Note: Descriptions are shown in the official language in which they were submitted.
131~8
Background of the Invention
Field of the Invention
This invention relates to a porous hollow
fiber membrane and a method for the removal of a
virus by using the same. More particularly, the
~ present invention is concerned with a novel porous
hollow fiber membrane which is characterized by its
unique porous structure wherein the inner and outer
membrane surfaces have an in-a-plane average pore
diameter of 0.01 to 10 ~m and the porous membrane
wall has an in-a-plane porosity of not less than
10 % measured in every plane perpendicular to a
radial direction of the annular cross-section of
the hollow fiber membrane, said in-a-plane porosity
exhibiting at least one minimum value between the
inner and outer membrane surfaces. The present
invention is also concerned with a method for the
removal of a virus from an aqueous protein solution
containing a virus by the use of the above-
mentioned porous hollow fiber membrane. 'The novel
hollow fiber membrane and the method of the present
invention are especially useful because they are
extremely effective for the removal of a virus with
the great advantages that both an excellent virus
`~ 131~0~8
removal percentage and a high filtration speed can be
simultaneously attained.
DISCUSSIOM OF RELA~ED ART
Methods for the re~oval of viruses from
aqueous solutions by using a uniform and symmetrical
membrane (e.g. the microporous polyethylene hollow
fiber disclosed in USP 4401567) are dl~closed in
Japanese Patent Application Laid-O~en Specification
Nos. 60-142860 laid open July 29, 1985, inventors
Kawai et al., 60-142861 laid open July 29, 1985,
inventors Kawai et al. and 61-168367 laid open July
3, 1986, inventors Kawai et al. In these methods of
prior art, hollow fiber membranes having an effec-
tive thickness of 5~m or more and a uniform pore
structure are utilized to remo~e viruses. As an
example of such hollow fiber membranes, there can be
mentioned a polyethylene microporous hollow fiber
which has rectangular pores formed by microfibrils
that are oriented in the lengthwise direction of the
fiber and knotted portions that are aonnected to said
microfibrils sub~tantially at right angles thereto,
the averaqe width o~ the pores being in the range of
from 0~05 to 0.35 ~m, the pores being conti~uous with
each other from the inner wall surface to the
outer wall surface to form a stacked, multicellular
structure. In the specifications o~ these Japanese
- 3 -
` 131~8
patent applications, there is a description to the
effect that when the filtrate ohtained by the ~iltra-
tion of HBs antigen-positive fresh human plasma using
the above-mentioned hollow fiber membrane was observed
by an electron microscopy, there was detected no Dane
particle haYing a diametler of 0.042 ~m. In this
connection, however, it should be noted that there is
no description wlth respect to the actual virus
removal percentage (the measurable upper limit of
virus removal percentage by electron microscopy is
about 99~). Further, since in these cases the
transmembrane pressure which is one of the filtration
conditions is 50 mmHg or less, and the filtration
speed is extremely low, such a filtration method
cannot be commercially employed for removing viruses.
Furthermore, since the filtration speed is low, the
physiological activity of the filtrate ~ecomes
extremely low.
On the other hand, in the Japanese
Patent Application Laid-Open Specification No. 61-
254202 laid open November 12, 1986, inventors Manabe
et al., a method is disclosed for the removal of
tabaco mosaic virus from an aqueous solution contain-
ing ovalbumin by using a porous hollow fiber made of
cuprammonium regenerated cellulose which has an
-- 4 --
~ 3 ~
average pore diameter of 0.02 to 0.2 ~m and an in-
a~plane porosity of 10 % or more. However, with
this method, the virus removal percentage is about
99 % and the ovalbumin permeability is 43 %, which
is insufficient for practical use. This hollow
fiber has a relatively uniform pore structure.
With the above-mentioned conventional methods
in which an asymmetrical, or uniform, symmetrical
porous membrane is employed, high virus removal
percentage and high filtration speed (or high
permeability for protein) cannot be simultaneously
attained. The protein permeability of the
conventional porous membranes is about 50 %.
In general, if the average pore diameter of a
porous membrane is decreased, the virus removal
percentage is increased but the filtration speed
and the protein concentration o~ the filtrate are
lowered. If the average pore diameter is
increased, the virus removal percentage is lowered
to 99 % or less, which is insufficient for a
membrane to be used for the removal of viruses.
The virus removal percentage normally required for
a virus removing membrane is as high as 99.99 to
99.99g999 %. Thus, there has been a technical
dilemma that a porous membrane cannot be
-- 5 --
~3:~8~
simultaneously characterized by an excellent virus
removal percentage and a high filtration speed when
it is used for the removal of a virus from an
aqueous protein solution containing a virus.
Therefore, it has been desired to solve the dilemma
and develop a porous hollow fiber membrane having
both an excellent virus removal percentage and a
high filtration speed.
Summary of The Invention
With a view to developing a novel porous
hollow ~iber membrane free from the above-mentioned
drawbacks inevitably accompanying the conventional
porous membranes and a method of removing a virus,
the present inventors have conducted extensive and
intensive studies to attain these goals. As a
result, it has unexpectedly been found that these
goals can be attained by a novel porous hollow
fiber membrane having a specific pore structure in
which the porous polymer membrane has an in-a-plane
porosity of not less than 10 % measured in every
plane perpendicular to a radial direction of the
annular cross-section of the hollow fiber membrane
and the in-a-plane porosity exhibits at least one
minimum value between the inner and outer membrane
1318~
surfaces.
Accordingly, it is an object of tha present
invention to provide a novel porous hollow fiber
membrane which has an extremely high separating
ability and is especially effective for removing a
virus from an aqueous protein solution.
It is another object of the present invention
to provide a method for removing a virus from an
aqueous protein solution, which is effective not
only for removing a virus with an extremely high
virus removal percentage, but also for recovering a
protein with a high protein permeability and
without causing the protein to be denatured.
The foregoing and other objects, features and
advantages of the present invention will be
apparent to those skilled in the art from the
following detailed description and appended claims
taken in connection with the accompanying drawings~
Brief Description of The Drawinqs
In the drawings:
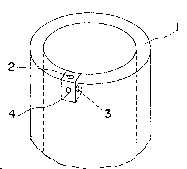
Fig. 1 is a schematic illustration of one end
portion of the porous hollow fiher membrane
according to the present invention, in which
~3~a~
numeral 1 denotes the porous polymer wall of the
porous hollow fiber membrane, numeral 2 denotes a
portion of a transverse cross section o~ the porous
polymer wall, numeral 3 denotes a portion of a
longitudinal cross section of the porous polymer
wall and numeral 4 denotes a portion of the outer
wall surface of the porous hollow fiber membrane;
Fig. 2 is an enlarged schematic illustration
of a scanning electron photomicrograph of the
portion indicated by numeral 2 in Fig. 1;
Fig. 3 is an enlarged schematic illustration
of a scanning electron photomicrograph of the
portion indicated by numeral 3 in Fig. 1;
Fig. 4 is an ~nlarged schematic illustration
of a scanning electron photomicro~raph of the
portion indicated by numeral 4 in Fig. l;
Fig. 5 is a graph showing the variation of the
in-a-plane porosity with the variation of the
distance of the plane from the inner wall surface
~0 with respect to various types of porous hollow
fiber membranes according to the present invention;
Fig. 6 is a flow diagram illustrating one mode
of the method of the present invention in which
viruses are removed from an aqueous protein
-- 8 --
~31~8
solution.
Detailed Description
In one aspect of the present invention, there
is provided a porous hollow fiber membrane
comprising a porous polymer wall having a
substantially annular cross-section and a hollow
space defined by the inner wall surface of said
porous polymer wall which hollow space extends in
the longitudinal direction of said porous polymer
wall, said porous polymer wall having pores which
form through-passages passing from the inner wall
surface to the outer wall surface of said polymer
wall, and w~erein the inner and outer wall surfaces
of said porous polymer wall have an in-a-plane
lS average pore diameter of 0.01 to 10 ~m, said in-a-
plane average pore diameter being an average pore
diameter as measured in a plane perpendicular to a
radial direction of said annular cross-section, and
said porous polymer wall has an in-a-plane porosity
of not less than 10 % measured in every plane per-
pendicular to a radial direction of said annular
cross-section, said in-a-plane porosity varying
con~inuously between said inner wall surface and
said outer wall surface, wherein said in-a-plane
porosity increases in the vicinity of each of said
~3~,a~
inner and outer wall surfaces and exhibits at least
one minimum value between said inner and outer wall
surfaces, said in~a-plane porosity at each of said
inner and outer wall surfaces being at least 1.5
times the lowest value of the in-a-plane porosity
within said porous polymer wall.
The most c'naracteristic feature of the porous
hollow fiber membrane of the present invention
resides in that the porous hollow fiber membrane
comprises a porous polymer wall having a
substantially annular cross-section which membrane
has a specific pore structure. The porous hollow
fiber membrane of the present invention has the
following pore structure characteristics:
(1) the in-a-plane average pore diameters in
the inner and outer wall surfaces of the porous
hollow fiber membrane as measured by scanning
electron photomicrography, are in the range of from
0.01 to 10 ~m;
(2) the in a-plane porosity is not less than
10 % with respect to every plane perpendicular to a
radial direction of the annular cross-section;
~3) the in-a-plane porosity varies continu-
ously between the inner wall surface of the porous
hollow fiber membrane and the outer wall surface of
- 10 -
131~8
the membrane, which porosity increases in the
vicinity of each of the inner and outer wall
surfaces and exhibits at least one minimum value
between the inner and outer wall surfaces; and
(4) the in-a-plane porosity in each of the
inner and outer wall surfaces of the porous hollow
fiber membrane is at least 1.5 times the lowest
value of in-a-plane porosity within the porous
polymer wall.
The term "in~a-plane average pore diameter" as
used herein is intended to mean an average value of
the pore diameters in a plane within and on the
porous polymer wall, which plane is perpendicular
to a radial direction of the annular cross-section
of the porous polymer wall. The "plane" includes
an inner and an outer wall surface of the porous
polymer wall, and also includes a plane within the
porous polymer wall which plane is parallel to the
inner and outer wall surfaces of the porous polymer
wall. Therefore, exactly stated, the plane is not
flat but curved. ~owever, since the measurement is
conducted by scanning electron photomicrography
with respect to an extremely limited area as will
be described later, the term "plane" is used in the
present invention for the sake of convenience.
1 1
Likewise, the term "in-a-plane porosity" as
used herein is intended to mean a porosity in a
plane within and on the porous polymer wall, which
plane is perpendicular to a radial direction o~ the
annular cross-section of the porous polymer wall.
The "plane" has the same meaning as defined above.
The term "minimum valuel' as used herein in
connection with the in-a-plane average pore
diameter and the in-a-plane porosity is intended to
mean a value of a varying quantity that is less
than any value which immediately precedes and
follows it in accordance with mathematics, and the
minimum value is not necessarily equal to the
lowest value. However, in a case where there is
only one minimum value~ the lowest value is equal
to the minimum value. ~ikewise, the term "maximum
-value" as used herein in connection with the in-a-
plane average diameter and the in-a-plane porosity
is intended to mean a value of a varying quantity
that is greater than any value which immediately
precedes and follows it in accordance with
mathematics, and the maximum value is not
necessarily equal to the highest value.
Referring now to Figs. 1 to 4, there is shown
schematic illustrations for the purpose of
3 ~ ~ 8
illustrating the specific pore structure of the
porous hollow fiber membrane. In Fig. 1, numeral 1
denotes the porous polymer wall of the porous
hollow fiber membrane, numeral 2 denotes a portion
of a transverse cross section o~ the porous polymer
wall, numeral 3 denotes a portion of a longitudinal
cross section of the porous polymer wall and
numeral 4 denotes a portion of the outer wall
surface of the porous hollow fiber membrane. In,
Fig. 2, there is shown an enlarged schematic
illustration of a scanning electron photomicrograph
of the portion indicated by numeral 2 in Fig. 10
In Fig. 3, there is shown an enlarged schematic
illustration of a scanning electron photomicrograph
of the portion indicated by numeral 3 in Fig 1.
In Fig. 4, there is shown an enlarged schematic
illustration of a scanning electron photomicrograph
of the portion indicated by numeral 4 in Fig. 1
As is apparent from Figs. 2 to 4, pores (vacant
portions) are uniformly present in the surface of
the porous polymer wall~ but non-uniformly present
in the thicknesswise direction of the porous
polymer wall. That is, the in a-plane porosity
varies continuously between the inner wall surface
and the outer wall surface. Further, the in-a-
- 13 -
1~8~
plane porosity increases in the vicinity of each of
the inner and outer wa:Ll surfaces toward a value of
the in-a-plane porosity at each of the inner and
outer wall surfaces, and exhibits at least one
minimum value between the inner and outer wall
surfaces. The in-a-plane porosity at each of the
inn~r and outer wall surfaces is at least 1.5 times
the lowest value of the in-a-plane porosity within
the porous polymer wall. The pore characteristics
as mentioned just above will be more clearly
understood from Fig. 5.
In Fig. 5, there is shown a graph showing the
variation of the in-a-plane porosity with the
variation of the distance of the plane from the
inner wall surface (hereinafter often referred to
simply as "variation of the in-a-plane porosity")
with respect to various types of porous hollow
fiber membranes according to the present invention.
Curve A indicat~s the variation of the in-a-plane
porosity with respect to a porous hollow fiber
having two minimum values of the in-a-plane
porosity. Curve B indicates the variation of the
in-a-plane porosity with respect to the porous
hollow fiber of Example 1 (given later), which has
one minimum value of the in-a-plane porosity at a
- 14 -
~3~a~8
middle portion between the inner and outer wall
surfaces. Curve C indicates the variation of the
in-a-plane porosity with respect to a porous hollow
fiber which is similar to the porous hollow fiber
of Curve B in that both the hollow fibers of Curve
C and Curve B have one minimum value of the in-a-
plane porosity. However, the hollow fiber of Curve
C is similar to that of Curve A in the virus-
removing effect because both of these hollow fibers
have a minimum value of the in-a-plane porosity in
a portion deviated from the middle portion to the
side of the outer wall surface. Curve A corres-
ponds to a porous hollow fiber membrane in which
there are two occurences of minimum values of the
in-a-plane porosity and those minimum values are in
the range of from about 1~ % to about 20 %, i.e.
the minimum values are small. In such a hollow
fiber membrane, in the portions at which the in-a-
plane porosity is at a minimum, the in-a-plane
average pore diameter is likely to be extremely
small as compared to those in the inner and outer
wall surfaces, that is, a skin construction is
likely to be formed. Therefor~ althou~h such a
hollow fiber membrane has a slightly low protein
permeability (for instance, about 70 %), the hollow
13~ sa~
fiber membrane is preferably used ~or the removal
of viruses in which the virus removal percentaqe is
required to be especially high (for instance, more
than 99.999999 ~
Porous hollow fiber membranes having various
in-a-plane porosity variations can be obtained by
controlling various conditions in the process of
producing the porous hollow fiber membrane, that
is, they can be obtained by controlling the
compositions of the injection liquid and the
coagulating bath, the period of time for which the
spinning solution is in contact with the injection
liquid and the coagulating bath and the like.
The minimum value of the in-a-plane porosity
can be mathematically determined on the basis of
the curve as obtained by dividing the membrane wall
into 10 sections in the radiai direction from the
inner wall surface of the membrane to the outer
wall surface of the membrane and plotting the value
of the -in-a-plane porosity, as measured by the
method described later, at each of the points
against Z/d, in which ~ represents the distance
from the inner wall surface and d represents the
thickness of the membrane (see Fig. 5).
- 16
g8
The porous hollow fiber membrane is made
mainly of a polymer. Examples of a polymer include
homopolymers and copolymers of acrylonitrile,
sul~one, vinylidene chloride, vinyl chloride, vinyl
acetate, methyl methacrylate, urethane, styrene and
vinyl alcohol; polytrifluorochloroethylene;
polytetrafluoroethylene; polyolefins such as
polyethylene and polypropylene; polyamides;
polyesters; cellulose acetate, cellulose nitrate,
cellulose butyrate and cellulose acetate butyrate;
regenerated cellulose; and cellulose and
mucopolysaccharides. The composition of the porous
polymer wall of the hollow fiber membrane may
comprise 50 % by weight or more of a polymer and
50 % by weight or less of a suitable additive, for
example, inorganic low molecular weight compounds
such as silica and activated carbon, and organic
low molecular weight compounds such as plasticizers
and surfactants.
A preferred process for producing the porous
hollow fiber is now described as follows. In the
process, a spinning solution of a polymer is
extruded through an annular orifice to form a fiber
extrudate with a hollow space while simultaneously
injecting an injection liquid into the hollow space
- 17 -
13~Q~
of the fiber extrudate through an in;ection tube
provided in the center of the annular orifice. In
this instance, the fiber extrudate should be
immediately immersed into the coagulating liquid.
During the above-mentioned process, there occurs a
microphase separation in the wall of the fiber
extrudate by the action of both the injection
liquid and the coagulating liquid. The microphase
separation initially occurs at the inner and outer
wall surfaces of the fiber extrudate and progresses
into the interior of the wall. The term
"microphase separation" as used herein means a
state wherein a polymer-rich phase or a polymer-
lean phase is stably dispersed as particles having
a diameter of about 0.01 to about 5 ~m in a polymer
solution. Due to the formation of the particles,
the pol-ymer solution first loses its transparency,
and then gradually undergoes coagulation and
regeneration. The resultant porous membrane has
such a characteristic structure that the surface of
a frozen fracture of the porous membrane consists
of many particles linked together having a diameter
in the range of from 0.1 to several ~m (see Figs. 2
and 3).
- 18 -
88
In producing the porous hollow fiber membrane
of the present invention, it is necessary that the
spinning solution be free from bubbles and
undissolved residues, that the composition of the
spinning solution be strictly controlled and the
temperature of the spinning solution be maintained
strictly at 10 to 40 (', desirably at a temperature
near the ambient temperature, and that the
injection li~uid delivered from the injection tube
disposed in the center of the orifice and the
coagulating liquid have the same or similar
strictly controlled composition, and the
temperature thereof be maintained at 10 to 40 C,
desirably at a temperature near the ambient
temperature. The process of producing the porous
hollow fiber membrane of the present invention will
be further illustrated, taking an example in which
a cuprammonium regenerated cellulose solution is
used as a polymer. The porous hollow fiber
membranes of the present invention using other
polymers can also be produced in substantially the
same manner as described below.
Cellulose linters are dissolved in a cupr-
ammonium solution so that the cellulose concent-
ration becomes from 2 to10 % by weight, to obtain
- 19 -
~ 3 ~ $
a spinning solution. The spinning solution must be
homogeneous and completely filtered and deaerated,
and the cellulose concentration must be maintained
at a predetermined level within ~ 0.05 % by weight
during the spinning. It should be ensured that the
filtration of the spinning solution is complete
and, therefore, no impurities and undissolved
cellulose are contained in it. The temperature of
the spinning solution is maintained at a predeter-
mined point within + 0.1 C, and the temperature of
the atmosphere of the spinning machine is control-
led so as not to cause unevenness in the
temperature of the spinning solution. Mainly by
changing the cellulose concentration of the
spinning solution, the in-a-plane porosity and ln-
a-plane average pore diameter in the inner and
outer wall surfaces of the porous hollow fiber
membrane can be changed. The thus prepared
spinning solution is allowed to be extruded from
the orifice (e.g. 2 mm in diameter) at a fixed rate
(e.g. 1.0 to 5.0 ml/min). In this instance, it
should be ensured that undulation is not caused.
Simultaneously with the extrusion of the spinning
solution, a solution which causes microphase
separation to the spinning solution (e.g. a ternary
- 20
~3l$a~
system consisting of acetone, ammonia and water in
a ratio such as 35:1:50) as an injection liquid is
injected from the injection tube (e.g. 0.6 mm in
diameter) at a fixed rate (e.g. 2.0 to 20 ml/min).
The composition and the temperature of the
injection liquid are required to be controlled at
least as strictly as those of the spinning
solution. In this connection, by changing the
composition of the injection liquid, not only the
in-a-plane porosity and in-a-plane average pore
diameter in the inner wall surface but also those
within the wall can be changed. The fiber shaped
extrudate (the inner part is the injection liquid
and the outer part is the spinning solution) is
immediately immersed into the coagulating bath.
The composition of the coagulating liquid (a
ternary system consisting of e.g. acetone, ammonia
and water) is formulated to be identical or similar
to that of the injection liquid, and the composi-
tion and the temperature must be controlled as
strictly as those of the injection liquid. By
changing the composition of the coagulating liquid,
not only the in-a~plane porosity and in-a-plane
average pore diameter in the outer wall surface but
also those within the wall can be changed. The
- 21 -
depth of the coagulating bath and the take-up
speed is set so that the ~iber-shaped extrudate is
immersed in the coagulating bath for a fixed period
of time (e.g. 1 to 30 min~. After being taken up,
the resultant fiber is subjected to regeneration
using an aqueous sulphuric acid solution having a
concentration of 2 ~ by weight, followed by water
washing and drying.
The porous hollow fiber membrane of the
present invention is capable of completely removing
the viruses contained in an aqueous protein solu-
tion even when the membrane has in-a~plane average
pore diameters larger than the diameter of the
viruses in the inner and outer wall surfaces of the
membrane, due to the above-mentioned pore structure
characteristics of items ~1) to ~4) above of the
membrane. The porous hollow fiber membrane of the
present invention is highly effective in inhibiting
the passage of vi~uses therethrough due to the pore
characteristic change in a radial direction of the
annular cross-section of the membrane wall and to
the presence of a minimum value of the in-a-plane
porosity at least at one portion between the inner
and outer wall surfaces as mentioned in item ~3)
above~ Moreover, the porous hollow fiber membrane
- 22
~ 3 ~ 8
of the present invention does not adversely affect
the permeation of proteins therethrough due to
the pore structure characteristics as mentioned in
items (1), (?) and t4) above. Therefore, in
removing a virus from an aqueous protein solution,
the physiological activity of the protein recovered
using the porous hollow fiber membrane of the
present invention is extremely excellent as
compared with the physiological activity of the
protein recovered by the conventional methods.
The protein permeability and protein
permeation rate of the present porous hollow fiber
membrane can be improved by causing the porosity of
one surface of the membrane from which a protein is
permeated to become larger than that within the
membrane wall. For example, no matter what kind of
polymer is employed as the wall material of the
membrane, the value of VA/Vw, in which VA
represents the permeability for an aqueous 5 % by
weight albumin solution and Vw represents the
permeability for purified water, is significantly
improved when the ratio of the in-a-plane porosity
in one surface of the membrane, from which
permeation of the water and albumin solution is
performed, to the lowest value of the in-a-plane
13~$~
porosities within the membrane wall, is at least
1.5, as compared with that when such ratio is 1.
The value of VA/Vw is rlemarkably improved, and
som~times becomes twofold or more, when the above-
mentioned ratio is at least 2.
From the viewpoint of further improving the
capabllity of inhibiting the passage of viruses
through the membrane, it is preferred that the
porous polymer wall have a pore structure in which
the in-a-plane average pore diameter varies
continuously between the inner wall surface and the
outer wall surface so that from the inner wall
surface toward the outer wall surface/ the in-a-
plane average pore diameter alternately decreases
and increases at least two times, thereby
experiencing occurrences of at least a minimum
value, a maximum value and a minimum value, in this
order, in the continuous variation of the in-a-
plane average pore diameter between the inner and
outer wall surfaces, wherein the in-a-plane average-
pore diameter increases in the vicinity of said
outer wall surface and in which the wall portion of
the membrane has a layer structure as described
later. With the last increase in the above-
mentioned alternate decrease and increase of the
- 24 -
~31~8
in-a-plane average pore diameter, the in-a-plane
average pore diameter increases toward a value of
the in-a-plane average pore diameter at the outer
wall surface.
The in-a-plane average pore diameter within
the porous polymer wall is in the range of 0.005 to
10 ~m.
The in-a-plane average pore diameter at a
plane exhibiting a minimum in-a-plane porosity
value with respect to the porous hollow fiber
membrane of the present invention may be larger
than the diameters of the viruses to be removed.
Generally, when the number of layers constituting
the layer structure of the wall portion of the
present porous hollow fiber membrane is large, even
if the in-a-plane average pore diameter at the
plane exhibiting a minimum in-a-plane porosity
value is about twice the diameters of the viruses,
the membrane is capable of highly effectively
inhibiting the passage of the viruses therethrough.
The porous hollow fiber membrane of the present
invention may advantageously have a layer structure
in the direction of the wall thickness, so that the
membrane has the following characteristics:
(1) uniformity in pore characteristics such
as pore diameter distribution and pore config-
uration is obsarved at any portion of any parti-
cular plane which is in parallel with the inner and
outer wall surfaces, and any of such planes is
approximated to a screen filter in the light of
filtering proper-ties;
(2) in any particular plane which is in
parallel with the inner and outer wall surfaces,
the pores are randomly disposed or regularly
arranged only along the direction of the fiber
axis;
~33 the specific pore diameter distribution,
in-a-plane average pore diameter and in-a-plane
porosity are measurable with respect to any of such
planes;
(4~ each of such planes has a different pore
diameter distribution, different average pore
diameter and different in-a-plane porosity,
depending on the distance, in a radial direction of
the annular cross-section of the membrane wall, of
the plane from the inner wall surface of the
membrane. In the light of the principle governing
the formation of the present porous hollow fiber
membrane, the above-mentioned planes are each
approximated to a section of a layer having a
thickness of formula
~ x (2S2)
wherein 2S2 represents the diameter of fine
particles formed by the microphase separation as
described hereinbefore,
which section is in parallel with the surface of
the layer. It is preferred that the number of
layers constituting the porous polymer wall of the
porous hollow fiber membrane of the present
invention be in the range of from about 10 to about
300. The number of layers is preferably not larger
than about 300 from the viewpoint of ensuring high
permeability for proteins or the like. The l'number
of layers" as used herein is defined as~ d/4S2 in
which d represents the wall thickness of the
membrane and S2 is as defined above. On the other
hand, a plane perpendicular to the fiber axis which
plane is represented by the cross section of the
porous hollow fiber membrane is approximated to a
layer-form accumulation of particles having a
diameter of from 0~1 to 2 ~m~
To improve the capability of inhibiting the
passage of viruses through the membrane, it is
- 27 -
g ~ ,
preferred that the average shear rate of the
filtrand flow on the inner wall surface of the
hollow fiber be increased. For example, when the
shear rate ~s 1000 sec~~1 or more, the capability of
inhibiting the passage of viruses is about iO times
that exhibited when it is 0.
The ratio of the maximum value of the in-a-
plane average pore diameter to the minimum value of
the in-a-plane average pore diameter with respect
to the porous hollow fiber membrane of the present
invention is preferably in the range of from 1.2 to
10, more preferably from 1~2 to 2. The presence of
a maximum value with respect to the in-a-plane
average pore diameter is desirable from the
viewpoint of increasing the filtration rate of an
aqueous protein solution. It is also desirable
from the viewpoint of improving the mechanical
property of the hollow fiber membrane since it
provides a cushion against a mechanical deformation
of the hollow fiber membrane. However, when ~he
maximum value of in-a-plane average pore diameter
is too large, the capability of the membrane of
inhibiting the passage of viruses therethrough
tends to become poor. For this reason, the above-
mentioned ratio of the maximum value of in-a-plane
- 28 -
g
avera~e pore diameter to the minimum value of in-a-
plane average pore diameter is preferably 10 or
less, more preferably 2 or less.
The upper limit of the in-a-plane porosity
with respect to the porous hollow fiber according
to the present invention is not limited but may
preferably be about 80 % from the viewpoint of ease
of production of the membrane.
The hollow fiber membrane of the present
invention preferably has a wall thickness of from
10 ym to 200 ~m. If a membrane having a wall
thickness of less than 10 ym is employed, the virus
removal percenta~e is lowered. On the other hand,
if a membrane having a wall thickness of more than
200 ~m is employed, the protein permeability
decreases, leading to a lowering of the recovery of
protein.
The bulk porosity of the hollow fiber membrane
of the present invention is preferably 30 to about
75 %l more preferably 40 to about 75 %. As the bulk
porosity increases, the permeation rate of protein
increases. The ratio of the increase in the permea-
tion rate of protein to the increase in the bulk
porosity becomes large as the bulk porosity becomes
30 % or more and this ratio becomes even larger as
- 29 -
~3~8
the bulk porosity becomes 40 % or more. On the
other handr if the bulk porosity is more than about
75 ~, the performance of the membrane with respect
to the removal of viruses becomes poor.
The number of pores which are present in each
of the porous inner and outer surfaces of the
polymer wall of the porous hollow fiber membrane of
the present invention is preferably 106/cm2 or
more.
The apparent average pore diameter in the
porous polymer wall is in the range of 14 to
150 nm.
If the liquid to be subjected to the removal
of viruses has a protein concentration of 3 % or
more, adsorption of the protein onto the polymer
constituting the membrane has a large effect on the
permeability of protein, recovery thereof and
filtration rate. In this connection, it is noted
that a membrane made of a hydrophilic polymer has a
large value of VA/Vw in which VA and Vw are as
defined above and, hence, preferable. Further,
with respect to porous hollow fiber membranes
having substantially the same in-a-plane porosities
and in-a-plane average pore diameters at the inner
and outer wall surfaces, a porous hollow fiber
- 30 -
~31~8
membrane produc~d by a microphase separation method
has a higher filtration rate and a higher
filtration capacity than porous hollow fiber
membranes produced by other methods such as a
method in which a substance having a low molecular
weight is emulsified and mixed with a polymer
solution to obtain a spinning solution, the
obtained solution is spun into a hollow fiber and
then the substance having a low molecu ar weight is
removed from the hollow fiber. Therefore, a porous
hollow fiber membrane produced from a hydrophilic
polymer by a microphase separation method is
preferably used for removing viruses from an
aqueous protein solution having a high protein
concentration.
Blood plasma is an example of an aqueous
protein solution which may be subjected to the
removal of viruses according to the method of the
present invention. In the removal of viruses from
blood placma~ which has a protein concentration of
5 % or more, the chemical structure of the polymer
constituting the porous hollow fiber membrane has a
large effect on the performance of the membrane.
In this connection, a membrane made of a polymer
containlng a large number of hydroxy groups, such
- 31 -
~ 3 ~ 8
as a regenerated cellulose, is preferable from the
viewpoints of the filtration capacity of the
membrane and the recovery of proteins. A membrane
produced from a cuprammonium regenerated cellulose
by a microphase separation method is especially
preferable.
Viruses in human blood plasma, for example,
hepatitis virus, AIDS (acquired immune deficiency
syndrome) virus, etc., are generally highly infec-
tious to human beings and have a serious effect on
human bodies after infection. Therefore, in the
removal of these viruses from human blood plasma,
the removal percentage is re~uired to be 99.999 ~
to 99.999999 %. For removing viruses with such a
high removal percentage, there may preferably be
used a porous hollow fiber membrane, wherein the
membrane has a layer structure consisting of 10 or
more layers and the ratio of the minimum value of
the in-a-plane porosity (%) to the value of the
wall thickness (~m) is in the range of from 0O05 to
2Ø Of the above-mentioned membraner a membrane
having a layer structure consisting of 100 or more
layers is more preferable.
In another aspect of the present invention,
there is provided a method for the removal of a
- 32 -
~ 3 ~ 3 ~
virus contained in an aqueous protein solution,
which comprises contacting an aqueous protein
solution containing a virus with a porous hollow
fiber membrane comprising a porous polymer wall
having a substantially annular cross-section and a
hollow space defined by the inner wall surface of
said porous polymer wall which hollow space extends
in the longitudinal direction of said porous
polymer wall, said porous polymer wall having pores
which form through-passages passing from the inner
wall surface to the outer wall surface of said
polymer wall, and wherein the inner and outer wall
surfaces of said porous polymer wall have an in-a-
plane average pore diameter of 0.01 to 10 ~m, said
in-a-plane average pore diameter being an average
pore diameter as measured in a plane perpendicular
to a radial direction of said annular cross-
section, and said porous polymer wall has an in-a-
plane porosity of not less than 10 % measured in
every plane perpendicular to a radial direction of
said annular cross-section, said in-a-plane
porosity varying continuously between said inner
wall surface and said outer wall surface, wherein
said in-a-plane porosity increases in the vicinity
of each of said inner and outer wall surfaces and
- 33 -
~ 3i~
exhibits at least one minimum value between said
inner and outer wall surfaces, said in~a-plane
porosity at each of said inner and outer wall
surfaces being at least 1.5 the lowest value of
the in-a-plane porosity within said porous polymer
wall, said contacting of the solution with the
membrane being conducted on either of the inner and
outer wall surfaces of the porous polymer wall
while applying a trans-membrane pressure, thereby
causing the virus contained in the aqueous protein
solution to be captured in the porous hollow fiber
membrane while allowing the aqueous protein
solution to permeate throu~h the porous hollow
fiber membrane.
The method of the present invention can be
suitably applied to a virus-containing aqueous
protein solution such as plasma, particularly human
plasma, or the like. The aqueous protein solution
to be treated by the method of the present
invention has a protein concentration of 0.5 to
30 % by weight in terms of total protein concentra-
tion which protein is part of useful components of
the solution. As examples of such aqueous protein
solutions, there may be mentioned human or animal
blood or plasma, materials and intermediates for
- 34 -
plasma derivatives, aqueous solutions containing
plasma derivatives, growth hormone, injections
containing physiologically active substances such
as growth hormone, vaccine and the like, cell
culture fluid, aqueous product solutions in the
fermentation îndustry and intermediates therefor,
diagnostics, serums for cell culturing, vaccine and
the like. As viruses to be removed by the method
of the present invention, there may be mentioned
hepatitis virus, AIDS virus, influenza virus,
poliomyelitis virus and the like which are patho-
genic to human beings and/or animals.
In the method of the present invention, an
aqueous protein solution containing a virus is
contacted with either the inner wall surface or the
outer wall surface of the porous hollow fiber
membrane. The contact of the aqueous protein
solution with the wall surface of the porous hollow
fiber membrane may be effected either by flowing
the solution along the wall surface or by applying
the solution in the stationary state onto the wall
surface. In the ultrafiltration involved in the
method of the present invention, a trans-membrane
pressure of about 0.1 to 1 atm is applied.
11 3~$~
By the method of the present invention, the
virus is effectively captured in the porous hollow
riber membrane and, thus, can be removed from the
aqueous protein solution, not only with an extreme-
ly high virus removal percentage but also with a
high protein permeability. Therefore, the high
filtration speed is realized. Further, it should
be noted that according to the method of the
present invention, the protein contained in the
aqueous protein solution is not denatured and there
is no danger that the biological activity of the
protein is loweredO
Referring to Fig. 6, there is a flow diagram
illustrating one mode of the present invention in
which viruses are removed from an aqueous protein.
A plurality (about 10,000) oE the porous hollow
fiber membranes of the present invention are
bundled to obtain a module. Two modules are
designated by F1 and F2. Plasma obtained from a
plurality of persons is pooled in a tank T1~ The
temperature of the plasma is maintained at about
4 C. The plasma is supplied to the module F1 or
F2 through a line L1 by the manipulation of
switches S4, S5 and S1 at a predetermined flow rate
by a pump P1(in this case, the plasma is flowed
~,4},~
into the hollow space of the hollow fiber
membrane~. The arnount of the pressure applied to
the module F1 or F2 is given as the difference
between the pressure values at an entry port side
pressure gage G1 and an outlet side pressure gage
G2 and a vacuum line. By manipulating the switch
- S1, the module F1 or F2 is selected. A filtrate
from the module F1 or F2 flows to a tank T2 through
a line L4 by the manipulation of a switch S3.
Any residual filtrand flows through a line L2, and,
by the manipulation of the switch S~, is then
supplied to the module F1 or F2 again by the pump
P1 -
By the manipulation of a switch S2 and the
operation of a pump P2, the module Fl or F2 is back
washed by a buffer solution stored in a tank T3.
The initial part of the liquid flowing back into
the hollow space of the hollow fiber membranes by
the back-washing enters the line L~, and the latter
part of the liquid is led out of the system through
a line L5 by the manipulation of switches S1 and
S5. By this procedure, not only the back-washing
of the hollow fibers is conducted, but also an
excessive increase in the protein concentration
within the line L2 is prevented, thereby enabling
37 -
~L 3 ~
the filtration speed in the module F1 and F2 to be
stably maintained, so that the recovery of a virus-
free protein solution can be attained with high
efficiency.
As applications oE the method of the present
invention, there may be mentioned (1) separation of
viruses from human plasma (production of plasma for
transfusion), (2) removal of viruses from pooled
plasma or plasma preparations (production of plasma
preparations), (3) removal of viruses from drugs
and aqueous solutions used in genetic engineering,
(4) removal of viruses from a cell culture fluid,
(5) removal of viruses from reagents for clinical
laboratory tests, (6) removal of viruses from
injection reagents, (7) and removal of unneeded
viruses in the production process of vaccine, or
concentration of viruses. Thus, the present
invention can be advantageously utilized in fields
such as medicine, biochemistry, animal husbandry
and the like.
In the Examples, the in-a-plane average pore
diameter, the in-a-plane porosity, the bulk
porosity and the apparent average pore diameter
were measured by the following methods.
- 38 -
(1) In-a-plane average pore diameter (2r) and
in-a-plane porosity (Pre)
Water in the interior of a porous hollow fiber
membrane in the wet state is replaced by a water-
soluble organic solvent such as acetone and the
hollow fiber membrane is subjected to air-drying.
After drying, the hollow fiber membrane is embedded
in a polymer resin such as an acrylic resin to
obtain a resin-embedded hollow fiberv Using an
``~ ultramicrotome (Ultratome III 8800 type
manufactured and sold by LKB Ltd., Sweden) equipped
with a glass knife, sections which are
perpendicular to a radial direction of the hollow
fiber and have a thickness of about 1 ~m in the
radial direction are cut out from the resin-
embedded hollow fiber, at a plurality of
predetermined distances from the inner wall surface
in the radial direction. Then, the resin used for
the embedment of the hollow fiber is dissolved out
from the sections with a solvent such as
chloroform. Thereafter, an electron micrograph of
each section is taken.
The in-a-plane average pore diameter (2r) with
respect to a plane in the polymer wall of the
hollow fiber is represented by the formula
~ ~m~ ~ - 39 -
13~3~8
j_ _
2r = 2V r3~r4
wherein r3 and r4 are respectively represented by
the formulae
_ ~ r3N(r)dr
3 ~ r2N(r)dr
.~o
_ ~ r4N(r~dr
4 ~ r3N(r)dr
in which
r is the pore radius on the surface of the
section corresponding to the plane; and
N(r) is a pore radius distribution function
defined on the basis that the number of pores having
a pore radius falling within the range of r to r +
dr per 1 cm2 area of the surface of the section is
expressed ~s N(r)dr.
The pore radius distribution function [N(r)3
is determined by the electron photomicrograph of
the section as follows. With respect to the area
of the section of which the pore radius
distribution function is to be determined, a
scanning electron photomicrograph is taken and an
enlarged print thereof having an appropriate size
(for instance 20 cm x 20 cm) is made. On the thus
obtained print, 40 straight test lines are drawn at
- ~0 -
l3~a~s
an equal interval. Each line crosses over a number
of pores. With respect to every pores which have
been crossed over by a straight test line, the
length of the portion of the straight line which
lies within the pore is measured. Using the
frequency distribution function with respect to the
length thus measured, N(r~ is determined by, for
example, the method of stereology ~see, for
example, Norio Suwa, "Teiryo Keitaigaku
(Quantitative morphology)" (published by Iwanami
Shoten, Japan), p.185-272~.
The in-a-plane porosity (Pre~ with respect to
a plane in the polymer wall of the hollow fiber is
obtained by calculation from the following formula
Pre(%) = {7~ Jo r2N~r)dr} x 100
wherein r and N(r) are as defined above. The pore
radius distribution ~N(r)~ is determined in the same
manner as mentioned above.
(2) Bulk porosity (Prp)
Water in the interior of a porous hollow fiber
membrane in the wet state is replaced by a water-
soluble organic solvent such as acetone and the
hollow fiber membrane is subjected to air-drying.
After the air-drying, the hollow fiber membrane is
further dried in vacuo to reduce the moisture
- 41 -
i 3 ~ 8
content of the membrane to 0.5 % or less. The bulk
porosity of the membrane is obtained by calculation
from the following formula
P (%) = {1 - W 2 -~-} X loo
rp p/4 X ~(Ro - Rl) X Q
wherein
Ri, Ro, ~ and W respectively represent the
inner diameter (cm), outer diameter (cm), length
(cm) and weight (g) of the hollow fiber after drying
in vacuo; and
f is the density of the polymer constituting
the porous hollow fiber.
(3) Apparent average pore diameter (2rf)
Water is applied to the inner wall surface of
a porous hollow fiber at a predetermined trans-
membrane pressure ~P (cm Hq~. The filtration flux
J (ml/cm2/sec) is obtained. The apparent average
pore diameter (2rf) can be obtained by calculation
based on the following formula derived from
Poiseuille's equation
2rf (cm) = 406 x 10-2 (~Jd/~Prp~P)1/2
wherein:
d is the wall thickness (cm) of the porous
hollow fiber membranej
- 42 -
a~s
is the viscosity coefficient (centipoise);
Prp is the bulk porosity of the porous hollow
fiber membrane (%); and
J and ~P are as defined above.
S ~he present invention will now be described in
detail with reference to the following Examples,
which should not be construed to limit the scope of
the present invention.
- ~3 -
l3l~a~s
Example 1
Cellulose linter having a viscosity average
molecular weight of 1.50 x 105 was prepared accord-
ing to a customary known method. The prepared
cellulose linter was dissolved in an aqueous cupr-
ammonium solution having ammonia and copper concen-
trations of 6.8 % by weight and 3.1 ~ by weight,
respectively, so that the final concentration of
the cellulose linter in the resulting cellulose
linter solution became 7.0 % by weight. Then, the
cellulose linter solution was filtered, followed by
degassing, thereby to obtain a spinning solution.
The spinning solution was extruded through a spin
neret having an orifice diameter of 2 mm, an injec-
tion-tube outside diameter of 0.8 mm and an injec-
tion-tube inside diameter of 0.6 mm at a delivery
rate of 2.0 ml/min to form a fiber extrudate with a
bore, while simultaneously injecting an injection
liquid through the injeCtiQn tube disposed in the
center of the orifice into the bore at a delivery
rate of 5.0 ml/min. During this procedure, both
the spinning solution and injection liquid were
maintained at 25 ~ 0.1 C. As the injection
liquid, a solution whose composition was strictly
controlled so that the proportions of water,
- 44 -
~3~a~8
acetone and ammonia were 100.0 : 70.0 : 1.0 by
weight was employed. The fiber extrudate was imme-
diately introduced into a coagulating bath
maintained at a temperature of 25 + 0.1 ~C whose
composition was strictly controlled so that the
proportions of water, acetone and ammonia were
100.0 : 70.0 o 1.0 by weight, followed b~ reeling
up at a velocity of 7.0 m/min from the bath. In
the coagulating bath, the fiber extrudate which had
been transparent, blue upon extrusion thereof
gradually became white showing occurrence of a
microphase separation, followed by coagulation
thereby enabling the extrudate to solidify in a
hollow fiber form. Then, the fiber was regenerated
in a 2 % by weight aqueous sulfuric acid at 20 ~
0.1 C and subsequently washed with water, followed
by drying. The resulting hollow fiber had an
annular cross-section, and had an outside diameter
of 305 ~m, a wall thickness (d) of 30 ~m and an
inside diameter of 245 ~m. The in-a-plane porosity
(Pre? in every plane perpendicular to a radial
direction of the annular cross-section of the
hollow fiber was measured and plotted against the
value of the distance of the plane from the inner
wall surface divided by the wall thickness of the
- 45 -
~ 3 ~ 8
hollow fiber to obtain Curve B of Fig. 5. The
minimum value of Pre (in this case, it was also the
lowest value~ was 23 ~, while the Pre's in the inner
and outer wall surfaces of the hollow fiber were
both about 60 %. As a result of the observation of
a frozen fracture surface (obtained by freezing the
fiber in liquid nitrogen and breaking it in the
same) of the hollow fiber by means of a scanning
electron microscope it was confirmed that the hollow
fiber of the present invention had an average
particle diameter (2S2) of 0.50 ~m. Further, as a
result of the observation of planes parallel to the
membrane surfaces of the hollow fiber by means of a
scanning electron microscope it was confirmed that
the hollow fiber of the present invention had a
layer structure.
The porous polymer wall of the hollow fiher
had a pore structure in which the in-a-plane
average pore diameter continuously varied between
the inner wall surface and the outer wall surface
so that with a distance from the inner wall
surface, the in-a-plane average pore diameter
alternately decreased and increased twice, thereby
experiencing occurrences of a minimum value (D2), a
maximum value ~D3) and a minimum value (D4) in this
- 46 -
1 3~Q~8
order. The in-a-plane average pore diameter (D1)
in the inner wall surface was 0.61 ~m, and the in-
a-plane average pore diameter (D5) in the outer
wall surface was 0.60 ~Im. D3 was 0.15 ~m, D3/D2
was 1.32 and D3/D4 was 1.31. The bulk porosity of
the hollow fiber was 48.2 %. The apparent average
pore diameter ~2rf) was 50 nm. With respect to
this hollow fiber, a filtration test was conducted
using as a model substance of a virus a 5 % by
weight aqueous albumin solution containing
colloidal silica particles (Cataloi ~ S180P
manufactured and sold by Catalysts and Chemicals
Industries Company limited, Japan) having a
particle size of 70 to 90 nm. The filtration test
was carried out at 31 C by flowing the aqueous
albumin solution in the hollow fiber at a trans-
membrane pressure of 200 mmHg. The removal
percentage (X) of the colloidal silica particles by
the hollow fiber was calculated from the ratio of
the silica concentration (A) of the filtrate to
that (B) of the filtrand, which concentrations were
determined in terms of Si concentration by atomic-
absorption spectroscopy, according to the formula X
= ( 1 - A/B ) x 100. The results obtained are
~5 shown in Table 1. Further, the filtrate was
- 47 -
~3~'~$~
sprayed over a mesh for electron microscope which
was coated with carbon, and then the existence of
particles on the mesh was examined by means of an
electron microscope. As a result, complete removal
of the colloidal silica particles was confirmed.
The permeability of albumin was about 98 % as
measured by liquid chromatography.
Example 2
Cellulose linter having a viscosity average
molecular weight of 2.00 x io5 was dissolved in the
same cuprammonium solution as 4mployed in Example 1
so that the concentration of the cellulose linter in
the resulting cellulose linter solution became 8.0 %
by weight. The cellulose linter solution was
filtered, followed by degassing, thereby to obtain a
spinning solution. From this spinning solution, a
regenerated cellulose porous hollow fiber membrane
was obtained under substantially the same conditions
as in Example 1. The porous polymer wall of the
hollow fiber had substantially the same pore
structure as described in Example 1. With respect
to the particulars of the pore structure of the
hollow fiber, D1 was 0.325 ~m; D5 was 0.310 ~m; D3
was 0.092 ~m; D3/D2 was 1~40; and D3/D4 was 1-38-
~8 -
The in-a-plane porosities in the inner and outer
wall surfaces of the hollow fiber were 54 % and
56 %, respectively. The lowest value of the in-a-
plane porosities in ten planes taken at equi-
distance which planes are perpendicular to a radial
direction of the annular cross-section of the
hollow fibers was 17.5 % and the bulk porosity was
45.0 ~. The apparent average pore diameter (2rf)
was 20 nm.
With respect to this hollow fiber, a filtration
test was conducted in substantially the same manner
as in Example 1, except that use was made of
colloidal silica particles (Cataloi ~ SI45P manu-
factured and sold by Catalists and Chemicals
Industries Company Limited, Japan) having a particle
diameter of 35-55 nm. The results of the filtration
test are shown in Table 1. The alhumin permeability
as measured by liquid chromatography was about 98 ~.
Moreover, using the above-obtained hollow
fiber, a virus removal test was conducted with
respect to the plasma derived from hepatitis B
virus positive blood which plasma had a total
protein concentration of 5.9 % and a relative
concentration of hepatitis B virus in terms o DNA
of 1000 units. That is, the plasma was subjected to
_ 49 _
$ 8
perpendicular filtration in which the plasma was fed
into the hollow fiber at its one end, while the
other end of the hollow fiber was closed, at a
trans-membrane pressure of 100 mmHg. The filtration
rate was 0O030 /m2 hr mmHg. The relative
concentration o~ the hepatitis B virus in the
filtrate in terms of DNA was evaluated by the
hybridization method using an isotope-labeled cDNA
coding for hepatitis B virus . The relative
concentration was below the detection limit value,
thereby enabling compolete removal of the virus from
the plasma to be confirmed. Further; the
permeability of the total proteins as measured by
liquid chromatography was 90 %.
Comparative Example
In the weight proportions as indicated in
Table 2, a cellulose diacetate having a degree of
acetylation of 54.2% and a polymerization degree of
190 was dissolved in a mixed solvent comprised of
acetone and methanol in a weight ratio of 5/1
containing CaCl2 2H2O and cyclohexanol, thereby to
obtain a spinning solution. Under the spinning
conditions as indicated in Table 2, a hollow fiber
was spun~ The in-a-plane porosities in the inner
- 50 -
$
and outer wall surfaces of the hollow f ber were
28 % and 15 %, respectively, and the in-a-plane
porosities within the wall of the hollow fiber were
20 % or more. An in-a-plane porosity decrease from
20 % to 15 % was observed in the vicinity of the
outer wall surface within the wall. The in-a-plane
average pore diameters in the inner wall surface and
the outer wall surface of the hollow fiber were
0.52 ~m and 0.54 ~m, respectively. The in-a-plane
porosities within the wall of the hollow fiber had
no minimum value but a maximum value, which was
0.60 ~m. The apparent average pore diameter (2rf)
was 35 nm. With respect to this hollow fiber, a
filtration test using the same colloidal silica-
containing aqueous albumin solution as used in
Example 2 was conducted in substantially the same
manner as in Example 2. The results are shown in
Table 10
- 51 -
1318~8
Table 1
Particle Removal Filtration
Size of Percentage Rate (I/m2
Colloidal of Colloidal hr mmHg)
Silica (nm) Silica (%)
. .
- Example 1 70-90 99.99 or more 0.523
Example 2 35-55 99.99 or more 0.035
Comparative 35-55 90 0.025
Example
., . _ _
- 52 -
~L 3 ~ 8
Table 2
Composition of Spinning Conditions
Spinning Solution
.
Cellulose SpinnerQt
diacetate 45 g Orifice Diameter 1.5 mm
Injection-tube 0.5 mm
Outside Diameter
Acetone/
Methanol 150 g Delivery Rate of 4.3 ml/min
Spinning Solution
CaCl2 2H2 25 g
Temperature of 30 C
Cyclohexanol 130 g Spinning Solution
Composition of Aqueous
Injection Liquid Methanol
Solution
of 50~ by
Volume
(25 Cl
Delivery Rate 2.0 ml/min
of Injection Liquid
Composition of Aqueous
Coagulating Bath Methanol
Solution
of 50% by
Volume
~25 C)
Temperature of 25 ~C
Coagulating Bath
Distance between 100 mm
the spinneret
orifice and the
surface of the
coagulating bath
Reel-up Velocity 15.4 m/min
~ ~ , , , . . _ _ _ _ _ . . _ _ _
- 53 -