Note: Descriptions are shown in the official language in which they were submitted.
~L 3 '~ r~ ~
-- 1 --
This invention relates to a process and a system for the
production of an aromatic decaffeinated tea.
The increasing reservations against caffeine, which used to
be called "theine", causes a rising demand for black tea or green
tea with a reduced caffeine content. The demand for
decaffeinated tea would be expected to rise substantially if the
difference in taste between the treated teas and the untreated
teas is hardly noticeable. The problem of the production of a
high quality decaffeinated tea is a serious one, because the tea
to be treated is a finished product ready for use. Further
treatment should at least not deteriorate the quality.
In the past, the caffeine content was reduced by adding a
solvent to previously moistened tea leaves. Subsequently, the
tea was dried and either as much solvent as possible was removed,
or the tea was subjected to a further aqueous extraction for the
production of instant tea.
The use of methylene chloride ~dichloromethane) as solvent
has never been satisfactory, because a major portion of aroma and
flavour was removed together with the caffeine. According to EU~
PS 0 050 482 of 1984, reduction of the loss of aroma has been
attempted by saturating the methylene chloride with the tea
constituents (except caffeine) before extraction.
Owing both to potential danyers to health and legislative
measures against the use of methylene chloride for the
decaffeination of tea, ethyl acetate is now used as a substitute
(cf. Food Chemical News, July 16, 1984, page 25A). But this
solvent, too, not only fails to meet a sufficient number of the
.~ "'`' ~,,_
:iL 6~ ~ $~ ~ r~ i
~ 2
expectations made for it, to be used for the production of a high
quality product, but furthermore, the plants for the production
of ethyl acetate must be explosion-proof.
From DE-PS 21 27 6~2 of 1975, a process is known according
to which the caffeine was removed from fermented black tea by a
physiologically-acceptable solvent; it was said that flavour and
aroma were left completely unimpaired. For this purpose, in a
first step, the aroma substances were removed by means of a dry,
supercritical (with respect to pressure and temperature) gas,
preferably carbon dioxide. In a second step, the caffei.ne was
likewise removed from the moistened tea by supercritical, water-
saturated carbon dioxide. Finally, the dried tea was re-
impregnatèd with the aroma removed in the first step by charging
the gas stream at a supercritical pressure with the aroma
separated previously, which was liberated from the tea by
demixing or li~uifying the gas. As a prominent feature, it was
stressed that those constituents of the tea which were
responsible for flavour and colour, e.g., tanning agents,
falvines and rubigenes, were not extracted, i.e., they remained
in the decaffeinated tea.
For three out of the five steps of the above-described
process (extraction of the aroma, moistening, decaffeinating,
drying, re-impregnation of aroma) viz. the first, third and fifth
step, expensive, high pressure vessels were required;
furthermore, the tea had to be transferred to other vessels
during the process. Regrettably, the ultimately desired aim was
not achieved as comparative tests of taste and flavour
demonstrate.
~ 3 ~ ~?3 ~
Further, the rather minor improvement in quality achieved
by the re-impregnation of the aroma did not appear to justify the
high cost; therefore, a further improvement, DE-PS 26 37 197 of
1983 disclosed a selective separation of the caffei~e from the
charged solvent. To achieve this end, the supercritical CO2
charged with caffeine and other accompanying substances was
separated from the caffeine and the accompanying substances not
by a reduction of pressure but, rather, by passing the charged
CO2 over an acidic ion exchanger which selectively absorbed the
caffeine. A further improvement was achieved when the extraction
became selective for caffeine by the use of liquid CO2 as solvent
(DE-OS 34 13 869 of 1985).
Providing an economical process was the main object of two
further processes, according to which the extraction was again
effected with supercritical CO2 and the adsorption of the
caffeine was effected by the less selective activated carbon (DE-
OS 33 39 181 of 1985 and DB-OS 34 15 844 of 1985).
The problem of impairing the aroma may be avoided by
decaffeinating green tea before it is fermented into black tea
(DE-OS 34 14 767 of 1985), or by decaffeinating tea which, though
fermented, has not yet been dried (EU-A- 0 167 399 of January,
1986). Both methods are similar to the decaffeination of coffee,
where green beans are treated and flavour and aroma develop only
by the following process of roastin~. However, it is only in the
countries of origin that these possibilities would be realized
advantageously.
~L 3 ~ L r~ j~
- 3a -
All the methods described so far cannot combine the three
requirements (use of a harmless solvent for extraction, little
impairment of taste and flavour, and dispensability of high
pressure steps, i.e., low costs). In fact, such a combination
of features is the object of a main aspect of the present
invention.
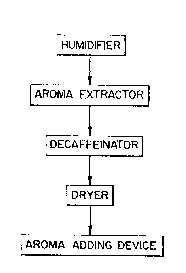
According to a main aspect of the present invention,
dealkaloidation, suitably decaffeination, is effected gently
after removal of the aroma fraction in a manner known per se;
however, the tea is previously rnoistened before the aroma
-
satisfactory tea in a small number o~ different steps
whlch, as far as taste and flavor are concerned,
surprisingly surpasses all other tea samples produced
according -to di~ferent methods.
In the accompanylng drawings:
Figure 1 ls a ~chema~lc dlagram lllustratlng the
process steps oE the present lnventlon.
Figure 2 ls a scllamatlc diagram showing a semi-
contlnuous flow ~rrangement for carrying out tl~e process 10
of the present lnventlon.
Flgure 3a S]lOWS the arrangement of Flgure 2 ln the
flrs~ cycle mode wherelil aroma ls extracted from fresh tea
ln a ~lrst proces~ tank and the tea from which the aroma
has already been extracted ls decaf~elnated in a second 15
process tank. Ullused flow llnes are indicated ln phantom.
Figure 3b shows the ~econd cycle whereln aroma-free
tea from the first cycle is dec~ffeinated ln the ~lrst
process tank and fresh tea ls dearomatlzed in the second
proce~s tank. Simllarly, unused flow llnes are 6hown in 20
E~l~alltom .
Figure 4 is a schematlc dlagram o~ showing the drylng
and rearomatlzing steps.
Figures 5a ~nd 5b show ~wo arrang~nts for different modes oE
re-establishing the pressure oE the carbon dioxide after pressure 25
r~luction in the ara~ separation step.
Figure 6 shows an arrang~nt for an alternatel~e of carrying
out the decaEfeination by means oE a washing step.
Figure 2 shows an arrange~ent for a semicontinuous
operation of the process of the present invention. This
arrangement comprises two interchangeable and
interconnected circult systems. The primary circult 35
comprises an input pump 12 connected to high pressure
line 14, joined at the other end thereof to liquified qas
pump 18 which in turn i5 connected to first process tank4
2~ via high pressure line 22 containing valve
26. ~0
-4-
~L 3 ~
First process tank 28 is connected to pressure
regulator 36 via high pressure line 34 containing
valve 32. Aroma separator tank 42 i~ connected to
pressure regulator 36 via high pressure line 38 and to
input pump 12 via high pressure line 48 which contains 5
valve 46, it is also provided with a
discharge valve 44.
The second circuit comprises input pump 52
connected to ca~feine absorber 56 which in turn is 10
conne,cted to the second process tank 66 via high pressure
line 58 which containssequentially circulating pump 62 and
valve 64. The circuit is completed by high
pressure line 68 containing valve 72
connecting the second process tank 66 to high pressure 15
line 54.
The interconnection between the circuits is provided
by high pressure line 82 containing valve 84 which
connects tank 28 to high pressure line 68 and high 20
pressure line 86 containing valve 88 which
connects tank 28 to high pressure line 58.
Similarly, high pressure line 92 containing
valve 94 connects second process tank 66 to high 25
pressure line 22 and similarly high pressure line 96
containing valve 98 connects tank 66 to high
pressure line 34.
The process of the present invention is suitably 30
carried out in two alternating cycles illustrated in
Figures 3a and 3b respectively.
As shown in Figure 3a, fresh tea whose moisture
content has been raised to a predetermined level, is 35
charged to first process tank 28,~'Carbon dioxide at a
predetermined pressure is pumped by input pump 12
into high pressure line 14 and circulated by liquiEied gas
pump 18, through high pressure line 22 and open valve 26
--5--
3.~4~
to tank 2~ where i-t absorbs moisture and aroma. ~he C02
stream then passes vla line 34 and open valve 32 into
aroma separation tanlc 42, the pressure whereof ls
reduced by pressure regulator 36 whereby the content of
tank 42 is split into three fractions, namely, a liquid 5
aqueous phase, a liquid carbon dioxide phase, and a
gaseous carbon dioxide phase. The aqueous phase
containing the aromatic components is removed through
discharge valve 44 and utilized for rearomatiYa-tion Of the
decaffeinated tea in a later step. 10
In one modification oE this ~x~ent the liquid carbon
dioxidc is evapora~ed by heating by heating device 43
ancl is passed through a condenser45(shown in Figure 5a)and
reintroduced into high pressure line 14. 15
~lternatively, the evaporated carbon dioxide is
passed to a compressorll8(shown in Fiyure 5b) which, after
compression, again introduces the carbon dioxide into the
cycle.
It will of course be clear to those in the art that the 20
important step in both of these recycling modes is the
evaporation step, so that the recycled carbon dioxide does
not carry any aroma material, hence, is enabled to absorb
the maximum amount from the mixed batch of tea.
Contemporaneously with the foregoing cycle, the
decaffeination is carried out in the second circuit. This
second circuit operates under isobaric conditions. In the
operation of this circuit during the first cycle, valves
72 and 64 are open and valves 84, 88, 94 and 98 are 30
closed. Thus, the carbon dioxide introduced into
said second circuit through second input pump 52 will flow
into the entire system to a predetermined pressure level.
Circulation pump 62 is then activated, whereby the carbon
dioxide passes through tank 66 which con-tains 35
damp dearomati7ed tea. ~he carbon dioxide
solvent is thus circulated into the caffeine absorber 56
which has previously been charged, either with activated
-6- ~0
-- 7 ~
carbon or a suitable ion exchanger, for the removal of
caffeine.
In an alternate mode shown in Fiyure 6, adsorber 56 may be
a washing tank, where water is pressed into the carbon dioxide
stream containing caffeine by a pump 57. The a~ueous wash is
removed from the system via discharge valve 59. The water
pressed in from reservoir 55 by pump 57 may contain a complexing
agent, e.g. a solution of tanning agents, forminy a precipitate
with caffeine. This mode of operation is more effective than
washing out the caffeine by water alone.
When the predetermined amount of aroma has been removed from
the first processing tank and similarly, a predetermined amount
of caffeine has been removed from the second processing tank, the
operation goes into the second cycle as shown in Figure 3b. In
this cycle, after closing the appropriate valves, tank 66 is
taken out of the system and is replaced with a similar tank 166,
containing fresh tea moistened to the predetermined level. First
processing tank 28 is left in place. Valves 26, 32, 64 and 72
are closed and valves 84, 88, 94 and 98 are opened. The effect
of this r~arrangement is to place the second processing tank 166
into the aroma extraction circuit which will operate as described
hereinabove. The now dearomatized tea in the first processing
tank 28 is placed into the caffeine removal circuit which, again,
is operated as previously described. When the predetermined
degree of aroma and caffeine removal have been reached, valves
84 and 88 are closed, first processing tank 23 is disconnected
1 ~ ~3
- 8 ~
from the system and replaced with a similar tank 128, again
containing fresh humidified tea.
It will be noted that tank 166 now contains dearomatized tea
and original tanks 66 and 28 contain dearomatized and
decaffeinated tea, which can be processed as described
hereinbelow. The system is now ready to recommence the two-cycle
operation as described hereinabove. It will be understood by
those skilled in the art that tanks 66 and 28 can be replaced by
fresh tanks as the progress of the operation proceeds.
The apparatus for the final step is illustrated in Figure
4 and comprises a hopper means 142 comprising a discharge means
143 issuing onto a continuously-heated belt 144. The belt is
oriented to provide a discharge into a mixing tank 152 provided
with mixing means 154 and discharge means 156.
In the operation of the final step, the moist dearomatized,
decaffeinated tea 140 is discharged into hopper 142 from first
and second process tanks 28, 66. The moist tea then passes
through discharge means 143 to provide a layer 146 on belt 144
where it is dried hy the application of a predetermined amount
of heat (Q) by rising the temperature of the tea to 40 to 80C
and is dischar~ed into mixing tank 152 wherein it is agitated
while the blend of aromas 150 obtained from aroma tank 42, are
added thereto.
The exact process conditions for carrying out the extraction
of the aroma fraction and the removal of the caffeine will depend
upon the nature of the tea to be processed. It is preferred that
prior to the actual processing step, the conventionally-obtained
~ 3 ~
_ 9
dry tea be humidified to a point where the humidified tea
contains between 25 to 50%, preferably 40% by weight (based upon
wetted material) of water. In the aroma extraction step, the
moist tea is subjected to carbon dioxide extraction at a pressure
of between 60 to 150, suitably 90 to 110 bar at a temperature of
between 20 to 70C, suitably between 50 to 60C. The extract
range will depend on the tea utilized. The extraction conditions
are chosen to dissolve the aroma but:to prevent any caffeine from
being dissolved. The amount of carbon dioxide utilized in this
step will of course vary, but it has been found that between 10
to 30 kg. of carbon dioxide/kg. of air-dried tea may be utilized.
While the extraction may be carried out in the lower end of this
range, namely, between 10 and 15 kg. of carbon dioxide/kg. of
tea, it is preferred to operate in the higher end of the range,
namely, utilizing between 20 to 30 kg./kg. of tea.
In the decaffeination circuit, the carbon dioxide may ~e
utilized in the pressure range of from about 150 to about 500
bar, suitably from 250 to 350 bar, at a temperature which may
range between about 10 to 100C, most suitably from 60 to 70~C.
Again, the amount of carbon dioxide will vary. When purification
is carried out ~tilizing activated carbon as the caffeine removal
agent, it has been found useful to utilize between 250 to 500
kg., most suitably between 300 to 350 kg. of carbon dioxide/kg.
of air-dried tea treated. It will be understood by those skilled
in the art that while it is preferred to remove the caffeine from
.~
1~ ~ 8 JL ~ ~:
- 9a -
the carbon dioxide solvent under isobaric conditions by
adsorption on carbon, separation can a]so be effected by
reduction of density of the solution by means of reduction of
pressure and/or rise of temperature (US Patent No. 4,167,589
patented September 19, 1979 by Vitzthum et al).
While the art dlscloses a prejudice against the stability
of tea aroma in water (EU-PS 00 50 482 of 1984), column 1, lines
41 - 43), it has been found that when utilized in the process of
10 aspects of the present invention, the aqueous aroma suspension
is sufficiently stable to impart a fresh flavour to the dried tea
when the tea is impregnated with it, in accordance with the
procedures of aspects of the present invention.
While it is preferred to rearomati~e the tea by adding the
15 aqueous extract to it as described hereinabove, an alternate mode
may also be employed. ~he aroma
A
~ 3~ 8 ~
containing extract may be microancapsulated in accordance
with procedures well known in the art and the thus
obtained microcapsules mixed wlth the decaffeinated,
dearomatized, dried tea, as described hereinabove.
In a sensorial test, samples o~ tea were
decaffeinated according to the above~mentloned known
processes and compared to untreated tea and to tea which
had been decaffeinated according to thP invention. The
tea used was "range Broken". Five tea tasters judged the 10
tea in accordance with "J. Schormuller, Handbook of Food
Chemistry, Volume VI, Springer-Verlag~ 1970". The
judgments were rated according to a scale of grades from l
to 5; the untreated tea was attributed the highest grade
of l. 15
Method of DecaffeinationGrad_
None (untreated tea)
According to the invention 1.5 20
According to DE-PS 21 27 642 3
According to DE-PS 26 37 197 2
According to DE-OS 34 13 869 2
According to DE-OS 34 15 844 3
Decaffeinated with dichloromethane 5 25
Decaffeinated with ethyl acetate 5
The ratings show that tea decaffeinated according to
the invention was preferred to all others. It was
particularly surprising to find that there was hardly any 30
difference in taste between the untreated tea and the tea
which had been treated according to the invention.
--10--