Note: Descriptions are shown in the official language in which they were submitted.
~ ~2129~
1 ~ ~2301-1544
Health care costs are increasing dramatically in
the United States and other countries. A significant component
of these escalating costs is tooth and gum disease.
Advances in dentifrice compositions and Modalities
for treatment of peridontal disease have greatly assisted in the
prevention and treatment of these conditions.
However, the primary tool for every day cleaning of
teeth remains the common toothbrush. Since the toothbrush fre-
quently comes into contact with the oral environment and may be
subsequently left in a non-sterile environment until subsequent
use, the toothbrush bristles can harbour bacteria and upon re-use
of the toothbrush, the bristles can become a source for introduc-
ing bacteria into the oral cavity.
Additionally, the toothbrush has not been used as
a source of medication but, rather, as an applicator for dentif-
rice. There would be merit in using the toothbrush as such a
medication source, preferably as a complement to the dentifrice
if a viable and practical low-cost means for doing so could be
found.
Although the technical problem of designing a
toothbrush capable of delivering dentifrice and other agents has
long been recogni~ed, no satisfactory workable system has been
developed to meet this long~felt ~eed.
The prior art illustrates that this is so. For
example, United States Patent 914,501 is an early approach
wherein a reservoir is formed along the sides of the bristles to
, .. , . .. ... ~ . . -. . . .
, ,, . . , : . : -
:. , . ~ :, : ,, . , . . ~ :
' ': ' ' ' . . , ! .. .. . ..
' ~ ' . ,''; ' .
. ' ' , ', . ' ' :
.
~ ~ 21 2 9 0 62301-1544
contain liquid dentifrice. This disclosure fails to provide
long-lasting means for release of dentifrice or other agents
since the liquid dentifrice would be washed from the oral cavity
with each use.
Another attempt to solve the problem is disclosed
in United States Paten-t 1,238,883 wherein the "bristles" of the
brush are vulcanized rubber and incorporated polishing and clean-
ing substances.
In United States Patent 1,214,556 a cotton insert
containing dentifrice is placed in the brush. This is believed to
be impractical as it would require consumers to undertake the
cumbersome task of replacing inserts after every use. A similar
insert device is described in United States Patent 4,588,089
wherein an envelope containing too-thpaste is inserted in a tooth-
brush and released by a spike. This technique is also employed
in United States Patent 3,316,580 which suggests the use of an
envelope disposed between the bristles. Such devices requiring
inserts are complicated to use.
Similar reservations would apply to United States
Patent 4,453,679 in which the handle of the toothbrush has a
separate attachment for releasing various agents. Indeed, the
handle-dispensing approach is the subject ofa number of earlier
efforts to solve the problem such as United States Patents
1,896,982; 2,077,758 and 2,303,667, plus United Kingdom Patent
259,268.
Another approach believed to be unsuccessful is to
- . ~ , ,
~ 3 2 ~ 2 9 62301 1544
coat or to spray the bristles with various agents~ Representatives
of this group are United States Patents 1,982,660; 3,302,230;
3,691,585 and Canadian Patent 549,168.
It is evident from examination of the above patents
that none of the devices solves the twin problems of maintaining
bristles of the toothbrush sterile in à practical manner and pro-
viding a viable slow release mechanism for anti-bacterial agents
or other medications.
A different approach is shown in British Patent
259,268 wherein a disinfectant is disposed to communicate with the
tuft holes of the bristles. However, even in Figures 7-9 of
the patent, the reservoir is not located within the tuft holes
themselves, nor is there any suggestion of the use of medications
other than disinfectants. Importantly, this patent fails to
teach how the material to be transferred to the bristles is, in
fact, so communicated thereto and absorbed by them. In view of
the fact that virtually all toothbrushes on the market today
employ hydrophobic plastic bristles, it is not understood how the
device of this patent would operate to transfer disinfectant or
other medications up through the bristles of such modern tooth-
brushes.
Figure 3 of United Kingdom Patent 1,026,738 discloses
recesses 6 disposed annularly around the upper interior perimeter
of tuft holes 4 to provide a source of dentifrice when the brush
is immersed in water. This patent, again, fails to teach how a
long-lasting source of disinfectant and/or other medications can
-- 3 --
,
13 21 2 9 ~ 62301-1544
be maintained over extended periods or be absorbed by the bristles
over such long periods, i.e., over a period of extended use, as
opposed to prior art teachings which disclose systems which would
be eEfective Eor only a single use.
The invention provides a toothbrush comprising a brush
head, said head having a plurality of tuft holes for the reception
and retention of respective multiplicities of bristles in the tuft
holes, each multiplicity of bristles being attached in a
corresponding tuft hole by a corresponding one of a plurality of
anchors, and at least one of said tuft holes containing therein an
agent releasing means which is structured for containing an agent
and for slowly releasing the agent over repeated brushings, said
agent releasing means being activated by entry into said tuft
holes of liquid during brushing, said agent being selected from
the group consisting of disinfectants and medications and mixtures
thereof, wherein said agent releasing means consist solely of a
coating applied on at least one of said anchors.
The invention also provides a method of manufacturing a
toothbrush comprising the steps of forming a brush head with a
plurality of tuft holes, providing an anchor which is coated with
an agent selected from the group consisting of disinfectants,
medications and mixtures thereof, and anchoring a plurali-ty of
bristles in one of said tuft holes by inserting said anchor
therein.
The toothbrush is preferably capable over long periods
of time of releasing anti-bacterial agents and other medications. -
Disinfectants and/or other materials can be provided in
, , . :: ~
,. , :: : .
. .
~ 32~ 29~ ~301-154~
slow-release for~ incorporated in the toothbrush structure so that
the materials are released through the bristles over extended ti~e
periods to keep the toothbrush sterile and clean and, if
medications are used, to assist in the application thereof to the
teeth during brushing.
4a
, , : , ., : . :
.
.
~3~,~2 ~ ~ 62301-1544
These and further features will be observed from
the following detailed description, the drawings and the claims.
The drawing illustrates, in schematic form, a
cross~section of a portion of the head of a toothbrush suitable
for the present invention.
As indicated, it is known that toothbrushes of con-
ventional manufacture are not sterile and, in fact, harbour
bacteria which transfers into the oral cavity during repeated
brushings. Thus, the primary instrument for tooth cleaning itself
can be source of infection within the mouth.
It would be desirable, in addition to eliminating
the foregoing infection problem, to provide a toothbrush which is
capable of releasing medication effective against tooth and gum
disease over extended periods of time, that is, during multiple
uses of the toothbrush.
Certain of the prior patents discussed above recog-
nize the problem of toothbrush sterility and the desirability of
using the toothbrush to deliver medication.
The solution to these long-recognized needs is the
device of the present invention wherein disinfectant, medication
or a mixture thereof is contained in slow-release capsules and the
latter, either as a plurality of small capsules, which might be
termed "microspheres", or within a single larger element or cap-
sule, is placed within the tuft hole of the toothbrush head
prior to the placement of the bristles therein. Subsequently,
during repeated brushings, the water and saliva present in the
: ~ , . ~ . , , - . .
.. , ~ .. . .. .
, . : . - ,
::
. `
~321~
62301-1544
oral cavity during brushing seeps into the tuft holes and causes
the release of measured amounts of the disinfectant and/or
medication. These agents travel up the tuft hole and out of it
and into the oral cavity during brushing. Moreover, at the end
of brushing, there is sufficient disinfectant remaining on the
bristles and toothbrush head and adjacent portions oE the handle
to sterilize the same so that bacteria will not build up on the
brush and contaminate the mouth upon succeeding toothbrushings
over an extended period of time, say about 2 to 3 months.
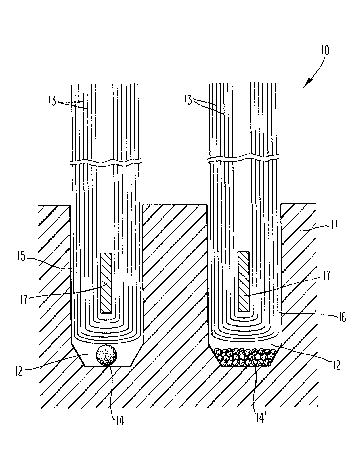
To illustrate one embodiment of the invention, the
drawing shows, in schematic form, a cross-section of a portion of
the head of a toothbrush suitable for the present invention. Thus,
the device is generally designated 10 and has a brush head 11.
Two tuft holes for the bristles, 15 and 16, are depicted in brush
head 11.
As shown the bristles or filament 13 are inserted
into the holes 15 and 16, most commonly by an anchor 17, typically
composed of aluminum or an alloy such as brass or silver-nickel.
In the present invention, the tuft holes are part-
cularly designed to incorporate a chamber or additional area 12
below the bottom of the bristles. Within the chamber 12 there
are placed slow-release capsules of disinfectant and/or medication.
Thus, within chamber 12, there is placed a large unitary capsule
14 having a number of microspheres therein or, alternatively,
being one large element capable of slow release of its contents.
Within chamber 12', a plurality of relatively smaller capsules 14'
` :
. .
~ 3 2 ~ 2 ~ ~ 62301-1~44
of the slow-release type are placed. It will be understood that
the capsules 14 and 14' are placed in the tuft holes during manu-
facture of the toothbrush prior to the insertion of the bristles
13.
Although the bristles are tightly anchored there is
still ample room left within the tuft hole, circa. 20 - 30 %.
Otherwise in the tufting operation the force of insertion of
filaments and anchor wire would cause the cracking of the plastic
around the hole~ The space left may be expected to have fluid
present during and after brushing. There will be also a film of
water on the filaments. Thus, by capillary action, the active
ingredient can come into contact with the filaments as well as the
area in the tuft hole where the microspheres are located. Though
nylon can be considered hydrophobic the preferred material , nylon
6.12 still absorbs about 1 - 2% w/w water.
In operation, the toothbrush is employed in the usual
fashion, i.e., dentifrice is applied to the bristles and the brush
is inserted into the oral cavity for brushing. The water and
saliva generated during this process causes measured amounts of
disinfectant and/or medication to pass from their capsule enclo-
sures and up along the brist].es out of the tuft holes and into the
mouth. At the end of brushing, sufficient disinfectant remains on
the bristles to render the same sterile, thereby preventing the
formation of bacterial colonies and fungal growth which, in the
case of prior art toothbrushes, infect the oral cavity when the
toothbrush is next used.
-- 7
. , . . .. :
:
; . - :: ~,
.
: , . .
~ 3 2 ~ 2 9 0 62301-1544
In the case of the smaller capsules 14', the micro-
spheres, a preferred size is on the order of about 75 - 500
microns in diameter. Both capsules 14 and 14' are of the slow-
release type and, as such, may be of natural or synthetic polymers,
e.g., gelatin, polyvinylpyrrolidone and hydroxyethylmethacrylate.
As disinfectants to kill bacteria and fungal colonies
on the bristles and brush, antimicrobial agents such as chlor-
hexidine, Triclosan, or bromochlorophene may be employed, as well
as other known agents~ A wide variety of medicaments effective to
destroy bacteria and fungus may be employed, both for the purpose
of preventing bacterial/fungus growth on the brush and to deliver
such ingredients into the oral cavity.
As for disinfectants/antibacterials, others that
could be included are:
Hexetidene
Phenols in general
Trichlorophenyl
Formaldehyde
Quaternary Ammonium compounds
(e.g. Benzalkonium Chloride)
Pyridine Derivatives
(e.g. Cetylpyridinium Chloride)
Hexachlorophane
Indeed, although the toothbrush of the present
invention is well-suited for conventional brushing with a
dentifrice, it may be used without the latter and thus be employed
., : ,
~ ~ 2 1 2 9 ~ 62301-1544
as a means of delivering suitable medications into the oral cavity.
The capsules used in the present invention may be
of a number of known types. Generally, these systems are either
those which release agents when the capsule wall is ruptured, or
those which have wall material which dissolves in contact with
water or other liquid. The slow-release type preferred in this
invention is a plurality of small microspheres which may be placed
in a single outer coating of dissolvable material, as in 14, or
placed in the tuft hole in relatively large numbers as in 15. The
preferred system is to use a large number of microspheres which
have increasing wall thicknesses. In such a system, the micro-
spheres which have the thinnest walls release their contents
first--because the thinnest walls dissolve first. Thereafter,
microspheres release their contents in increasing order of wall
thickness. In this fashion, using suitably configured microspheres,
the toothbrush releases disinfectants and medications over long
periods and over many brushings. The total duration is a matter
of selecting the type and number of microspheres to be employed.
Another preferred capsule is one formed of synthetic
material which is cross-linked. In this type, the rate of release
of the agent(s) is determined by their rate of diffusion from the
capsule, which is, in turn, controlled by the degree of cross-
linking. Further, certain types of cross-linked capsules are
essentially solid and swell in water (rather than dissolve), so
that pores are created through which the agent(s) can be released.
In this type, the rate of release is controlled by the amount of
_ g _
,
:
. . , . -
,
.,
1 3 2 1 2 9 ~ 62301-1544
swelling which, in turn, is a function of the degree of cross-
linking.
Microspheres can be manufactured by a variety of
ways, and include polymer spheres that have varying pore sizes.
Microspheres suitable for use in this invention preferably are
about 74-500 microns in diameter. The distribution and size of
the microspheres dictate the rate of release. Also, larger
structures (e.g., lmm or so in diameter) can be manufactured via
standard tablet manufacturing processes and may include an inner
non-absorbable material, giving a "matrix tablet". Whatever means
are selected, the release of a drug or similar agent in any
system depends on the rate of diffusion, which can be controlled.
References relating to the overall subject of drug
release is further covered in the session at the 6th Pharmaceutical
Technology Conference, Harrogate, England, April 1986 (some
papers published in Pharmaceutical Technology: Controlled Drug
Release Vol. I (+ II). Ed. M.H. Rubenstein, published by Ellis
Horwood Ltd. ISBN 0-7458-0178-1). Though the actives are not
necessarily drugs the concept is the same.
One method of making suitable microspheres would be
via the method used by Lee et al. as set forth in Science 213;
233-234, 1981, which involves mixing gelatine and an active
ingredient with water which is then added to an oil phase to pro-
duce spheres of gelatine/active/water. In this system, the speed
of mixing controls the size of the spheres. After purification,
filtration, etc., the spheres can be cross-linked--at least on
-- 10 --
, "
, . . .~ : : -
13212~0
62301-1544
their external surfaces--by a glutaraldehyde solution. Thus, in
this case, the active ingredient leaches out of the spheres and
the rate of release will be dependent on the degree of cross-
linking and active concentration, i.e., the amount and strength
oE the active ingredient.
In one embodiment of the invention, the capsule(s)
or tablet(s) are colored with a water-soluble dye and the brush
head is visually clear, thereby enabling the user to be aware when
the contents of the capsule(s) or tablet~s) have been depleted
and, thus, that the sterilization and medicinal efficiency of the
brush head is correspondingly depleted, so that the user will
know that the toothbrush should be replaced.
Another embodiment of the invention is to coat the
anchor 17--which is usually metal such as nickel-silver, brass,
aluminum, etc.~- with the disinfectant and/or antibacterial/fungus
agents described above.
~ ore particularly~ a water-swellable coating, or
a coating capable of releasing the above agent(s~ in concen-
trations sufficient to achieve the disinfecting and medicinal
purposes described when wet, is applied to the metal. For
example, the rnetal anchors are usually derived from a continuous
spool of the metal(s~ mentioned above and are passed to the
tufting machine and cut to size in situ. The coating material
may be, for example, a polyvinyl or similar polymer capable
of forming a film, e.g., cast from alcohol. The coating can
be applied to the spool of metal at the source of manufacture or
- ~ ' , '
'~ ' ' ' '' ~ `
'
132~29~
62301-1544
just prior -to the tufting process via a coating bath or spray.
To retain sufficient material, a groove may ~e formed on one or
both sides of the metal anchor to hold the coating material.
A variation of the above is to form the metal anchor
in two or more strips (not shown) and to apply the coating as a
"sandwich" between two of the strips.
Further, the anchor can be made of high strength
plastic which then can be coated with the agent(s) described above.
Alternatively, such agent(s) can be incorporated in the plastic
anchor itself.
- 12 -
~ ..... ... . .
: : ~ :. ~' :
: :, ::
:, ~ :