Note: Descriptions are shown in the official language in which they were submitted.
1~'5 ~ 19~8'7 ~
METALORGANIC CHEMICAL VAPOR D~POSITION GROWT~ OF
GROUP II-VI SEMICONDUCTO~ MATERIALS HAVING IMPROVED
COMPOSITIONAL UNIFORMITY
Back~round of the Invention
This invention relates generally to epitaxial growth
techniques~ and more particularly ts growth of Group II-VI
- semiconductor crystalline materials~
As is known in the art, Group II-VI semiconductor
epitaxial mater;als such as cadmium telluride and mercury
cadmium telluride have important applications as photodetector
elements for detection of electromagnetic energy in the
spectral range from approximately 0.8~ m to 30~ m. By adjust-
ing an alloy composition of cadmium and mercury, photodetector
elements are provided which are sensitive to different wave-
length ranges withi~ the 0.8 ~ m to 30 ~m wavelength band.
Several different techni~ues have been suggested for
providing cadmium telluride and mercury cadmium telluride
suitable for use in photodetector applications. One method
suygested is metalorganic vapor phase expitaxy (MOVPE), also
referred to metalorganic chemical vapor deposition (MOCVD).
As it is known, the MOCVD technique for growing mercury
cadmium telluride involves directing vapors of mercury,
dimethylcadmium, and diethyltelluride into a reactor vessel
and chemically reacting the directed vapors to provide the
epitaxial material.
~ 3 ~ 7
Several problems are enco~ntered in the art of growing
mercury cadmium telluride epitaxial layers by the MOCVD
technique! One problem of particular importance is the
compositional uniformity of the deposited epitaxial layers
provided by the MOCVD technique.
Generally, the composition of these layers varies from
the downstream portion of the substrate to the upstream
co~ ~ Os ~-h`o~ a/
, ` portivn. This ~osit-on-a~- variation generally involves a
progressively increasing depletion of Cd towards the downstream
or back portion of the substrate whereas the upstream portion
or front portion of the substrate is generally excessively
rich in Cd. Variations in the lateral and side to side
compositional uniformity Cd are ~enerally also present.
The prevalent view regarding the chemical reaction
mechanisms which occur in Group II-VI materials yrown by
MOCVD is set forth in an article entitled "Organometallic
Growth of II-YI Compounds~ by J.B. Mullin et al~ Journal of
Crystal Growth, Volume 55r 1981~ pp~ 92-106~ In this article,
the authors suggest that the directed alkyls of tellurium and
cadmium may not pyrolyse independently. Rather, the authors
suggest that adducts or complexes of these compounds are
produced because D~Cd (dimethylcadmium) and DETe
(diethyltelluridP) are attracted to one another in the vapor
phase forming a weak bond~ In the authors' v;ew, the decompo-
sition of these adducts leads to the formation of the Group
II ~I materials and o~her productsO
2 --
~3~L9~8~
62901-710
Aecording to the presen~ invention there is provided a
method of providing a layer comprising a Group II-VI semiconductor
material over a substrate, comprises the step of:
directing a flow comprising a Group II metalorganic yroup,
and a Group VI organic comprising a Group VI moie-ty attached to at
least one organic group towards the substrate wherein the said
organic group attached to the Group VI moiety is a large and bulky
organic group, characterized in tha~ the said organic group
attached to the &roup II moiety is a sufficiently large and bulky
organic group to sterically repulse the large bulky organic group
of the Group VI organic, such that the Group II metalorganic and
the Group VI organic pyrolyse substantially independently.
The invention further provides a method of providing a
layer comprising a Group II-VI material which comprises the steps
of:
directing a flow comprisiny a Group VI organic incorporating
a Group VI moiety towards a substrate;
directing a flow comprising a Group II organic towards the
substrate, with said Group II organic comprising a Group II moiety
with sufficiently large and bulky organic groups attached thereto
to sterically repulse the Group VI organic, such that the Group II
organic and the Group VI organic pyrolyse substantially
independently; and
reacting the Group VI and Group II moieties thereby released
from the said organics ~o form the Group II-VI material over the
substrate.
- 2a -
, :`,
:.:
1 3 1 ~ ~ g 7 62901-710
The invention additionally provides a method ~or growing
a layer comprising Group II-VI material over a subs~rate which
comprises the steps of:
directing a first flow comprising a Group VI oryanic towards
the substrate;
directing a Group II organic towards the substrate, with saicl
Group II organic having a Group II moiety with organic groups
bonded directly to the Group II moiety which provicle electron
transfer to the electroposi~ive Group II moiety, such that the
Group II organic and the Group VI organic pyrolyse substantially
independently.
- 2b -
~,
. . .
8 7 6290l-7l0
Ideal MOCVD Growth of HgCdTe, for example, may be viewed
as an irreversible pyrolyRis in which the primary alkyl ~1
order) of the tellurium and the 0 order alkyl of the cadmium
independently decompose into elemental telluriu~ and elemental
cadmi~m. The ~lemental tellurium react~ with the elemental
cadmium and elemental mercury provided in the vapor stream to
form mercury telluride ~nd cadmium telluride. The mercury
telluride and cadmium telluride are then deposited over the
Rubstrate to form the mercury cadmium tellur~de as ~how in
Reactions 1-5 below:
DETe - ~ Te ~ H.C. ~Reaction 1)
Te ~ H~ HgTe tReaction 2)
DMCd - ~ Cd ~ H.C. (Reaction 3)
Cd ~ Te - ~ CdTe (Reaction 4)
HgTe ~ CdTe ~ Hgl_xc~xT~ ~Reaction 5)
where ~.CO stands for hydrocarbons
Although there i~ evldenc2 that mercury tellurlde i~
grown by the independent pyroly~i~ oF the primary allcyl of
tellurium and the rcaction between mercury and elemental
tellurium ~Reaction~ 1 and 2~, cadmium telluride i~ now
be1ieved to be grown by ~ different proces~ which occur.~ at
elevated temperatures. ThQ simpllfled overa11 reaction i~ as
shown in ~eaction 6,
DMCd ~ DETe ~ CdTe ~ ~C ~Reaction 6)
:.. ..
~ ~9~
In this reaction, the electropositive cadmium atom in
dimethylcadmium (DMCd) is attracted to the electronegative
tellurium atom in diethyltelluride. At low temperatures,
this attraction is not significant enough to form a bond
creating a stable adduct. How~ver, at the ele~ated tempera
tures over the growth region in the reactor vessel, this
attraction leads to a chemical reaction in which the alkyls
of cadmium and tellurium react to form cadmium telluride plus
other hydrocarbons. This chemical reaction leads to a rapid
depletion of cadmium in thP downstream portions of the HgCdTe
layers formed over the substrate. Accordingly, the nonpyrolytic
behavior of the cadmium alkyl is seen as a first cause of
compositional nonuniformity in H~CdTe films formed by metal-
organic ch mical vapor deposition. In general, therefore, the
nonpyrolytic behavior of the Group II alkyl, caused by
attraction between the electropositive Group II atom and the
electronegative Group VI atom, is seen as the first cause of
compositional nonuniformity in MOCVD growth of Group II-VI
materials.
A second cause of comDositional nonuniformity in Group
II-VI materials , particularity materials such as HgCdTe
which involve two Group II elements, is believed to involve a
A reversible, exchange reaction in the vapor phase between the
primary alkyl of the ~roup IX atom and elemental Group II
atom. In particular in ~gCdTe, the primary alkyl of Cd
`8 ~
and the elemental mercury are involved in the following
reaction:
DMCd + Hg --3 DMHg + Cd ~Reaction 7)
Typically, with the MOCVD technique, the reactor walls and
the mercury source are heated to a temperature of about 220C
to prevent condensation of the mercury from the vapor stream.
At these wall temperatures, Reaction 7 has an equilibrium
constant in which the reaction is primarily driven towards
the right, that is in a direction sn which dimethylcadmium
decomposes. At these temperatures, however, the rate of the
reaction is relatively low, and accordingly the reaction i5
not a significant cause of Cd depletion. However, when the
vapor stream arrives at the substrate, there is a sudden
increase in temperature which causes the phase composition o
the vapor stream to change since Reaction 7 is now driven-
very strongly to the right. Consequently, there is a strong
variation in Cd concentration in which elemental cadmium is
produced by the reaction. The elemental Cd is even more
attracted to the diethyltelluride than DMCd~ As shown in
Reaction (8), it reacts with diethyltellurid~ to form cadmium
telluride over the upstream portions of the susceptor. That
- is, CdTe may be deposited out of the vapor stream prior to
reaching the substrate. This arran~ement again lead to a
cadmium depletion in downstream portions of he layers grown
over the substrate.
1 319~87
Cd + DETe ~ CdTe + H.C. (Reaction 8)
Accordingly, the nonpyrolytic behavior of the cadmium
alkyl is seen as a major cause of compositional nonuniformity
in Group II-VI materials such as ElgCdTe. This compositional
nonuniformity is also believed present with other Group II-VI
materials. For example, mercury zinc telluride has been
proposed as a replacement material for mercury cadmium tellu-
ride. Zinc is a significantly more electropositive atom than
cadmium. Accordingly, alkyl zinc compounds should be signifi-
cantly more reactive towards electronegative species such as
~ellurium than alXyl cadmium compounds. As a consequence of
this attraction, the first cause of compositional nonuniformity,
i.e. the chemical reaction between the Group II alkyl and the
Group VI alkyl, maybe even more significant for a material
such as mercury zinc telluride. This mechanism is also
believed present in mercury manganese telluride, a second
potential replacement or mercury cadmium telluride. Manganese
is also significantly more electropositive than cadmium, and
consequently~ a manganese alkyl would be significantly more
reactive towards the Group VI alkyl than the cadmium alkylO
With mercury zinc telluride, since zinc is significantly more
electropositive than Cd, there results a negligible exchange
reaction with mercury, and accordingly, Reaction 9 occurs
readily with substantial no reverse reaction present.
- 6
- ~3~9~8~
Zn ~ DMHg ~ DMZn ~ ~g (~eaction 9)
In accordance with the present invention, a Group II-VI
layer is provided over a substrate. A first flow comprising
a selected Group II organic is directed towards the substrate
and a second flow comprising an organic of the Group VI
el~ment is also directed towards the substrate. At least one
of the Group II and Group VI organic compounds have at least
one organic group that sterically repulses the organic groups
of the second one of the Group II and Group VI organic compounds.
Preferably, the Group II organic has large, bulky organic
groups which surround the Group II atom and sterically repulse
the organic groups of the selected Group VI organic, thus
reducing or substantially eli~inating reactions between the
Group II organic and the Group VI organic in the vapor stream.
Preferably still, the selected Group II organic includes a
,., ,~e~.lsi~r1
branched organic group which increases the steric r-s~
of the Group VI organic compound. With this particular
- arrangement, by providing a Group II or Group VI organic
source, having large organic groups surrounding the ~roup II
element or Group VI element, steric hindrance is provided
between the Group II organic, and the Group VI organic source.
That i5, by surroundin~ the Group II and/or Group VI element
with large, bulky groups which sterically repulse one another,
the Group II and/or Group VI atoms are prevented from getting5
close to each other, and ~hus the reactions involving the
L 9 ~ 8 7
Group II organic, and the Grou~ VI organic described above,
are substantially reduced. The selected Group II and Group VI
organic sources are thus substantially less reactive towards
each other in the vapor stream when compared to conventional
S Group II organics and Group VI organic sources. Accordingly,
the Group II organic and Group VI organic pyrolyse or decompose
substantially independently of one another over the growth
region above the su~strate. Since the Group II and Group VI
organics decompose substantially independently over the
substrate, the concentration of Cd varies significantly less
in the vapor stream, and hence the deposited layer has a
substanti~lly more uniform composition.
In accordance with a still further aspect of the present
invention, the Group II organic sterically repulses the Group
VI organic source which includes an organic group having a
relatively low activation energy compared to the activation
Pnergy of a primary alkyl of the Group VI element for the
formation of a free radical during pyrolysis of the Group VI
organic compound. A flow comprising an elemental source of a
Group II metal is also directed towards the substrate~ The
selected Group II organic having the large bulky organic
groups is sterically hindered from reactin~ with the elemental
Group II metal. Further, the sterically hindered Group II
organic is selected to have a thermal stability comparably to
the thermal stability o~ the selected Group VI organic. With
- 8
~ 3 ~
this particular ar~angementr low temperature growth of
compositionally uniform Group II-VI materials is provided.
The low growth temperatures should kinetically limit the rate
of the exchange reaction involviny the selected Group II
organic and elemental Group II metal, and the steric replusion
- effects should further kinetically limit this exchange reaction~
Thus, the second additional cause of compositional nonun~formity
in MOCVD growth of Group II-VI materials is also substantially
reduced, since the rate constant at low temperatures for the
exchange reactio~, is relatively small in the direction with
provides the exchange of Group II metals. Side to side
compositional uniformity is also believed improved, since
with prior approaches, any cause of side to side nonuniformity,
such as, variations in flow patterns and temperature gradients
were amplified due to the uncontrolled and non-independent
character of the chemical reactions which were occuring~
In accordance with a still further aspect of the present
invention, à mercury ca~mium telluride crystalline layer is
grown over a crystalline substrate by directing a plurality
of vapor ~lows ~owards the substrate. A first vapor flow
comprises a source of mercury, a second vapor flow comprises
an organic source of cadmium selected from the group consisting
of diethylcadmium (DECd), di-N-propylcadmium (DPCd~, di-iso-
butylcadmium (DIBCd) r and di-neopentylcadmium ~DnPCd). The
.
_ g _
1319~8~
tellurium organic includes at least one organic group
selected from the group consisting of a secondary alkyl, a
tertiary alkyl, an allyl, a benzyl, and a cycloallyl group
bonded to the tellurium atom. The selected Cd organic, Te
organic, and ~g are reacted at a temperature at which the
j exchange reaction involving the Cd organic and Hg is kineti-
cally limited. With this arrangement, by providing a cadmium
organi~ having organic groups surrounding the cadmium atom
:j .
which-~terically hinder the tellurium atom from reacting with
the cadmium atom, a substantially reactive-free transport of
these vapors through the reactor tube towards the elevated
temperature region over the substrate is provided. Further,
since the temperature over the growth region is such that the
exchange reaction is kinetically limited ~i,e. the rate of
the reaction is low) substantially more uniform growth of
HgCdTe is provided since there is less elemental Cd available
to react with the Te organic prior to the substrate. At the
growth temperatures over the substrate, the eadmium organic
and tellurium-organic pyrolyse substantially independently,
providing ~ree tellurium and cadmium. The free tellurium
reacts with the elemental mercury and free ~admium to form
mercury cadmium telluride. Since cadmium is not lost in the
:j vapor stream prior to pyrolysis of the cadmium organic over
the su~strate, the front to back compo~itional uniformity of
the deposited layers will be substantially more uniform. Due
-- 10 --
~ 31~8'~
to the controlled reactions which are occuring, i.e. substan~
tially independent pyrolysis of the cadmium and tellurium
alkyls, improvement is also anticipated in the side to side
compositional uniformityO
In accordance with a further aspect of the present
invention, a Group II-VI layer is provided over a substrate,
A first flow comprising a selected Group II organic is directed
towards the substrate and a second flow comprising an or~anic
of the Group VI element is also directed towards the substrate.
At least one of the Group II and Group VI organic compounds
have organic groups which provide either electron transfer to
the electropositive Group II element or electron transfer
from the electronegative Group VI element. Preferably, the
Group II organic has an electron releasing organic group bonded
directly to the Group II element. The electron releasing
group should preferably be a stable group where the potential
for incorporation of unintended dopants could be a problem.
An example of an electron releasing group is the phenyl group
(C6Hs). With this particular arrangement, by providing an
organic Group II source having electron releasing groups
bonded to the Group II element, the electropositivity-of the
Group II atom is reduced by the transfer of electronic charge
from the selected electron releasing group. In particular,
since the electropositivity of the Group II element is reduced
~5 by the presence of the phenyl groups, this will concomitantly
~ 31~58~
reduce the attractive force between Group II atoms and Group
VI atoms while the Group II organic and the Group VI organic
are in thP vapor stream. The electronegative nature of ~he
phenyl group should also reduce the interaction between an
organic Group VI compound and the organic Group II compound
having the phenyl group attached.
In accordance with a still further aspect of the present
invention, the Group II compound is selected having an organic
group bonded directly to the Group II atom which provides
electron transfer to the electropositive Group II atom and
provides steric hinderance between the Group II atom and
Group VI atom in the Group VI organic sourceO Preferably,
the Group II element has a pair of phenyl groups bonded
directly to it with at least one hydrogen atom of one of the
phenyl groups replaced by an organic group. Preferably,
electrophilic substitution at a hydrogen position on the
phenyl ~roup is from a species belonging to the class of
groups which are generally classified as activating, ortho,
para directors. Examples of organics classified as ~eakly
activating groups include phenyls and alkyls (methyl, ethyl
e t~ ro~Ds
etc.). ~e~e~o~ that is groups containing atoms other
than hydrogen and carbon and classified as moderately and
strongly activating groups include alkoxides, -OCH3, -OC2Hs
etcO; -NHCOCH3; -OH and -NH2 ~-NHR, NR) where R is a radical,
may also be used, keeping in mind the potential for in~roduction
~3 ~ ~87
of oxygen and nitrogen. For example, a methyl group may be
used to replace a hydrogen at one of the ortho positions or
the para position of each one of the phenyl groups. The
presence of the large organic groups will sterically hinder
the Group II atom from reacting with the Group VI atom by the
presence of out-of-plan~ hydrogen atoms associated with the
methyl group. That is, these hydrogen atoms will partially
shield the Group II atom. Furth~rmore, with this shielding
of the Group II atom by the out-of-plane hydrogen atoms the
vapor pressure of the Group II organic will also increase,
since the inten~olecular attraction between a cadmium atom of
one molecule and a phenyl group of a similar molecule is
reduced. Further, the use of ~he activating ortho and para
directors will increase the electron transfer and, hence,
further reduce the electropositivity of the Group II atom.
In accordance with a further aspect of the present
invention, the hydrogens at both ortho positions of each
phenyl group are replaced by a large organic group such as a
phenyl group, or an alkyl group such as a methyl, ethyl etcu
Substitution of a methyl group, for example, in each ortho
position of each phenyl group will sterically repulse the
methyl groups of the other phenyl groups and, accordingly,
the methyl groups will be rotated 90 from each other resulting
in a nonplanar molecule. With this particular arrangement,
in addition to the steric hinderance provided by the presence
- 13 -
9 ~ ~ 7 ~
of the substituted groups, and the electron releasing of the
phenyl groups, an additional feature of a molecule having
both ortho positions of each phenyl group substituted is tha~
the central Group II atom is enclosed by a cage fonmed by the
rotated methyl groups. This structure results in a molecule
whicht although heavier, is believed to have a higher vapor
pressure thanr a nonsubstituted Group II phenyl, or the
single ortho position substituted &roup II phenyl, since the
cage of methyl groups around the Group II atom should signifi-
cantly reduce intermolecular attractions between the Group II
atom of one molecule and a phenyl group of a second molecule.
Moreove., the cage of methyl groups surrounding the Group II
atom should make this atom particularly unreactive towards
Group VI alkyls.
In accordance with a still further aspect o~ the present
invention, a mercury cadmium telluride crystalline layer is
grown over ~ substrate by directing a plurality of vapor
flows towards the substrate. The first vapor flow comprises
a source of mercury, the second vapor flow comprises an
organic source o- cadmium selected from the group consisting
of di-phenylcadmium ~DPCd), di-orthotolylcadmi-~ (DOTCd),
di-(2,6 xylyl)cadmium (DXCd), and di mesitylcadmlum (DMSCd).
The Group VI organic includes organic groups selected from
the group consisting of a primary alkyl, a secondary alkyl, a
tertiary alkyl, an allyl, a benzyl r and a cycloallyl group
- 14 -
~3~8~
bonded to the Grou~ VI element. With this particular arrange-
ment, by providing a cadmium organic having organic groups
which sterically hinder reactions with the tellurium organic,
and provides for electron transfer to the electropositive
cadmium atom, the attraction between ~he cadmium organic and
the tellurium organic is concominantly reduced. The steric
hinderance will also reduce the exchange reaction between the
cadmium organic and mercury. Accordin~ly, substantially
independent pyrolysis of each organic source over the substrate
is provided, thereby, providing a mercury cadmium telluride
layer having improved compositional unifor~ity.
. 15
- 15 -
,
13~93~ ~
~ .
Brief Descr_ tion of ~
The foregoing features of this invention, as well as the
invention itself, may be more fully understood from the
detailed description of the drawings, in which;
FIG. 1 is a plan view of a photodetector element, here a
photoconductive element including cryst~l layers comprising
Group II-~I semiconductor materials;
FIG~ 2 is a cros~-sectional view taken along line 2-2 of
FIG. l;
FIG. 3 is a view showing the relationship between FIGS.
3A and 3B;
FIGS. 3A, 3~ are schematic diagrams oE a growth apparatus
for use in growing the epitaxial la~er shown in FIG. l; and
.~5
FIG. 4 is a schematic diagram of an alternate reactor
vessel having a reservoir for those Group II organic sources
having a high melting temperature.
- 16 -
a 8 7
Descri~tion of the_Preferred Embodiments
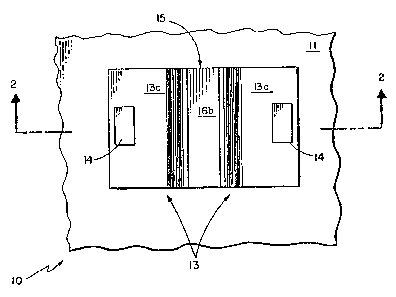
Referring now to FIGSo 1 and-2, a typical photoconductive
element 10, suitable for use in a photoconductive array (not
shown) is shown to include a substrate 11, here comprising
cadmi~m telluride (CdTe) or gallium arsenide ~GaAs), indium
- antimonide (InSb1 or other suitable Group II-VI or Group III-V
; ~ubstrate materials or sapphire (A12O3)~ Disposed over and
here on a substrate 11 is a Group II-VI epitaxial buffer
layer 12a here comprising cadmium t~lluride ICdTe), and a
second epitaxial layer 12b of cadmium telluride (CdTe) or
mercury cadmium telluride tH9cdTe)~ or other suitable Group
II-VI material such as HgZnTe, or a material such as HgMnTe.
Disposed on portions of the epitaxial layer 12b are a pair of
electrical ohmic-type contacts 13 each provided from a patterned
composite layer comprising sequentially deposited layers 13a~
: 13b, and 13c respectively, of indium ~In) 10,000 A thick,
chromium (Cr) 500 A, and gold ~Au) 5,000 A thicko Pads 14
comprising gold each 1.5 m thick are disposed over the
contacts 13 to provide a bonding point for external components.
Disposed in a channel region 15 between the ohmic
contacts 13 is a passivation layer 16a, here of an in situ
anodic oxide formed from a portion of the HdCdTe layer 12b as
is known, 800 A thick and an anti reflection coating layer
16b. Layer 16a, 16b are used to protect the channel region
15 and to provide a composite layer window 16 which is trans
- }7 -
3 1 ~
parent to incident electromagnetic energy 17 generally in the
wavelength range o approximately 0.8~ m to 30~ m which is
directed towards the window 16. In response to such incidence
radiation 17, the conductivity of the epitaxial layer 12b
changes, thus permitting the photoconductive element to
det~ct the presence of the incident electromagnetic radiation
17. Further, the ratio x of Cd to Te may be adjusted, as is
known, to sèlectively cover different ranges of wavelengths
within the band of approximately 0.8~ m to 30~ m.
Referring now to FIGS. 3, 3A, and 3B, a schematic
representation of a vapor phase epitaxial apparatus 20 ~FIG.
3) used for growing epitaxial layers 12a, 12b of cadmium
telluride or mercury cadmium telluride, as described in
conjunction with FIGS. 1 and 2 above, includes a vapor
apparatus 2~a (FIG. 3A) having a manifold 26 with mass flow
controllers 26a-26~, and bubbler apparatus 39 and 55, as
shown. During operation, hydrogen is fed, via H~ purifier 22
and valve 24, to manifold 26, whereas, helium is fed through
apparatus 20 when the apparatu~ 20 is inoperative and exposed
to air. The vapor phase apparatus 20 also includes a vapor
phase epitaxial reactor 20b ( FIGo 3B), here including an open
quartz reaction tube 60, as shown. Suffice it here to say
that a graphite susceptor 63 i5 disposed in the quartz reaction
tube 60 and the susceptor is inductively heated by an r.f. coil
620 R. f . coil 62 is disposed around the periphery of quartæ
- 18 -
13~9~7 :~
;
reactor tube 60 and is activated to raise the temperature of
the susceptor 63, the substrate 11 disposed on the susceptor
63, and the immediate region 61 around the substrate 11 to a
~- predetermined temperature. The temperature of the substrate 11
S is monitored, via a thermocouple ~not shown), embedded in
the susceptor 63. Prior to the susceptor 63 and the substrate
11 being heated, however, the system is purged of atmospheric
gasses by introducing helium, then hydrogen into the interior
of the furnace tube 60 and vapor apparatus 20a. Then vapors
, from lines 27e-27g, 31c and 47c are fed in~co rhe tube where
th~y decompose and react to provide the epitaxial layers 12a,
12b. Quartz reaction tube 60 also includes a cap 72 at an
opposite end from lines 27e, 279, 31c and 47 which is coupled
to a quartz exhaust line 74 used to exhaust gasses from tube
60.
Referring now particularly to FIG. 3A, the vapor apparatus
20a provides tubes 31c, 47c and 27e-27g which feed vapors to
the quartz reaction tube 60 (FIG. 3B), as shown.
Tube 31c the Group II organic source ~H2 tube is fed
from a junction member 32. Junction member 32 is u ed to mix
flows from two gas sources delivered to a pair of ports
thereof, and direct said mixed gas flow to third port thereof,
which is coupled to the quartz tube 31c. The first port of
junction 32 is fed from the bubbler apparatus 39~ Bubbler
apparatus 39 includes a pair of solenoid control valves 28,
~ 19 --
~3~9~7 `
30. A first one of.the solenoid contxol valves, 28, 30, hore
solenoid control valve 28, has a first port coupled to a
first mass flow controller 26a, via tubes 27a and has a
second port coupled to a bubbler 36, via tube ~9a. Bubbler
36 here has disposed therein the selected Group II organic
compound, as will be described hereina~ter. The bubbler 36
is provided ln recirculating temperature control bath 40
which provides a constant flow of a liquid around the bubbler
to maintain the organic Group II compound 36 at a predetermined
temperature to provide a sufficient vapor pressure. This
range of temperature may extend but not necesarily be limited
to the range of -20C to 100C. A second tube 29c is disposed
into bubbler 36, above surface of the organic Group II source
and is coupled to a port of solenoid control valve 30. A
15 third tube 2~b is coupled between the remaining ports Qf
solenoid control valves 28 and 30~
The normally deactivated state of solenoid control
valves 28 and 30 enables hydrogen gas to pass from the
hydrogen source, here the mass flow controller ~6at via tube
27a, to tube 29b and on through to tube 31c to purge ~he
reactor vessel of atmospheric gasses, as described above~
During epitaxial growth of cadmium telluride or mercury
cadmium telluride, for example, solenoid control valves 28
and 30 are placed in their activated state enabling hydrogen
gas to pass through tube 29a into bubbler 36 which contains a
- 20 -
:~19~87
selected organic cadmium source 37. The hydrogen gas bubbles
through the organic cadmium source 37 and picks up molecules
of the organic cadmium source 37. Therefore, a mixture oE
the organic cadmium source and hydrogen (Cd-organic ~H2)
emerges from bubbler 36, via line 29c, and is routed by
solenoid control valve 30 to line 31a. A second mass flow
controller 26b is activated to provide a predetermined flow of
a carrier gas, here hydrogen, through a valve 36, via line
31b, to junction member 32. Therefore, emerging from line
31c is a diluted vapor flow of the Cd organic with respect to
the carrier gas, here hydrogen.
Tube 47c, the "Group VI-organic tube," is fed from a
junction member 48. Junction member 48 is used to mix flows
from two gas sources and delivers said mixed gas flow to a third
port coupled to tube 47c. The first port of junction member
48 is fed from the bubbler apparatus 55. Bubbler apparatus
55 includes a pair of solenoid control valves 44, 46.
first one of said solenoid control valves, here solenoid
control valve 44, has a irst port coupled to a third mass
flow controller 26c, via tube 27c, and has a second port
coupled to a bubbler 52, via tube 45a. Bubbler 52 here has
disposed therein a Group VI organic 53 as will be described
hereinafter, Suffice it to say here that the Group VI organic,
~ay be a primary alkyl of the Group VI element or alternatively
is selected to have an activation energy for the formation of
- 21 -
1319~8
a free radical during dissociation o the the Group VI-organ;c
that is lower than the activation energy during disassociation
of a primary alkyl of the Group VI element. The bubbler 52
is provided in a recirculating temperature control bath 56
which provides a constant flow of a liquid around the bubbler
52 to maintain the tellurium organic 53 in bubbler 52 at a
predetermined temperature sufficient to provide ade~uate
vapor pressureO This range may extend to but is not necessar-
ily limited to the range of -20C to +100C. A second tube
45c is disposed into bubbler 52, above the surface of the
Group VI organic, and is coupled to a port of solenoid control
valve 46. A third tube 45b is coupled between remaining ports
of solenoid control valves 44 and 46.
The normally deactivated state of solenoid control valves
44 and 46 enables hydro~en gas to pass from the hydrogen source,
here the mass flow controller 26c, via tube 27c, to tube 45b,
and on throu~h tube 47c to purge the reactor ve~sel of atmos-
pheric gasses, as described above. Duxing epitaxial growth of
cadmium telluride or mercury cadmium telluride over substrate
11, valves 44 and 46 are placed in their activated state,
enabling hydrogen gas to pass through tube 45a into bubbl~r
52 which contains the Group VI organic 53. The hydrogen gas
bubbles through the Group VI organic 53 and picks up molecules
of the Group VI organic 53. Therefore, a mixture of the
Group VI organic and hydrogen (Group VI-organic ~ H2) emerges
~- ~ 319~8~1 ~
from the Group VI organic 53, via line 45c, and is routed by
solenoid control valve 46 to line 47a. A fourth mass flow
controller 26d is activated to provide a predetermined flow
of a carrier gas, here hydrogen, through a YalVe 50 and via
S line 47b to junction member 48. Therefore, emerging from
, line 47c is a diluted vapor flow of the Group VI organic with
respect to the concentration of the carrier gas, here hydrogen.
Tube 27e is fed from a fifth mass flow controller 26e
to a quartz reservoir 66 (FIG. 3B) containing a liquid source
of a Group II element such as mercury. Hydrogen gas is
directed over the surface of the liquid mercury, and vapor
; molecules of mercury over the liquid mercury surface are
picked up by thç hydrogen gas flow, providing a vapor flow of
~ mercury and hydrogen (Hg+ H2). The vapor flow is fed to a
quartz junction element 70 (FIG. 3B). A second input port of
quartz junction element 70 is fed via a quartz tube 71a which
is coupled to a sixth mass flow controller 26f, via a valve
72 and tube 27f. Emerging from junction element 70 via tube
71b and into tube 60 is, therefore, a diluted flow of mercury
vapor and hydrogen.
Referring particularly now to FIG. 3B~ as previously
mentioned, the susceptor 63 is heated by an r.fO coil disposed
-~ around the quartz reaction tube 60.
A quartz reservoir 66 containing the liquid elemental
mercury and the region adjacent thereof is resistively heated
!
- 23 ;
1 3 ~ 7
by a resistance heat source 68, as shown, to a temperature of
at least 100C, but generally less than 250C preferable
within the range of 150C to 180C. The zone immediately after
the reservoir 66 and past the substrate 11 is then heated by
banks of infrared lamps 64 to a temperature in the range of
lOO~C to 250C with 150C to 180C being the preferred range~
Heating of the walls prevents premature condensation of
~ mercury from the vapor stream.
; The outwardly exposed surface of the substrate 11 is
degreased and cleaned using appropriate solvents and then
polished us;ng an appropriate material which will etch the
material of the substrate. For example, a bromine methanol
solution is used to chemically polish CdTe or GaAs before
growth of the various epitaxial layers. The substrate 11 is
lS then placed on the susceptor 63 which is then disposed in the
quartz reaction tube 600
In operation, furnace tube 60 is purged of atmospheric
gasses by introduction of helium and then hydrogen gas as
described abovc. The susceptor 63 is then inductively heated
by the r.f. coil 62, the reservoir 66 by the resistive heating
element 68, and reaction tube 60 by the infrared lamps 64.
Each is then allowed to reach the growth temperaturesO When
the apparatus 20b has reached the growth temperatures, valves
28, 30, 34, 44, 46, 50, and 72 are activated enabling diluted
mix~ures of hydrogen gas + Group II organic, hydrogen gas +
- 24 -
3~ 9~87
the Group VI organic, and hydrogen gas + mercury to
emerge from tubes 31c and 47c and 71b, respectively, upstream
from the substrate 11.
The hydrogen r mercury, and organic vapors are at the
desired operating temperature provided by the uniform heating
of the substrate 11 and the region 61 around the substrate
11. It is believed that the directed, selected organic
source will pyrolyse substantially independent of one another
and produce mercury cadmium telluride in accordance with
chemical Reactions lA-5A below:
Te organic -~ Te + ~.C. (Reaction lA)
Te ~ ~g ~ HgTe ~Rèaction 2A)
Cd organic -~ Cd ~ H.C. (Reaction 3A)
Cd + Te ~ CdTe ~Reaction 4A)
HgTe ~ CdTe ~ Hgl_xCdxTe ~Reaction 5~-)
where H.C. stands for hydrocarbons
The composition x is controlled by regulating the flow
of H2 into the Hg reservoir, the temperatu~e of the Hg
reservoir and the concentration of cadmium organic and the
tellurium organic.
The mole fraction (i.e., concentration of Cd-organic,
Te organic and Hg) is given by:
MF(Cd organic)= H2 t~.ru bubbler 36 x Cd or~anic Va~or Pressure~Torr)
Total H2 Flow in Tube 60 760 (Torr)
MF(Te organic)- ~ thru bubbler 52 x
Total H2 Flow in Tube 60
Te orqanic Va~or Pressure_(Torr)
- 25 -
~ ~3~8~ f
MF(Hg) = H2 oveE reservoir 66 x Hg Vapor Pressure (Torr)
Total H2 Flow in Tube 60 760 ~Torr)
Only a portion of the organic vapors which are directed
over the substrate 11 is actually reacted. Unreacted organic
vapors are exhausted from the reactor tube 60, via the exhaust
line 74, and are directed towards an exhaust cracking furnace
~not shown) which cracks the remaining organic gasses into
the elements and provide a gas stream which comprises substan-
tially hydrogen and various hydrocarbons.
In accordance with one aspect of the invention, the
cadmium source is an organic source having organic groups
which are selected to sterically hinder the cadmium atom from
reacting with a tellurium atom provided in the organic tellurium
sourc~. Preferably, the selected organic group is not bonded
directly to the cadmium atom since bonding of the organic
group directly to the cadmium atom will increase the reactivity
of the cadmium organic.
The selected Cd organic has a gen~ral chemical structure
as:
Rl - Cd -R2
where Rl, R2 may or may not be the same, and at least
one of Rl, R2 has the general chemical structure as set forth
below:
- 26 -
~ ~3~9~
Y --C--
l2
where Xl~ X~ may or may not be the same and preferably
are ~elected from the group of hydrogen, a halogen, or an
organic~ Y has the general chemical structure as set forth
below:
Yl
Y2-- I--
where Yl, Y2, Y3 may or may not be the same and are
preferably hydrogen, a halogen, or an organicO
As shown below, the Cd organic has an organic group
which incorporates the carbon atom at the Q position of the
chain of the Cd organic.
Yl
Y2 C - C - -Cd - C - C - Y2
Y3 X2 X2 Y3
With this particular arrangement, the large bulky groups at
the ends of the chain will sterically hinder the cadmium atom
from reacting with the tellurium atom in the tellurium organicO
One preferred example of a Group II organo having a large
bulky group at the ~ position carbon in the organic groupr
thereof, is the chemical di-neopentylcadmium (~CH3)3CCH2)2Cd.
- 27
31~87
Di-neopentylcadmium has a general chemical structure as set
forth below:
cl3 H ~ C~3
C~3 - ~ _ C --Cd - C ~ - CH3
CH3 H H CH3
The molecule contains two tertiary butyl groups which are
separated from the cadmium atom by a ~ position carbon atom,
here a CH2 group. Since the tertiary ~utyl groups are not
bonded directly to the cadmium atom, they do not signiicantly
destabilize the di-neopentylcadmium molecule. Accordin~ly,
di-neopentylcadmium ~DNPCd) should have a thermal stability
comparable to diethylcadmium (DECd). D~PCd has several
advantages. DNPCd is ~elieved to reduce reactions between
itself and the selected tellurium organic due to steric repul
sion provided by the tertiary butyl groups in the DNPCd
molecule. The presence of these tertiary butyl group~ makes
it difficult for the two molecules and, in particular, for
the two atoms of the two molecules to come within a close
enough distance to react. Furthermore, by selecting an
appropriate tellurium source, low temperature ~rowth of
mercury cadmium telluride will be provided. Accordingly,
Reaction 7, the exchange reaction between the Group II
organic and mercury should be kinetically limited and,
therefore, not be a major c~use of cadmium depletion~ The
- 28 -
~319a~7
neopentyl groups ((~H3)3CCH~) due to their weight and size
should also reduce the rate of free radical chain reactions
and, therefore, provide a molecule which is substantially
less reactive in the vapor stream than dimethylcadmiumO
A second preferred example is the chemical diisobutyl-
cadmium ((CH3)2CHCH2)2Cd which has an isopropyl group separated
from the Cd atom by a c~ position carbon atom, here a CH2
group. Di-isobutylcadmium has a general chemical structure
as set forth below:
CH3 H H ~ CH3
CH - C - Cd - C - -CH
t I ~
CH3 . H H CH3
Other examples include di-N propylcadmium and diethyl-
cadmium, each has the respective general chemical structure
set forth below:
CH3 -CH2- CH2- Cd - CH2~ ~H2- CH3 - di N-propylcadmium
CH3- CH~- Cd- CH2 - CH3 - diethylcadmium
Alternatively, the cadmium organic may have organic
groups bonded directly to the cadmium atom which transfers
electron charge to the electroposi~ive cadmium atom. By
reducing the electropositivity of the cadmium atom with the
electron releasing organic ~roup, the cadmium organic will be
less reactive towards the organic tellurium molecule than
prior known dimethylcadmium An example of such a compound
~ 2~ -
` ~3~87
is diphenylcadmium having the general chemical formula set
forth below:
H H H H
H--~-- Cd --~--H
H H H H
As shown, diphenylcadmium contains two phenyl groups which are
sources of electrons because of their ~ level electron
clouds. The central cadmium atom is electropositive.
Consequently, the electropositivity of the cadmium atom
should be reduced by the presence of the phenyl groupsO This
will concomitantly reduce the attractive force between the
cadmium organic and tellurium organic. The negative charge
nature of the phenyl groups should further reduce the inter-
action between a selected tellurium organic and diphenylcadmium~
since the phenyl groups should repel the electronegative
tellurium atom. Another feature of using an aromatic cadmium
compound such as diphenylcadmium is that typically aromatic
cadmium compounds are relatively s~able. Accordingly, it can
- be stored for long periods of time without decomposition.
Furthermore, the phenyl groups themselves are also stable
entities, and it is believed that the rings will not be
broken during pyrolysis. Accordingly, it is also believed
that MOCVD growth using diphenylcadmium should result in
little carbon incorporation into the mercury cadmium telluride
- 30 -
- ~ 3 ~
films. Although diphenylcadmium has a relatively low vapor
pressure and is a solid having a malting point of 174C, it
is nevertheless believed that such a source may ba used.
- A heated reservoir arrangement such as shown in FIG. 4
may be used to provide a suitable vapor flow of diphenylcadmium,
in a similar manner as reservoir 66 pro~ides the Hg vapor
flow. That is the line 27a may be directed to a reservoir
87 containing the Cd organic 86~ Hydrogen gas is passed
over the reservoir and picks up molecules of the Cd organic
86 and directs this vapor stream into the reactor vessel via
tube 31c after predetermined dilution with H2 as described
above. The reservoir is disposed within a heated furnace at
a predetermined temperature, as shown. The furnace may be a
multizone furnace to heat the Cd organic reservoir and Hg
reservoir to selected temperatures.
Alternative oadmium sources having electron releasing
phenyl groups rings include di-orthotolylcadmium having the
gener21 chemical structure set forth below:
H~ C,H3 H~ H
H- ~ - Cd - ~ -H
H H CH3 H
Di~orthotolylca~mium is similar to diphenylcadmium except
that one ortho position hydrogen on each benzene ring is5
replaced by a methyl groupJ The methyl groups also increase
~ ~19~7
the trans~er of electron charge from the phenyl groups to
the cadmium atoms, consequently, reducing the positive charge
of the Cd atom. Although this molecule is heavier than
diphenylcadmium, it is believed nevertheless, di-orthotolyl-
cadmium (DOTCd) will have a higher vapor pressure, because by
attaching the methyl group at one of the ortho positions, the
planar symmetry of the molecule is altered by the ou~-of-plane
methyl hydrogens. These hydrogens atoms partially shield the
central cadmium atom, and as a consequence reduce the inter-
molecular attraction between a cadmium ato~ of one molecule
with a benzene ring of another molecule. Di-orthotolylcadmium
has a melting point of 115C which may indicae that DOTCd
will have a higher vapor pressure than DPCd. Furthermore, it
is believed that the partial shielding of the cadmium atom by
the out of-plane hydrogen atoms will result in reduced attraction
between the cadmium atom and a tellurium atom in the tellurium
organic.
Further alternate examples of cadmium compounds having
increased electron transfer and increased steric hinderance
are di-(2,6 xylyl~ cadmium (DXCd) and di-mesitylcadmium
(DMSCd). These molecules have the gen0ral chemical structure
as set forth below:
~5
~ 3~8~
H CH3 CH3 H
H--~-- Cd ~--H
DXCd
. H c~3 ~H3 H
H CH3 CH3 H
DPlSCd~ \ ~
CH3--~-- Cd ~ CH3
H ~H3 ~ CH3
These compounds are the same as di-orthotolylcadmium except
that DXCd has methyl groups at both ortho positions on each
benzene ring and DMSCd has methyl groups at both ortho posi-
tions and the para position of each benzene ring. With two
ortho groups attached to each benzene ring, the electron
charge transferred to the cadmium atom is 4urther increased.
Further, the ortho mPthyl groups attached to the benzene ring
should sterically repulse each other resulting in a nonplanar
molecule~ A further important feature of this structure is
that because of the steric hinderance, the cadmium atom will
be enclosed by a cage of ~our methyl groups. This increased
steric hinderance should concominantly increase the vapor
pressure of DXCd. Furthermore, the cage o~ methyl groups
around the cadmium atom should reduce the attraction between
the tellurium organic and DXCd or DMSCd and should prevent or
~ 33 -
~ 8 ~
substantially limit the exchange reaction between cadmium and
mercury.
Accordingly, the selected Cd organic having electron
releasing groups has the general chemical structure as set
forth below:
R3 Cd R4
where R3, R4 may or may not be the same and at least
one of Rl, R2 has the general chemical structure as set ~orth
below:
3 ~
H Y2
, 15
where hydrogen (H~ is generally, but not necessarily,
provided at the me~a positions and where Yl, Y2 are at the
ortho positions and Y3 i5 at the para position and are each
selected from the group of hydrogen and an organic group.
Preferably, the organics are activating groups such
as phenyls and alkyls (C6Hs, CH3, C~s etc~) or heteroatoms
such as the alkoxides, -OCH3, -OC2Hs etc; -NHCOCH3; -OH; and
h~te r~at~f~
~ NH2 (N~R, NR) where R is a radical. The he~o~ may be
~ ,, .
used where the potential for O or N incorporation into the
2S deposited films is not a problem~
- 34 -
~.31 9~87
62901-710
Preferably, in order to increase steric h~nderanc~
between the sterically hindered cadmium organic and the
tellurium organic, the tellurium organic i5 selected to
include large bulky organic groups which will likewise ste~i-
cally hlnder the tellurlum organic molecule from reacting
with the c~dmium organic. A tellurium source naving a
relatively low activation energy for ~orma-tion of a free
radical during pyrolysis whe~ compared to the activa-tion
energy for diethyl-telluride is th~ tertia~y alkyl
ditertiarybutyltelluride. Diter-tiarybutyltelluride has a
general cnemical structure given below: .
cl3 Cl3
CH3 ~ C Te - C ~ CH3
CH3 ~H3
Ditertiarybutyltelluride lncludes two tertiary butyl groups
bonded directly to the tellurium atom. Accordingly, the
presence of the tertiary butyl groups ln th~ tellurium organo
ditertiarybutyltelluride, destabilize the tellurium organic
and st~rieally hinders tha tellurium atom. Selection of
ditertiarybutyltellurld~ as the tellurium organie ~ource And
selection o one of the ~terically h~ndered cadmium ~ource
mention~d abov~ will provid0 lncreas~d ~teric hlnderanc~ and,
- 35 -
13~9~87
62901-710
there~ore, increase reactive ~r~a transport o~ tha cadmlum
and tellurium or~anic~ thru the reactor vess21.
Other sources o t~llurium (Group VI alement) ~nclud~
dllsopropyltellur1de tDIpTe) having the general chemical
structure given below-
CH3 ~ / CH3
CH -- T~ -- CH
CH3 . CH3
Di~.~opropyltellurld~ has a lo~er stability andr
hence, enhanced cracking efficiency when compared to the
cracking efEiciency of DETe. DIPTe is a preferred example
o~ a secondary tellurium alkyl. The bulky isopropyl als.o
sterically hinder the tellurium atom from reacting with
the Cd organic.
Greater delocatization and consequently ].owor
activation ene.rgies are provided using the overlap of the
p orbital of the unpaired electron with double bonds
instead of single bonds. The
- 36 -
... ~.
1319~7
` . 62901-710
allyl radical, the benzyl radical, and cycloallyl radical
each delocal~ze the fr~e ~lectron chargQ over the entire
carbon chain. Pr~ferr~d examples o~ allyls, benzyl-~, and
cycloallys of the Group VI element are shown ln the Table.
Therefore, tellurium organic sources such as diter-
tlarybutyltelluride or the a~orementioned secondary alkyls,
allyls, cycloallyls, or benzyls, each provide lower activation
energy for formation of a free radical and, c~nsequently,
reduced growth temperatures. ~y selecting the tellurium
organic ln ~unction w~th the ~elactlon of the cadmium organlc
compound, growth of Group II-VI matsrials such a~ mercury
cadmium telluride will occur at lowar ~rowth t~mpQrature~,
the ~elected organic~ will st2rlcally hinder each other, and
the exchange react~on b~tween tha Group II or~anic 80urc3 and
mercury will be k~netically limited.
Growth of Gr~up
II-VI ~em~conductor materlals usln~ dltertiary~utyltellurld~
as the tellur~um organic can occur at temperatures as low a~
about 230C. It is believed that at thi3 temperature~ the
exchange reactlon (Reaction 7) between mercury and the cadmium
organic, a~ previously mentlone.d, will be substantially
kinetically limited. Thu~, the exchange re~ction will not be
a significant source of cadmium depletion a~ in prior
tQchn~que~. Accordlngly, wlth the abova described arran~ement,
; 37
3 t'~ 8
a
.~
h
~ ~ a
hal I I Cl~
O
0 ~i_' I I h
o
-V
0 r~
E~ a~ I o
~ ~ a) ~ ~a ~1
Z --I ~ O -- V
I ~ l ~ I
N rl ~ _I V --I
.c ~ s
Q JJ ~ ~ ~
~ E~ ~ E3 ~a
~D i
~: ~ C~
E~ O
U~ U
~ o ~o) 3:~
c ~ UE~ U ~)
h t~ U~_) V ~ ~
. ~ ~D ~ ~ I I
V
U C~
Q)
~ _ c.) c~
_~
_,
E u~ u v U ~ u
O
~D ~ 3
U ~ U ~r U ~
-- U ~ U
-- 38 --
8 ~ ~ -
substantially reactive free transport of the cadmium organic
and the tellurium organic will be provided, and the cadmium
organic and the tellurium organic will undergo substantially
independent pyrolsis over the elevated temperature region
of the substrate, thereby, providing`mercury cadmium telluride
films having improved front to back compositional uniformity.
It is also believed that the side to side compositional
uniformity of the deposited mercury cadmium telluride films
will also be improved.
To further reduce the attraction between the electrv-
positive Cd atom (Group II atom) and electronegative Te atom
~Group VI atom), electron withdrawal from the Te (Group VI)
atom may be accomplished by selecting the Te (Group VI)
organic source to have electron withdrawal groups, as shown
below for Te:
Y X2 X2 Y
X~ T~ Xl
Y X2 X2 Y
where Xl and X2 are ~enerally hydrogen and Y is a meta
position deactivating group selected from the group -NO2;
-N(CH3)3+; -CN; -COOH ~-COOR); SO3H -CHO (-CRO)where R is an
~ \ .
-~ ~ 3~9~87
organic group, keeping in mind the potential for N, o, S
etc. incorporation into the Group II-VI layers.
Alternatively, the para, ortho position hydrogens
``~ halacien
.~ (Xl, X2 positions) may be replaced by a ~a~n ~-F, -Cl, -Br,
I) and the meta positions groups are hydrogen.
Having described preferred embodiments of the invention,
it will now be apparent to one of skill in the art that other
embodiments incorporating their conce.pts may be used. It is
felt, thereforet that these embodiments should not be limited
to the disclosed embodiments, but rather should be limited
only by the spirit and scope of the appended claims.
- 40 -