Note: Descriptions are shown in the official language in which they were submitted.
:~ 3 ~
- - 1 - RK36a
WIRE
This invention relates to electrical wires, espe-
cially although not exclusively, to wires intended for
use in aircraft.
One phenomenon to which aircraft wires may be sub-
jected is tracking. Tracking is associated with the
formation of permanen~ and progressive conducting paths
on the surface of the material by the combined effects
of an electrical field and external surface pollution.
Once commenced, the carbonaceous conducting deposits
often extend quickly in dendritic fashion to give a
characteristic "tree" pattern until failure occurs
across the surface. Electrical tracking can occur when
a damaged energised bundle of wires become wet e.g.
from electrolytes or condensation. This tracking may
lead to flashover and arcing that causes additional
wires in the bundle to become damaged. A catastrophic
cascade failure can result from a fault to a single
wire i~ adjacent wires that are at a different electri-
cal potential are also susceptible to tracking or if
the bundle is in contact with a grounded structure.
Tracking can occur at low voltages e.g. 100V a.c. or
less but becomes less likely as the voltage is reduced.
~L 3 ~ 3
- 2 - RK368
A related phenomenon, to which these wires may be
subject, is that of breakdown due to arcing. In this
case a potential difference between two conductors, or
between a conductor in which the insulation has been
mechanically damaged, and ground, can result in the
formation of an arc between the conductors or between
the conductor and ground. The high temperature of the
arc causes the polymer to degrade extremely rapidly and
form an electrically conductive carbonaceous deposit
which can extend rapidly, as with wet tracking, and
lead to catastrophic failure in which many or all of
the wires in a bundle are destroyed. Arcing can occur
at very low voltages, for example 24V d.c. or lower,
and since, unlike tracking, no electrolyte or moisture
is involved, it is a particularly hazardous phenomenon.
Arcs may also be struck by drawing apart two conductors
between which a current is passing as described for
example by J.M. Somerville "The Electric Arc", Methuen
-1959.
Another phenomenon that can be associated with
tracking and arcing is erosion. In this case insu-
lating material is removed by a vaporization process
originated by an electrical discharge without the for-
mation of electrically conductive deposits so that
failure of the insulation will not occur until complete
puncture of the insulation occurs.
According to the present invention, there is pro-
vided an electrical wire which comprises an elongate
electrical conductor and electrical insulation that
comprises:
~ 3 ~ 3
_ 3 `_ RR368
ta) an inner insulating layer which comprises a poly-
amide that has a glass transition temperature of
at least 0C; and
(b) an outer insulating layer which comprises a
fluorinated polymer.
- The invention has the advantage that it enables a
wire to be formed that has a balance of properties such
as solvent resistance, scrape abrasion resistance,
toughness, weight and ability to strip in addition to
very high resistance to tracking, arcing and erosion.
For example the polymeric material (including any
fillers) forming the inner, polyamide layer, and pre-
ferably the materials forming both layers will normally
have an elongation to break of at least 50% and espe-
cially at least 100%, and, together, a cut through
value at 150C of at least 15, and preferably at least
-20 N.
The polyamide preferably has a molar carbon to
hydrogen ratio of not more than 1.0 and especially not
more than 0.8. This will normally correspond to a car-
bonaceous char residue of not more than 15%, preferably
not more than 10%, most preferably not more than 5%,
especially not more than 2% and most especially
substantially 0% by weight.
The char residue of the polymer components in the
electrical wire according to the invention can be
measured by the method known as thermogravimetric ana-
lysis, or TGA, in which a sample of the polymer is
heated in nitrogen or other inert atmosphere at a
~3~9~3
- 4 - RK368
defined rate, e.g. 10C per minute to a defined tem-
perature and the residual weight, which is composed of
char, is recorded. The char residue is simply the
quantity of this residual char expressed as a percen-
tage of the initial polymer after having taken into
account any non polymeric volatile or non-volatile com-
ponents. The char residue values quoted above are
defined as having been measured at 850C.
The polyamides employed in layer ~a) may be
entirely aliphatic or may have both aliphatic and aro-
matic moieties, and preferably have adjacent amide
groups that are separated from each other by an average
of at least 4 carbon atoms (excluding the amide car-
bonyl group carbon atoms) in the polymer backbone, that
is to say excluding any pendant groups but including
all the carbon atoms in any monocyclic or fused aroma-
tic rings in the polymer backbone. The most preferred
polyamides have adjacent amide groups that are
separated from one another by an average of at least 6
carbon atoms, but preferably by not more than 15 carbon
atoms in the polymer backbone. The preferred polyami-
des may be defined by the following general formula I:
H H O O (I)
- N-R-N-C-R'-C-
_ n
wherein each R and R' which can be the same or
different, each represents an alkylene group
having 1 to 30 carbon atoms, preferably 2 to 20
carbon atoms and most preferably 4 to 12 carbon
atoms; or a divalent group having one or more
~ 3 ~ 3
_ 5 - R~368
alkylene, cycloalkylene or arylene moieties and
from 3 to 30 carbon atoms, preferably 2 to 20 car-
bon atoms and most preferably 4 to 12 carbon
atoms; or a mixed alkyl-cycloalkyl radical having
from 4 to 30 carbon atoms, preferably four to 20
carbon atoms and most preferably 4 to 12 carbon
atoms; or R can also be represented by the general
structure (II):
R ~ (II)
\~/ R2 \~/
R3 R3
in which R2 and R3, which may be the same or dif-
ferent r each represents a hydrogen atom or an
alkyl radical from one to 15 carbon atomsl pre-
ferably one to five carbon atoms; a cycloalkyl
radical having from 3 to 16 carbon atoms, pre-
ferably five to 10 carbon atoms; a mixed
alkylcycloalkyl radical having from 6 to 20 carbon
atoms, preferably 6 to 10 or an aryl radical
having from 6 to 20 carbon atoms, preferably 6 to
10 carbon atoms. In a preferred embodiment, each
R2 represents a methyl group and each R3 repre-
sents a hydrogen atom. n is an integer and, pre-
ferably, falls within the range of about 10 to
about 500,000.
Examples of preferred polyamides include, but are
not limited to: a 50:50 mole ratio copolymer of
2,2'-(bis~4-aminocyclohexyl) propane and a 60/40 weight
~ 3 ~
270~5-177
percent mixkure of azelaic acid and adipic acid, (~or example as
prepared ln accordance with U.S. Patent No. 3,840,501, an
amorphous polyamide derived from a 50:50 ratio of hexamethylene
diamine and an equal mixture of terephthalic acid and isophthalic
acid, an amorphous polyamide derived from dimethylterephthalate
and a 50:50 mixture of 2,2,4- and 2,~,4-trimethylhexamethylene
diamine, a polyamide of m-xylylenediamine an adipic acid~ a
polyamide formed from the condensation of one or more
bisaminomethylnorbornane isomers with one or more aliphatic,
cycloaliphatic or aromatic dicarboxylic acids e.g. terephthalic
acid and optionally including one or more amino acid or lactam
comonomers, e.g. -caProlactam comonomers, and especially
polyamides based on units derived from laurinlactam, isophthalic
acid and bis-(4-amino-3-methylcyclohexyl) methane. Amorphous
polyamides prefera~ly have a crystallinity of not more than 2% as
measured by differential scanning calorimetry (DSC) e.g. as
described in U.S. Patent No. 4,528,335. Crystalline or semi-
cxystalline polyamides that may be employed according to the
invention include the nylons e.g. nylon 46, nylon 6, nylon 7,
nylon 66, nylon 610, nylon 611, nylon 612, nylon 11, nylon 12 and
nylon 1212.
If desired the polyamide may be blended with one another
or with other polymers. For example the polyamides may be used as
blends with polyesters, polyolefins such as polyethylene, ethylene
ethyl acrylate copolymers or styrene/diene bloc~ copolymers or
phenylene ether homo- or copolymers. In many cases it is highly
desirable for the polyamide to he blended wlth an aromat~c poly-
1 3 ~ 3
- - 7 - 27065-177
mer, pre~erably a wholly aromatic polymer or one that includes
aliphatic moieties e.g. pendant alkyl groups or alkylene groups in
the polymer backbone, such alkyl or alkylene groups preferably
having no more than 5, especially no more than 3 carbon a-toms e.g.
methyl groups or, as alkylene groups, methylene or isopropylidine
groups. Such composi-tions, and wires that employ such composi-
tions, are disclosed in our Canadian Patent Applications 571,477
and 571,478 filed on July 8, 1988.
The fluorinated polymer used in layer (b) preferably
contains more than 10%, preferably more than 25%, by weight of
fluorine. Thus the fluorocarbon polymer may be a single fluorine-
containing polymer, a mixture of two or more fluorine-containing
polymers, or a mixture of one or more fluorine-containing polymers
with one or more polymers which do not contain fluorine. In one
preferred class, the fluorocarbon polymer comprises at least 50%,
particularly at least 75% especially at least 85%, by weight of
one or more thermoplastic crystalline polymers each containing at
least 25% by weight of fluorine, a single such crystalline polymer
being preferred. Such a fluorocarbon polymer may contain, for
example, a fluorine-containing elastomer and/or a polyolefin,
preferably a crystalline polyolefin, in addition to the crystal-
line fluorine-containing polymer or polymers. The fluorine-
~3~9~3
- 8 - RX368
containing polymers are generally homo- or copolymers
of one or more fluorine-containing olefinically unsa-
turated monomers, or copolymers of one or more such
monomers with one or more olefins. The fluorocarbon
polymer usually has a melting point of at least 150C/
and will often have a melting point of at least 250C,
e.g. up to 350DC, the melting point being defined for
crystalline polymers as the temperature above which no
crystallinity exists in the polymer (or when a mixture
of crystalline polymers is used, in the major
crystalline component in the mixture). Preferably the
polymeric composition, prior to cross-linking, has a
viscosity of less than 104 Pa.s (105 poise) at a temp-
erature not more than 60C above its melting point. A
preferred fluorocarbon polymer is a copolymer of ethy-
lene and tetrafluoroethylene and optionally one or more
other comonomers (known as ETFE polymers), especially a
copolymer comprising 35 to 60 mole percent of ethylene,
-35 to 60 mole percent of tetrafluoroethylene and up to
10 mole percent of one or more other comonomers. Other
specific polymers which can be used include copolymers
of ethylene and chlorotrifluoroethylene; poly~inylidene
fluoride; copolymers of vinylidene fluoride with one or
both of hexafluoropropylene and tetrafluoroethylene, or
with hexafluoroisobutylene; and copolymers of tetra-
fluoroethylene and hexafluoropropylene. Alternatively
Cl-Cs perfluoroalkoxy substituted perfluoroethylene
homopolymers and copolymers with the above fluorinated
polymers may be used.
Either or both layers of the wire insulation may
be, and preferably are, cross-linked for example by
exposure to high energy radiation.
.
13~9~
_ g _ RK368
Radiation cross-linking may be effected by expos-
ure to high energy irradiation such as an electron beam
or gamma-rays. Radiation dosages in the range 2~ to
800 kGy, preferably 20 to 500 kGy, e.g. 20 to 200 kGy
and particularly 40 to 120 kGy are in general
appropriate depending on the characteristics of the
polymer in question. For the purposes of promoting
cross-linking during irradiation, preferably from 0.2
to 15 weight per cent of a prorad such as a poly-
functional vinyl or allyl compound, for example,
triallyl cyanurate, triallyl isocyanurate (TAIC),
methylene bis acrylamide, metaphenylene diamine bis
maleimide or other crosslinking agents, for example as
described in U.S. patents Nos. 4,121,001 and ~,176,027,
are incorporated into the composition prior to irra-
diation.
The insulation may include additional additives,
for example reinforcing or non-reinforcing fillers,
stabilisers such as ultra-violet stabilisers, antioxi-
dants, acid acceptors and anti-hydrolysis stabilisers,
pigments, processing aids such as plasticizers, haloge-
nated or non-halogenated flame retardants e.g. hydrated
metal oxides such as alumina trihydxate or magnesium
hydroxide if the processing conditions allow, zinc
borate or decabromodiphenyl ether, fungicides and the
like.
In many cases the wire insulation will consist
solely of the polyamide inner layer and the fluoropo-
lymer outer layer. However, if desired one or more
other layers may be present. For example an additional
inorganic layer may be provided directly on the conduc-
~ 3 ~
- 10 - 27065-177
tor, formed for example by deposition of an inorganic material on
the conductorO Alternatively or in addition a highly aromatic
polymer layer may be provided between layers (a) and (b) in order
to improve for example the high temperature properties of the
insulation. Examples of such aromatic polymers are disclosed in
our copending Canadian Patent Application No. 571,502 filed on
July 8, 19~8.
The wires and cables according to the invention may be
formed by conventional techniques. For example the polymers may
be blended with any additional components, in a mixer, pelletised,
and then extruded onto a wire conductor. Other, non-preferred,
wires may be formed by a tape-wrapping method although it is
preferred for both the fluoropolymer and the polyamide layers to
be melt extruded.
The wires may be used individually as equipment or
"hook-up" wires, or airframe wires, or in bundles and harnesses,
both jacketted and unjacketted, and may be used in multiconductor
cables. The wires, harnesses or cables may be unscreened or they
may be provided with a screen to protect them from electromagnetic
interference, as well known in the art. In addition flat cables
may be formed using the insulation materials according to the
invention, either employing flat conductors or round conductors.
The invention will be described by way of example with
reference to the accompanying drawings in which:
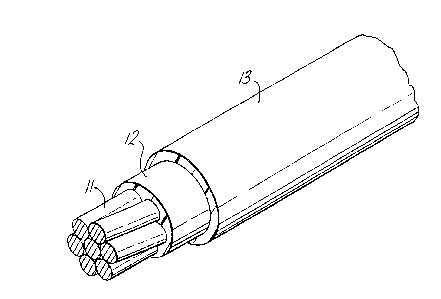
Figure l is an isometric view of part of an electrical
wire according to the invention;
~; . .
~ RK368
Figure 2 is a schematic view of the test arrange-
ment for wet tracking; and
Figure 3 is a schematic view of the test arrange-
ment for dry arcing.
Referring initially to figure 1 of the accompany-
ing drawings an electrical wire comprises a conductor
11 which may be solid or stranded as shown and is
optionally tinned. On the conductor an inner insu-
lating layer 12 or primary insulation has been
extruded. The insulation is formed from nylon 12 or a
blend of nylon 12 with a polyaryl ether imide which
contains about 5% by weight triallyl isocyanurate
crosslinking promotor. After the inner layer 12 has
been formed an outer layer 13 or primary jacket formed
from an ethylene-tetrafluoroethylene copolymer, con-
taining about 7% by weight triallyl isocyanurate cross-
-linking promotor, i9 extruded on the inner layer 12.
Each layer has a wall thickness of about 100 pm. After
both layers have been extruded the insulation is irra-
diated by high energy electrons to a dose of about 120
kGy.
The following Examples illustrate the invention.
In the Examples the following test procedures were
used:
9 ~ ~ ~
- 12 - RR368
WET TRACKING TEST
This test is designed to simulate the condition
occuring when a damaged wire bundle comes into contact
with an electrolyte. Under actual conditions, the
electrolyte may be moisture containing dust particles
or other ionic contaminant. Damage to the bundle may
occur through a number of reasons e.g. abrasion, hydro-
lysis of the insulation, ageing, etc. Current ~low
through the electrolyte results in heating and evapora-
tion of the solutionO This causes one or more dry
bands to appear across which the test voltage is
dropped, resulting in small, often intense, scin-
tillations which damage the insulation.
Figure 2 shows the sample set-up. A wire bundle 1
is prepared from seven 18cm lengths 2 of 20AWG tinned-
copper conductor coated with a layer of the material
-under test. The bundle 1 is arranged with six wires
around one central wire and is held together using tie
wraps 3 so that the wires are not twisted. Two adja-
cent wires are notched circumferentially to expose
0.5mm bare conductor on each wire. The notches 4 are
arranged such that they are 5mm apart with the tie
wraps 5mm either side of them. One end of each wire is
stripped -to enable connections to be made to the power
supply via insulated crocodile clips. The sample is
held at an angle of 30 degrees to the horizontal using
a simple clamp made of an electrically insulating resin
so that the damaged wires are uppermost and the
stripped ends are at the upper end of the bundle. A
piece of filter paper 5 20 x 10mm wide is wrapped
around the bundle approximately 2mm above the upper
.
~ 3 ~
13 - RK368
notch; th~s is best held in place with the upper tie
wrap.
A peristaltic pump conveys the electrolyte from
the reservoir to the sample via a dropping pipette 6,
and a power supply is provided to energise the bundle.
The electrolyte used is 2% sodium chloride and
optionally 0.02~ ammonium perfluoroalkylcarboxylate
surfactant in distilled or deionised water. The pump
is set to deliver this solution at a rate of approxima-
tely lOOmg per minute through the pi~ette 6 which is
positioned lOmm vertically above the filter paper 5.
The power is supplied by a 3-phase 400Hz 115/200V
generator of at least 5kVA capacity or a single phase
50Hz 115V transformer of at least 3kVA capacity. A
device for recording time to failure is provided which
records the time when either a wire goes open circuit,
-or when a circuit breaker comes out. Leakage currents
can be followed with the use of current clamps
surrounding the wires and connected to a suitable
oscilloscope.
In the case of the three phase supply, adjacent
wires of the bundle are connected to alternate phases
of the power supply via 7~5A aircraft-type circuit
breakers e.g.*Xlixon with the central wire connected
directly to neutral. In the case of the single phase
supply, alternate wires are connected to neutral with
the remaining wires including the central conductor to
live. A few drops of electrolyte are allowed to fall
onto the filter paper to ensure saturation prior to
starting the test. The power is switched on and the
time~ started. The test is allowed to continue until:
Trc~ Je Mark
14 ~3~a3 RR368
a) one or more circuit breakers come out;
b) a wire becomes open circuit; or
c) 8 hours have elapsed~
The condition of the final bundle and the time to
failure is noted in all cases. Where failure has
occurred due to breakers coming out, the power is then
reapplied and each breaker is reclosed in turn until
there is no further activity. The condition of the
bundle is again noted.
Failure due to the wire becoming open circuit
(result (b)) is indicative of erosion. If failure
occurs due to one or more circuit breakers coming out
(result (a)) then the absence of further crepitation on
resetting of the circuit breakers indicates failure due
to erosion, while further crepitation indicates
tracking failure.
Dry Arc Test
This test is designed to simulate what happens
when a fault in a wire bundle causes arcing under dry
conditions. A graphite rod is used to initiate the arc
which causes thermal degradation of the insulation.
Continuation of the fault current can only occur
through the wire bundle under test due to shorting
across adjacent phases through a conductive char, or
direct conductor-conductor contact such as might occur
if the insulation is totally removed by the duration of
the arc.
13~9~
~ 15 - RK368
Figure 3 shows the sample set-up. A wire bundle
21 is prepared from seven 10cm lengths 22 of 20AWG
tinned-copper conductor coated with a layer of the wire
insulation under test. The bundle 22 is arranged ~ith
six wires around one central wire and held together
with tie wraps spaced about 5cm apart. One of the
outer wires is notched circumferentially between the
tie wraps to expose 0.5mm bare conductor and one end of
each wire is stripped to enable connections to be made
via insulating crocodile clips.
A rod 23 is provided which is made of a spectro-
graphically pure graphite, diameter 4.6mm, with an
impurity level not more than 20ppm. It is prepared
before each test by sharpening one end using a conven-
tional pencil sharpener of European design to give an
angle of 10 degrees off vertical with a tip diameter of
0.4~0.1mm. A 100g weight 24 is clamped onto the top of
-the rod 23 to maintain contact during the arc ini-
tiation and also acts as a device to limit the depth of
penetration of the rod by restricting its downward tra-
vel. The rod passes through a PTFE bush which allows
it to slide freely up and down.
The arrangement of levers enables precise posi-
tioning of the rod 23 on the wire bundle 21 which is
held securely in place by means of a simple clamp 25
made of an electrically insulating resin and ~ounted on
a block 26 made of the same material.
The power source can be either:
a) a 3-phase 400Hz 115/200V generator of at
least 5kVA capacity
~3~03
- 16 - RR368
b) a single phase 50~z 115V transformer; at
least 3kVA capacity
c) 24V d.c. supplied by two 12V accumulators.
The fault current is detected by means of current
clamps surrounding the connecting leads and the voltage
at failure is measured using a 10:1 voltage probe. The
transducer signals are fed into a multi-channel digital
storage oscilloscope where they can be displayed and
manipulated to obtain power curves (voltage x current)
and energy tintegration o power curve).
The wire bundle 21 is positioned in the clamp 25
so that the notched wire is uppermost. Adjacent wires
of the bundle are connected to different phases of the
supply through 7.5A aircraft type circuit breakers, and
the central wire is connected directly to neutral. In
the case of single phase or d.c. supplies, alternate
-wires are connected to neutral or the negative ter-
minal, with the remaining wires, including the central
wire, connected through circuit breakers to live or the
positive terminal. The carbon rod is also connected to
neutral or the negative terminal and positioned so that
the point is in contact with the exposed conductor.
The gap between the lOOg weight and the PTFE bush is
adjusted to 0.4mm using a suitable spacer to limit the
penetration of the rod into the sample. A voltage
probe is connected across the damaged wire and the rod,
and current clamps positioned on each of the three pha-
ses, or on the wires connected to the live side of the
supply. A protective screen is placed in front of the
test set-up and the power switched on. The condition
of the final bundle i5 noted in all cases. Where
~ 3 ~ 3
- 17 - RX368
failure has occurred due to breakers coming out, the
power is then reapplied and each breaker is reclosed in
turn until there is no further activity. The condition
of the bundle is again noted. A material is deemed to
pass this test if:
a) no circuit breakers come out and the activity
is relatively non-eventful, or
b) there is no further activity on resetting the
breakers after a non-eventful test.
In addition, non-tracking materials will have
relatively few spikes in the current trace with a
correspondingly low total energy consumed. Tracking
materials, on the other hand, show many spikes usually
on all three phases, which are accompanied by violent
crepitation and large energy consumption.
Example l
A 20mm Baughan extruder was used to produce a wire
construction comprising a 20 AWG tin-plated copper con-
ductor with a lO0 um inner insulating layer formed from
an aromatic/aliphatic polyamide (Polyamide 1) based on
laurinlactam, isophthalic acid and
bis-(4-amino-3-methylcyclohexyl) methane containing 5
by weight triallyl isocynaurate and a lO0 um outer
insulating layer formed from ETFE containing about 7%
by weight triallyl isocyanurate. After formation the
wire was irradiated with high energy electrons to a
dose of 120 kGy to crosslink the insulation.
~ 3 ~ 0 3
- 18 - RX36a
The physical and mechanical properties of the
wires are shown in Table I together with those of a
conventional airframe wire having a 200 llm thick ETFE
insulation, from which it can be seen that the wire
according to the invention is lighter and has superior
cut through and scrape resistance than the ETFE wire.
I~BLE I
WeightCut ~rough(i~ Scrape(ii) Tensile
Wire (kal~ ) @ 150C (N) (cycles)Stren~h (MPa)
(a) 100 ~m Polyamide 1 + 1.10 55.2 170 69.0
100 ,IJm E~FE
(b) 200 ~m E~FE 1.32 25.5 50 51.7
Notes ~i) measured according to BS G230:1984 test 26
with crosshead speed of 5mm minute~l.
(ii) measured according to BS G230:1984 test 30
using a tungsten carbide blade with a 0.005
inch radius blade edge, an 800g weight and
conducted at 20C.
While the physical and mechanical properties of
the wire are considerably improved by replacing a pro-
portion of the ETFE with a polyamide, the ETFE cannot
be completely replaced with the polyamide in view of
the very poor erosion performance of the polyamide
~ 3 ~ 3
- 19 - RR368
wires when subjected to the wet tracking test and dry
arcing test as described above. The results of these
tests for wire construction (a) and for a wire having a
200 um thick insulation formed from the same polyamide
as used in construction (a) are shown in Table II.
T~EE ~
~T~K~C~T
reCI~lu~n C~x~itb~b~s t~eto ~tq~n O~pit~i~n
~t CLX~it(S) ~ ~divity
(a)(lOO~mp~y~del+
100 ~n E EE) O 54~0
(c)(200Fmp~y~i~ 1) 1
T~
~rear~lu~n on~itb~a~s ~.ofw~s O~k~n
~t q~nc~n~t cna~ivity
ta)(~m~y~del+
~c)(200pm~T~del) 0 2
~ 3 ~ 3
~ 20 - RK368
Example 2
Example 1 was repeated with the exception that the
following polyamides were used: nylon 12 (polyamide
II), an amorphous polyamide derived from
dimethylterephthalate and a 50 50 mixture of 2,2,4- and
2,4,4-trimethylhexamethylene diamine (ex Dynamit Nobel)
~polyamide III), an amorphous polyamide based on hexa-
methylene diamine and isophthalic acid (polyamide IV~,
and an amorphous polymer based on xylylene diamine and
adipic acid (polyamide V). In addition tests were per-
formed on single wall polyetherimide and polyetherke-
tone wires by way of comparison. The results for the
wet tracking and dry arcing tests are shown in Tables
III and IV.
~ 3 ~ 3 .~3
- 21 - RR368
~E m
~r
~re ~im ar~lit h~rs~re ~ l~k ~tatikn
a~ ~n ~ra~it(s) al r~
(d) (100 ~n pciL~rd~ Ir
(e) (200]mF~ ) O ~260 2
(f) (lDO ~n ~ciLya~rod~ m
100 pn EEE)
(g) (æ~mE~ m:) o
(h) (æO~n~L~-
i~rd~) 6 ~i Y~;
h~e) 4 36 Yes
( j) (lOO un pc~Lyardde IV
lQO ~n En~) 0 5040 2
(k) (~0 ~ L~ V
100 ~n EEE) O 1920
3 3
~2 - RR368
~E lV
r~ F~; ~
C~it }~rs ~tati~ N:~. of ~res
alt cn r~et~en cir~it at
~1 aE t~;t
td) (100 }m p~ e rr
;) 0 2; /A
(f) (100 ~np~rode m o
100
(h) (æO ,~m p~
imide) 2 ~s 7
(i) (200 ~n p~.~-
l~ne) 2 Y~s 7
( j) (100 ~n EriLy~e IV)
100 ~m 13EE) O ~A
(k) (lDO~mp~deV
100 pm E3~:) 0
Example 3
Example 1 was repeated with the exception that the
f luorocarbon layer comprised perf luoroalkoxy polymer
-Teflon PFA (Trademark) and a fluorinated ethylene-
propylene polymer - Tef lon FEP ( Trademark ) and that
~3~03
- 23 - RR368
neither layer contained any crosslinking promotor nor
was irradiated. The results are shown in Tables V and
VI.
TABLE v
Total insulation
Weight Cut through
Fluorocarbon layer (kqkm~l) @ 150C (N)
Teflon PFA 1.27 32.2
Teflon FEP 1.28 42.3
~ 3 ~
- 24 - RK368
TABLE Vl
WET TRACKING TEST
Wire construction Circuit breakerstLme to 1st open Crepitation out circuit(s~ on reset
Polyamide 1 +
Te~lon PFA 0 5160 N/A
Polyamude 1 +
Teflon FEP 0 12,000 N/A
~RY ARCING TEST
Circuit breakers No. of wires Crepitation
out open circuit on reset
Polyamide 1 ~
Teflon PFA 0 1 N/A
Polyamide 1 +
Teflon FEP 0 1 N/A