successfully designed. The results obtained are indicated in Table 2.
It will be understood, however, that the scope of the invention is set forth in the summary and
defined in the claims following.
THE GAS 4EHYDF~AT10~/DESICCANT R~GENERATION C:IRCUIT
The known DRIZO~ dehydration Circuit 1 is utilized as part of the present process. This Circuit
1 was selected because it is capable of dehydrating the wet natural gas to a degree that the latter
is capable of being chilled to a temperature in the range of -50F to -80F without plugging the
equipment by formation of hydrates.
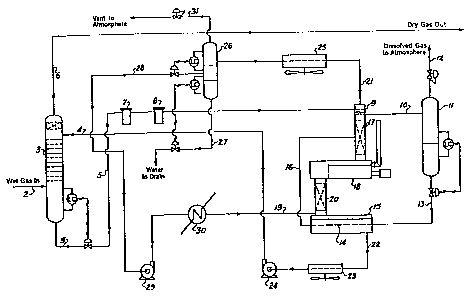
More particularly, wet natural gas, having a typical composition set forth in Table 1 and an
ambient temperature in the order of 60F is introduced via line 2 into the base of a contactor 3
(See Figure 2).
TABLE 1
Gas Composition in Mole %
He 00.02 iC4 00.61
N2 02.73 nC4 01.35
CO2 01.33 iCs 00.32
C1 79.19 nC5 00.32
C2 09.1 1 C6 00.15
C3 04.80 C7+ 00.07
~
~ . , .
.
,
'''
:. , , .. ' - :
.
-
1 3 .~
The gas flowrate is typically Z0 MMSCFD. The contactor 3 is a gas/liquid contactor of
conventional design. It operates at a pressure of 540 psia.
The wet natural gas contacts counter-currently in extractive relationship in the contactor 3 by a
liquid desiccant, specifically triethylene glycol, introduced into the top of contactor 3 by line ~.
The triethylene glycol, termed the lean glycol, contains less than 250 ppm of water, typically 200
ppm of water. The glycol is supplied at a rate of 60 Imperial gallons per hour at a temperature
of 75F.
The dried gas leaves the top of the contactor 3 through the line 6. Typically, the dried gas
contains less than 1.0 ppm of water. The dried gas then passes to the gas cooling and
liquefaction circuit shown in Figure 3, which will be fully described hereinafter.
Water-rich glycol leaves the base of the contactor 3, through line 5 to be led through the circuit,
the function of which is to regenerate rich glycol to lean glycol. On leaving the contactor 3, the
water concentration of the glycol is 4% for a gas temperature in the contactor of 60F, at a
pressure of 540 psia and a glycol circulation rate of 60 Imperial gallons per hour.
The water-rich glycol in line 5 is fed through serially connected filters 7 and 8 respectively, and
into a reflux coil 9 where it is heated from 60F to 90Fi
From coil 9, the water-rich glycol is fed at a rate of 60 Imperial gallons per hour via line 10 into
flash drum 11, which is maintained at a pressure of 45 psia. In the flash drum 11, dissolved
gases such as N2 and CO2 and some hydrocarbons, flash off and are vented to the atmosphere
through line 12.
The water rich glycol stream leaves the base of the flash drum 11 through line 13. It is fed
through a coil 14 positioned within the glycol accumulator vessel 15, where it is heated to 225F.
.". ~
. ., i- . ~ .. ,,,",.
9 13.~6~
From coil 14, the heated glycol is fed via line 16 into the central portion of an isothermal still
column 17. The water-rich glycol flows downward under gravity in the column 17 into a glycol
reboiler 18.
The bath temperature of the glycol reboiler is maintained at 400F. In the reboiler 18, a
substantial portion of the water is evaporated, leaving a glycol solution containing about 1.2%
water.
The glycol leav~s the reboiler through stripping still column 20 and enters glycol accumulator
vessel 15.
A stream of an azeotropic agent, specifically isooctane, is introduced via line 19 into a stripping
still column 20.
The stripping still column 20 is positioned beneath the g!ycol reboiler 18, whereby isooctane is
effectively introduced under the support of the packed section of the stripping still column 20 and
rises in counter-current contact with the reboiled glycol moving down in said still column. The
isooctane is introduced at a rate of 20 Imperial gallons per hour and at a temperature of 1 40F.
The isooctane forms an azeotropic mixture with the residual water in the rebolled glycol moving
down in the stripping still column 20. The azeotropic mixture flows through reboiler 18 and
upward through isothermal still column 17 and leaves the latter via line 21, The azeotropic
mixture is comprised of water and isooctane in the ratio 1:32 by volume.
The lean glycol flows downward via stripping still column 20 into the glycol accumulator 15. The
glycol is withdrawn from accumulator 15 via line 22 and passes via trim cooler 23 and pump 24
to contactor 3.
The azeotropic mixture withdrawn overhead via line 21 from the isothermal still column 17 is fed
to a condenser 25. The condenser 25 converts the azeotropic mixture to a liquid comprised of
water and isooctane, which is fed into a three phase separator vessel 26 where the pressure is
maintained at 16 psia.
~ `
... ,.. ,... . :
.
.. . .
, ,
,
: . . ~ ,
. . .
,~ . ,
. ~ .
~o 131~7
In the separator vessel 26, the water pl~ase settles to the base thereof and is withdrawn therefrom
through drainline 27.
The isooctane phase settles out above the water phase. It is withdrawn through line 28 and
recycled via pump 29 and heater 30 to the stripping still column 20, where it is introduced in the
vapour phase.
The gaseous phase of separator vessel 26 is vented to the atmosphere through line 31.
Dried Gas CLllin~/NGL Con~ensation, Refrigerant Cooling, Absorption and
Fractionation Circuit
In this circuit the dried natural gas product from circuit 1 (as shown in Figure 2) is chilled to -49F
and the liquifiable NGL's at that temperature are condensed out. The propane refrigerant used
is cooled to the desired temperature, using a two-stage compound mechanical refrigeration
system.
The hydrocarbon liquid and gas stream leaving the chiller is further cooled by expansion in a
Joule Thomson valve. The cooled stream is passed to a low ternperature separator where
hydrocarbon liquids and an overhead hydrocarbon gas stream are produced. The hydrocarbon
liquids are then fractionated to produce the NGL product and an overhead gas stream. The latter
stream is partially liquified by expansion and cooling in a Joule Thomson valve. This liquified
stream and the hydrocarbon gas stream from the low temperature separator are contacted
counter-currently in a Super Cooled Absorption Unit, thus flashing and absorbing propane and
heavier components from the gas stream. The liquids flow to the fractionator and the process
is completed with NGL collected at the base of the fractionator.
More particularly, on leaving circuit 1 via line 6 (see Figure 2), the dried natural gas at a
temperature of 60F, pressure of 540 psia and a rate of 20 MMSCFD, flows through a gas/gas
heat exchanger 32 to lower its temperature to 0F. The cooler gas stream is passed via line 33
`- X
11 13 ~
into chiller 34 where it indirectly contacts the propane in heat exchange relatlonship.
Liquid propane refrigerant at a temperature of -58F is fed to the chiller through line 35. The
vessel is maintained at a pressure of 12 psia. Propane vapour at a temperature of -55F and
pressure of 11 psia is withdrawn from chiller 34 via line 40 for recycling and cooling.
In order to recycle and cool the propane drawn from chiller 34, the vapour first passes through
line 40 to a suction scrubber 41, to remove liquids entraine~ therein. From scrubber 41, the
vapour passes ~hrough line 42 into the first stage compressor 43 where it is compressed to a
pressure of 62 psia and a temperature of 125F.
The hot vapour from compressor 43 is fed via line 44 into a desuperheater/economizer vesse! 45
In the desuperheater/economizer vessel 45, the liquid propane settles in the lower part thereof
and is withdrawn therefrom. Withdrawing the vapour from the top of vessel 45 causes propane
liquid to flash off, thus cooling the liquid from 125F to 27F.
The propane vapour, at a pressure of 62 psia, is fed through line 46 from the upper portion of
vessel 45. The vapour passes through a second stage compressor 47 which compresses the
vapour to 216 psia at a temperature of 1 50F.
The compressed vapour is sent via line 4~ to a condenser 49 to convert it to a liquid at 1 00F
using 90F ambient air. It is then passed via line 50 to a surge receiver 51 and on through an
expansion valve 52. In valve 52, the vapour is cooled to a temperature of 27F and a pressure
of 62 psia. From the expansion valve 52 the vapour is introduced through line 51 into the
desuperheater/economizer vessel 45.
;,,, ; ~ :
' '... .
' '' ,,' ~ . ' ,
- .
.. , . :
, . ,
' ' .,.,.,",;. - : . j,.. , .,.:: .,.
12 :~ 3 ~
As stated previously, the liquid propane settles in the lower portion of desuperheater/economizer
vessel 45 and is withdrawn therefrom. The temperature and pressure of the propane leaving the
vessel 45 through line 53 is 27F and 62 psia.
The liquid propane from desuperheater/economizer vessel 45 is passed through the heat
exchanger 39 and is cooled to -20F. It is cooled further to -58F and a pressure of 12 psia by
passage through expansion valve 54. Thus the cooled propane is then fed via line 35 into chiller
34.
The chilled dry gas and condensed hydrocarbons leave the chiller 34 via line 36 at a temperature
of -49F, at 53Q psia and pass through a Joule Thomson valve 37, where the pressure is reduced
to 300 psia. This reduction in pressure cools the stream further to a temperature of about -70F.
The chilled stream is then introduced into the low temperature separator vessel 55 via line 38.
In the low temperature separator vessel 55, the liquid fractions NGL's settle to the base and are
withdrawn through line 57. The chilled liquids are utilized for energy efficiency purposes in heat
exchanger 39, and then, via line 58, introduced through a pump 60 to the fractionator column 62
via line 61.
The overhead gas stream from the fractionation column 62 is discharged through line 63 at a
temperature of -14F. It is then cooled to partial liquefaction by means of exchanger 70, to a
temperature of -50F. The cooled stream is then passes through a Joule Thomson valve 66 to
reduce the pressure to 300 psia. The temperature of the stream is thereby lowered to -62F. On
leaving the valve 66, the cold two-phase stream flows through line 67 and is introduced into the
upper region of the SCAU (Super Cooled Absorption Unit).
A Super Cooled Absorption Unit (SCAU) is a pressure vessel wherein a liquid flows counter-
current to a gas strearn at extreme low temperature for the purpose of removing one or more
constituents from the gas. Most SCAU's are vertical with the liquid entering at the top
.~
. .
,
,. .; .~
-
13 13~6~7
and the gas at the bottom. The amount of contact that must be provided in this SCAU depends
on the system, the reiative flow rate of liquid and gas, and the composition of the gas involved.
The overhead gas stream withdrawn from the low temperature separator 55 via iine 56 enters the
lower region of the SCAU (Super Cooled Absorption Unit) 68 at a temperature of about -70F.
In the SCAU 68, the low temperature separator overhead gas stream contacts the partially
liquified gas stream from the fractionator 62 counter-currently. The lighter hydrocarbon liquid
fractions, namely methane and ethane, entering the SCAU via line 63 from the fractionator flow
down within the SCAU and preferentially absorb the propane and heavier components from the
contacted gaseous stream that flowed upwards, entering the SCAU via line 56 from the low
temperature separator. The methane and ethane vapourize by absorbing heat from the gaseous
propane and heavier hydrocarbons, thereby causing the propane and heavier hydrocarbons to
condense at the bottom of the SCAU.
The gaseous stream flows out at the top of the SCAU 68 via line 69 and is heated up via heat
exchangers 70 and 32. The gas stream then leaves the circuit for sales via line 72.
The hydrocarbon liquids are withdrawn from the base of the SCAU 68 via line 59 and are
combined with the liquids in line 58, for introduction into the fractionator'vessel 62. The liquids
from the bottom of the fractionator are removed through line 64 as natural gas liquids (NGL's)
which thereby consist essentially of propane and heavier hydrocarbon components.
The beneficial results (hi~h recoveries of NGL's) of the practice of the invention as just described,
are set forth in Table 2. As shown, in accordance with the invention, when the temperature of
the chilled gas is -49F and a SCAU is used, the recovery of NGL's is in the order of 47.5 API
BbllMMSCFD of natural gas processed based on 0.71 specific gravity gas with 2% of the ethane
remaining in the NGL's. This
~'
", . . .
- . . ~ .
.
... . .
1~ 13165~ i'
compares with applicantls earlier process yielding 44 API Bbl/MMSCFD from the same 0.71
specific gravity gas.
The following table is provided to illustrate the improved recovery yields using the present
improvement in comparison to applicant's earlier process mentioned hereinbefore.
TABLE 2
Recovery Improved Original
API Bbl/MMSCFD 47.5 44.0
% C3 77.8 70.3
% C4 97.4 91.3
% C5 100.00 97.6
% C6 100.00 99.5
% C7+ 100.00 99.9
.
.~ ,
......... ..
'' , ' '' '~ i ,
'
, ':
"