Note: Descriptions are shown in the official language in which they were submitted.
~32~
Backaround of the invention
Up to the development of low pressure methanol synthesis
processes dimethylether has been obtained in a quantity of
2 - 5 weight-% as a side-product in high-pressure methanol
production units and has been isolated by distillation from
mixtures r which contained additional low boiling side-pro-
ducts.
After introduction of lo~ pressure methanol processes, which
yield only negligible quantities of ~imethylether, special
synthetic processes have been developed, based on the cata-
lytic dehydration of methanol.
Numerous processes have been disclosed in the patent litera-
ture. For example, according to DE-PS 680 328, aliphatic
ethers are obtained by heatinq alcohols in the presence of
zinc chloride.
~ 1
~32~
- 2 - UK 372/373
Other suitable catalysts for the production of ethers from
alcohols are according to GB-PS 332 756, Gs-PS 350 010l
GB-PS 403 402, US-PS 1 873 537 and FR-PS 701 335, ferrous
and ferric chloride~ copper sulfate, stannic and stannous
chloride, manganese chloride, aluminum chloride and -sulfate,
chromic sulfate, alum, thorium con~pounds, aluminum oxide,
titanium oxide, bariu~n oxide, silica or aluminum phospate.
In "Industrial and Enginnering Chemistry", Vol. 41, No. 12,
page 2928 (1949) use of bauxite with a SiO2 portion of 4,40 -
13,99 weight-% is described.
In US-PS 3 036 134 an aluminum silicate-catalyst is dis-
closed for the production of dimethylether from methanol,
with a ratio of Al2O3 : SiO2 of 1 part : 1,35 - 0,3 parts.
The synthesis of dimethylether directly from synthesis gas
~CO ~ H2) has also been described (DE-PS 23 62 944, DE-PS
27 57 788 and DE-PS 32 20 547).
The technically most important cataLysts have turned out
to be aeeording to DE-PS 28 18 831, DE-OS 32 01 155, EP-A
0 099 676 and EP-A 0 124 078 in parl:icular, aluminum oxide
and aluminum silieate eatalysts with and without doping.
ïn DE-PS 28 18 831 a eatalyst fox the production of di-
methylether is disclosed, whieh can contain any aluminum
oxide as a base material, as far as it possesses a suffi-
eiently large surfae~ and additives of 1 to 30 weight-~ of
rare earthes.
Finally in EP-A 0 ~99 676 a eatalyst is disclosed, which
eontains 1 - 20 weight-~ of SiO2, preferably 1 - 10 weight-%
of SiO2 and more preferably 6 weight-% of SiO2.
~_
~ 3 ~
_ 3 _ UK 372/373
Crude dimethylether thus obtained contains reaction water,
unreacted methanol as well as small quantities of contami-
nations, like methyl formate, hydrocarbons, amines and sul-
fides, carboxylic acids and esters, amides, acetales ana
others.
In synthesis units of the state of the art, crude dimethyl-
ether is worked up in two distillation col~mns connected in
series. In the first column,~hich operates under pressure,
pure dimethylether is obtained. In the s~cond one unreacted
methanol is distilled off. Thus in EP-A 0 124 078 a process
is described, according to which dimethyleth~r is drawn off
in a first column, which is operated under pressure as a
sidestream, where as in a second column, which is operated
under lower pressure, contaminations with boiling points
between dimethyletherand methanol,are drawn off overhead
Methanol is obtained in the same column as a sidestream.
Catalysts are ~l2O3, SiO2,aluminum silicates and preferably
~ -Al2O3.
Although by this process dimethylet:her of higher purity com-
pared to the state of the art is obtained, it has; from an
economical point of view, a considerable disadvantage, be-
cause not only the first column, but also the second one
have to be equipped with a high number of trays.This leads
to high in~estment costs and in particular to high costs of
operation. Furthermore, there exists a considerable risk,
that the contaminations, boiling between dimethylether and
methanol, are not completely transferred to the second
column, but accumulate in the first column and are drawn off
with d;methylether. As a consequence a dimtheylether quality
is obtained, which is not free of odour.
.
.i
- 4 - UK 372/373
Since dimethylether gains increasing importance as a
propellant for sprays, very high demands are made with
regard to purity.
Thus, no irritating substanc~.s in dimethylether are permitted
in applications like cosmetic, human and household sprays.
Furthermore dimethylether has to be free of odour for these
applications~
Object of the instant invention therefore was the production
of a highly pure dimethylether by a more economlcal process
comparea to the state of the art and to convert methanol
nearly quantitatively into a highly pure product which is
suitable for the above named applications.
~227~ `
- 5 - UK 372/373
Preferred embodim~nt
Applicant has succeeded in solving the problems desc~ibed
above by the instant inventive process. According to~the in-
vention highly pure dimethylether is continuously produced by
catalytic dehydration of methanol a temperature of 140-500C
and a pressure of 1-50 bar and distillative workup of the de-
hydration product, Characterized in that the dehydration product
is fed to the distillation column for the production of pure
dimethylether below the 25th tray (from the head of the column)
at one tray or several trays, I
that pure dimethylether i5 dxawn off above the 15th tray
(rom the head of the column), p:referably above the 10th tray,
at one tray or several trays,
that a fraction, which contains contaminations boiling bet
ween dimethylether and methanol, is drawn off at one tray or
several trays, which is (are) situated at least 5 trays above
the highest feed (dehydration product) tray and at least one
tray below the 15tll tray (from the head of the column)
and
: that the dehydration product is fed to the column at least
1 tray abo~e the bottom of the column,
whereby in case of feed, which contains 1-S weight-% of dimethyl-
ether, the distillation is operated at a reflux ratio of 1:1
to 1:25,
whareby in case of feed, which contains 20-80 weight-~ of
dimethylether, the distillation is operated at a reflux
ratio of 1:0,4 to 1:5, preferably of 1:1 to 1:2,5,
~ one Qspect ~
- 6 - L3~ 2~ 6
28160-3
whereby in case of feed, which contains 6-19 weight-% oE dimethyl-
ether, the distillation is operated at a reflux ratio between
1:0.4 and 1:25 and whereby in case of feed, which contains more
than 80 weight-% and < 99 weight-% of dimethylether, the
distillation is operated at a reflux ratio between 1:0.01 to 1:5.
According to another aspect of the invention there is
provided a process for the production of pure dimethylether by
catalytic dehydration of methanol at a temperature of 140-500C
and a pressure o~ 1 to 50 bar, comprising dehydrating methanol in
contact with a y-A12O3-catalyst, which contains 0.0001 to
< 1 weight % of SiO2.
According to the state of the art, the determination of
substances wi-th an unpleasant odour is carried out predominantly
by empirlcal methods, in particular by sensory determination by a
trained team. Thus, the limit of inconvenience in case of H2S
has for example been identified in the Federal Republic of
Germany by 150 persons as 45 ~g/m3. (Schriftenreihe der
Landesanstalt fur Immissionsschutz des ~andes Nordrhein-
Westfalen, Heft 49 (1979), pa~e 77.) In those cases, when the
limit of perception of odour can be analyzed ~y instruments,
gaschromotography, electric conductivity, photometry or
fluorescence measurement are applied l''Erdol und Kohle-Erdgas-
Petrochemie, Vol. 32, Nr. 2, Febr. 1975, page 86). The determina-
tions of odour in the instant application are based on sensory
methods. Crude methanol from methanol synthesis units as well as
dimethylether produced catalytically from methanol, contain as
outlined above, numerous contaminations, with, in some cases,
strong odour. Whereas from high-pressure-methanol synthesis
i~
- 6a - 1322767
28160-3
units crude methanol is obtained, which in general contains 2-3,
however up to 5 welght % of dimethylether, crude dimethylether
which
~.
7 ~
- 7 - UK 372/373
is produced from crude or pure methanol in dimethylether syn-
thesis units, consits of 20 to 80 weight-% of dimethylether
and in addition the above named contaminations, reaction
water and unconverted methanol.
Since the boi.ling points of the contaminations, for example
of dimethylamine (b.p. 6,9C), dimethyl sulfid~ (b.p. 37,3C),
methyl mercaptane (b.p. 5,8C), formic acid (b.p. 100,75C),
formic acld methylester (b.p. 31,5C), formaldehyde (b.p.-21C)
formaldehyde dimethyl acetale (b.p. 45,5C) or acetic acid
methylester (b.p. 56,95C), as well as solubilities and
vapor pressures are very different and since the intensity of
odour of the individual compounds is also very different
and in ad~ition numerous azeotropic mixtures are formed, the
object of obtaining highly pure dimethylether in high yield
by a more economical process compared to the state of the
art, is very difficult to achieve.
The investigations of applicant, which have been carried out
during several years in numerous test sequences, in laboratory,
pilot plant and technical unit, have non-obviously led to the
result that highly pure dimethylether can be produced nearly
quantitatively in an particularly economical way by the in-
ventive catalysts and the inventive distillative purification.
Figur 1 represents exemplarily a unit for the production and
purification of dimethylether.
In Figur 2 exemplarily a distillation column for the pro-
duction of pure dimethylether is shown.
In Figur 3 exemplarily a column is shown which is equipped
with a side-stripper.
A 7 _
.~
- 8 - ~ 3 2 ~ 76 J
28160-3
Preferred inventive catalysts are catalysts of the
~-Al2O3-type which contain 0.0001 to < l weight-% of SiO2. Unlike
the catalysts of the state of the art, they contain only very
small quantities of SiO2 and lead to considerably better results
than the known catalysts. The preferred SiO2-concentration is
0.001 to 0.5 weight-% and a particularly preferred concentration
is 0.001 to 0.2 weight~6 SiO2.
~dditionally the inventive catalysts may contain other
components in small and very small quantities, for example ~a2
or other alkali- and alkaline earth oxides, alkali, alkaline
earth- or aluminum sul~ate, iron oxide, cobalt oxide, nickel
oxide and other compounds.
The reaction in the presence of the inventive catalysts
is carried out at a temperature of 1~0 to 500C~ preferably at
140 or 150 to 450C and pressure of 1 to 50 bar, preferably of l
to 25 bar. The reaction may be carried out in the gas or li~uid
phase, prefe:rably in the ~as phase. Preferably the pressure in
the synthesis reactor and in the distillation columns are
ad~usted to each other. Operation is carried out at a liquid
hourly space velocity (LHSV) of 0.2-16 1/l h, preferably of
0.5-13.5 l/l h.
The inventive process can be operated discontinuously
however preferably continuous.
Reactors, which can be used for the inventive process
may be the known reactors of the state of the art/ like fixed
bed-, fluid bed- or fluidized bed-reactors, but also modified,
new, and improved reactors, which are suited for catalytic
reactions.
~ 3 2 2 ~ ~ r~
- 9 - 23769-43
The reaction in -the presence of catalysts, resp. inventive
catalysts can be kinetically or thermo-dynamically controlled,
dependent on the Ye~c~ion parameters. As a consequence the
corresponding quantities of dimethylether are obtained at the reactor
exit besides -the respective unconverted quantities of methanol.
The investigations of applicant have led to the result,
that in order to obtain highly pure, odourless dimethylether by a
particularly economical process in nearly quantitative yield, crude
product from the reactor exit has to be fed to the distillation
column for the production of pure dimethylether below the 25th tray
(from the head of the column) at one or several trays. If the feed
product is introduced into the column at a higher tray, the desired
product purity can not be obtained, in particular a product free oE
odour can not be obtained
According to the invention crude dimethylether can be
fed to the column, depending on the t:otal number of trays in the
column, between the 25th and 50th tray (from the head of the
column~. For example in case of a column with a total number of 60
trays, feed is introduced between the 32nd and ~6th tray.
Since, according to -the invention, the feed must be introduced at
least one trav, preferably 5 trays and particularly 10 trays above
the bottom, the feed tray(s) can be chosen between the first tray
from the bottom and the 26th tray from the top of the column,
however preferably above the 5th and particularly preferable above
the 10th tray from the bottom.
~32~7~'~
- 9a - 23769-43
Furthexmore~ according to the invention, pure
dimethylether is withdrawn from the column from the 15-th tray
included up to the top of the col.umn, preferably above the 10th
tray at one or several trays.
L32~7~'~
- lo - UK 372/373
This may be for example the 6th tray, it may be alter~
natively a tray between the first and 5th or between the
6th and 15th tray. However it is also possible to with-
draw head condensate depending on the feed. As mentioned
above, withdrawel of pure dimethylether can also take
place at several trays.
Furthermore the investigations of applicant have led to
the result that components with boiling points between
methanol and dimethylether are withdrawn in the same
column at one or several trays, which are located at
least 5 trays above the highest feed tray for crude
dimethylether resp. feed mixtures of dimethylether,
methanol and contaminations resp. water, however below the
15th tray from the top of the column.
In case of a feed product, which for example contains
1 S weight-~ of dimethylether, besides methanol, other
components with boiling points between those of methanol
and dimethylether and optionally water and other oxygen
containing hydrocarbons, like for example alcohols with
a number of C-atoms ~1, the distillation column is
operated with a reflux ratio of 1:1 to 1:25V depending
on the quantity of dimethylether. Thus in case of a
dimethylether concentration of 1 weight-%, the reflux ratio
may be 1:20.
A reflux ratio of 1:1 means, that the quantity of dimethyl-
ether withdrawn, is 1 part and the quantitiy of dimethyl-
ether vapour which is condensed at the top condenser of
the column is 1+1 part (the first number represents the
quantity withdrawn).
A reflux ratio of 1: 5-8 is preferred for example in case of
a dimethylether concentration of 3-4 weight-%. These
quantities of dimethylether correspond to the concentrations,
which are present in crude high-pressure methanol.
A'l ~
"
:~3~7~J
~ UK 372 / 373
Dimethylether can only be obtained in highly pure,
nearly ~uantitative yield, if the inventive feed trays
for crude dimethylether and the trays for withdrawal of
pure dimethylether and contaminations boiling between
methanol and dimethylether, are chosen according to the
inverltion .
If the concentration of dimethylether is 20-~o weight-%
in the feed and also methanol, contaminations boiling
between methanol and dimethylether and optionally water
and other ~xygen ccntaining hydrocarbons like for example
alcohols with a number of C-atoms ~1 are present, the reflux
ratio is 1:o,4 to 1:5, preferably 1:1 to 1:2,5 depending on
the concentration of dimethylether. For example in case of
a mixture of 60 weight~-~ of methanol, besides water and
contaminations, a reflux ratio of 1:1,5 to 1:2,5 may be
chosen.
Such feeds are typical mixtures, which are obtained at the
reactor exit in synthesis-processes for the catalytic pro-
duction of dimethylether from methanol in the presence of
catalysts like A1203- and A1203/SiO2-Catalysts.
In case of dimethylether concent.rations between 5 to 20
weight-~ respectively ~80 weight-%, the inventive reflux
ratios have to be selected on the basis of the data out-
lined above. For example, in case of a dimethylether con-
centration of 5-20 weight-% r the reflux ratio is chosen
between 1:o,4 and 1:25 and in case of ~80 weight-% between
l:o,ol and 1:5.
The distillation column for the production of pure dimethyl-
ether is gen.erally operated at a pressure of 5-lo bar, where-
by the pressure is preferably adapted to the preceeding syn-
thesis reactor. However pressure beyond 5-lo bar can also
be applied according to the invention.
Throughput is determined as usual by column design, heat
supply and reflux ratio.
~' , , .
.~ I I ,i
~27~7
- 12 - UK 372/373
In order to obtain pure dimethylether nearly quantitatively,
in partieular in case of small concentrations in the feed
product, waste gas, which leaves the column at the top and
which contains small quantities of dimethylether mixed
essentially with C02, N2 and hydrocarbons, is washed in a
washing device. The washing liquid can be recycled to the
distillation column or to the synthesis reactor.
Suitable washing liquids are for example methanol or
bottoms of the dimethylether distillation column.
The waslling process can be operated in parallel flow or
countercurrently.
The distillate, which is withdrawn as a sidestream and which
contains the contaminations can be stripped i~n a side-stripper.
The stripping product, containing small quantities of dimethyl-
ether, ean be recycled to the column.
Methanol can be separated from wa-ter in a stripping column,
which succeeds the dimethylether column.
Aeeording to the invention, distillation eolumns of the state
of the art with the eapacity desired, can be used.
Trays can be those of the state of the art, for example
valve trays, sieve trays, bubble trays and other trays of
the state of the art.
In prineiple also paeked eolumns ean be used, whieh are
equipped for example with ceramic materials, glass materials,
wire mesh and other materials of the state of the art.
In case of packed eolumns the inventive locations for feeding
erude produet and withdrawal of dimethylether and contamin~
ation, ean be caleulated from the required number of trays
aeeording to the invention.
The invention is described in more detail with the aid of
figures 1 to 3.
A ~
.,
:~3`227~33
- 13 - UK 372/373
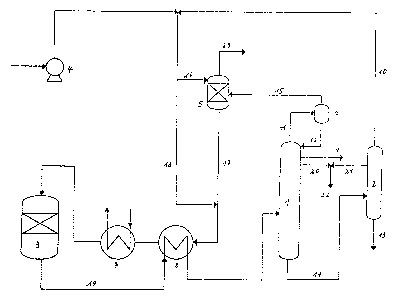
In figure 1 ~3) represents the dehydration (synthesis)
reactor. Fresh methanol is fed to the reactor through
pump (4~, pip~ (18) and heat exchangers (8) and (7).
(1) is the distillation column for the production of pure
dimethylether. The column usually is operated at a pressure,
which is related to the synthesis reactor, in general at a
lower pressure. If for example, the synthesis pressure is
8-12 bar, the column can advantageously be operated for ex-
ample at 6 11,5 ~ar.
These data are however not to be considered as limiting~ In
general it is preferred to operate reactor and distillation
Column at a pressure di~ference of 0 to 10 bar.
Highly pure dimethylether is wi.thdrawn at (9). Head vapours
are fed through (11) tolcondenser (6). Reflux is recycled
through (12) to column (1).
Waste gas is introduced through (15) .into washing device (5),
where .it i9 washed with methanol (16). In principle other
washing liquids can also be used like for example crude
methanol or bottoms of column (1~. In the latter case the
washing liquid~ which contains d.imethylether is recycled to
column (1).
Small quantities of dimethylether are fed to reactor ~3)
through (17), mixed with washing methanol.
~aste gas is withdrawn at (23).
Synthesis product (19) is fed to (1) through heat exchanger(~l.
At (20) contaminations, boiling between methanol and dimethyl-
ether are withdrawn and can ~e incinerated for example.
Bottoms of (1), essentially containing methanol and water are
fed to stripping column (2) through (14).
The stripping column usually is operated at normal pressure,
however it may also be operated at higher or lower pressure.
In general the pressure is lower than in column (1). Methanol
is withdrawn through (lo) and recycled to the synthesis r~actor.
~3
:.,
~ 3227 f~ ~1
- 14 - UK 372/373
From the bottom of 12), waste water is withdrawn. If necessary,
contaminations with boiling points higher than the boiling point
of methanol, can be withdrawn at (21).
In _gure 2, (l) represents the distillation column for the pro-
duction of pure dimethylether. Feed product is introduced at 12).
Pure dimethylether is withdrawn at (3).
Contaminations are withdrawn at ~4~.
Head vapours are fed through (5~ to condenser (lo).
Reflux is recycled through (7) to column (l).
~aste gas is discharged at t6). (8) represents the reboiler
cycle. At (9) bottoms are withdrawn.
In igure 3 the fraction, which contains the contaminations, is
passed to stripper t5) through (4). (5) is equipped with reboiler
cycle (7).
Stripped dimethylether is recycled to column (l) through (6).
~t (2) feed product is introduced. At (3) pure dimethylether is
withdrawn. Head vapours pass through (ll) to condenser tlo~.
Reflu~ is recycled through (8) to co:Lumn (l).
Waste gas is dischar~ed at (9).
At (12) contaminations free of dimethylether are withdrawn.
According to the instant invention h:ighly pure dimethylether
is obtained from the distillation column nearly quantitatively.
D methylether thus obtained is free of odour~ contains less than
lo ppm of methanol, a maximum of o,l weight-~ of hydrocarbons
and is of a purity of up to 99,9 wei~ht~% of dimethvlether.
Dimethylether obtained according to the invention is excellently
suited for any application in the field of aerosol sprays.
Furthermore compared to the state of the art, only one highly
efficient distillation column is needed, whereas it is sufficient
to operate a second column for the separation of methanol from
water with only low separation efficiency.
In addition, in the second column optionally contaminations with
boiling points between methanol and water can be withdrawn as a
side-stream. As a consequence waste water is obtained with only
small quantities of contaminations, which can be~purified more
A eaSily
~ - '
~ 3~?~ 7~ P~
- 15 - UK 372/373
Although in principle, operation of additional columns is
possible, a less economical process would result.
Examples
Work-up of crude synthesis product (3000 kg/h with 65 weight-%
of dimethylether) was carried out in examples 1-9 in-two
succeeding continuously operating distillation columns, the
first of which was e~uipped with 50 valve trays, whereas the
second one was a column packed with ceramic Raschig rings.
In the first column which was operated at 7 bar, highly pure
dimethylether was withdrawn at the 12th tray (from the top
of the column) at a reflux ratio of 1:2.
Crude product was fed to the column at the 22nd tray. In the
second column, unconverted methanol was recovered.
The dimethylether synthesis was carried out at 27c-290C and
a pressure of lo bar.
In example 1 a ~-A1203-catalyst was used, whieh contained
o,ol8 weight-% of SiO2.
Pure dimethylether was obtained nearly quantitatively,
(99,8 weight-% based on methanol corlverted) which was free of
odour.
In example 2 a~CAl 0 -catalyst was used with a SiO -content
2 3 2
of o,ooS weight-%, however a reflux ratio of l:o,4 was applied
and the crude feed to the distillation column contained 80
weight-% of dimethylether.
As in example 1 pure, odourless dimethylether was obtained
nearly ~uantitatively.
In example 3 the synthesis reaction was carried out with a
~-A1203-catalyst, containing o,4 weight-% of SiO2.
The same xesult as in examples 1 and 2 was obtained.
In e~ample 4 the ~-~1203-catalyst used, contained o,o25
weight % of SiO2 and o,c2 weight--~ of Fe2o3.
1S-,
.,
:~32 ~P~
- 16 - UK 372/373
Dimethylether ~hus obtained was ~ree of odour. The yield
was nearly quantitiative based on methanol converted.
In example S the same catalyst was used as in example 4,
however the dimethylether distillation column was packed
with wire mesh. Feed product was introduced at the calcu-
lated position and dimethylether and contaminations were
withdrawn at the calculated positions.
A pure, odourless dimethylether could be obtained in nearly
quantitative yield.
In comparative example 6 a ~-A12-03-catalyst with o,o2
weight-% of SiO2 was used.
The contaminations boiling between dimethylether and methanol
were withdrawn at the top of the second column.
No dimethylether free of odour could be obtained in this case.
In comparative example 7 , example 6 was repeated, however the
catalys-t contained 6 weight-~ of SiO2.
~o dimethylether ~ree of odour could be obtained.
In example 8 pure dimethylether was withdrawn at the 3rd tray
(~rom the top of the column) at a distillation pressure of
7 bar and a reflux ratio of 1:3.
Feed was introduced at the 30th (~as phase) and at the 36th
tray (liquid) (from the top of the column).
Contaminations were withdrawn at the 18th tray (from the top
of the column ).
d~ A1203-catalyst with 6 weight-~ of SiO2 was used.
Pure odourless dimethylether was obtained.
In example 9 , example 8 was repeated, howe~er re~lux in the
distillation column was 1:4.
As a ~atalyst aluminum silicate was used. Pure, odourless
dimethylether was obtained.
In the following examples 10-14, dimethylether synthesis was
carried out at a temperature of 260-280C and a pressure which
was 1-~ bar higher than in the dimethylether distillation column.
The latter was operated continuously.
16 , .i
~ 3 ~ J
- 17 - UX 372/373
Example lo
4000 kgJh consisting of a mixture of 2400 kg of dimethyl-
ether , 580 kg of methanol, 91o kg of water and llo kg of
contaminations was fed to a distillation column equipped
with 65 valve trays at the 49th tray (from the top of the
column).
The column was operated at 8 bar and a reflux ratio of
1 : 1 , 9 . ,
From the top of the column approx. 30 m3 of waste gas were
withdrawn, consisting essentially of C02, N2, hydrocarbons
and a small quantity of dimethylether.
At the 6th tray from the top, 2385 kg/h of pure dimethylether
were withdrawn with lo ppm of methanol.
At the 35th tray from the top, 9o kg/h of contaminations were
withdrawn.
From the bottom a mixture of 580 kg/h of methanol, lo kg/h of
higher boiling components and 91o kg/h of water were withdrawn
and fed to a second column, where methanol was distilled off.
Example 11
Example lo was repeated, however the contaminations were passed
to a side~stripper.
2395 kg/h of pure dimethylether and 80 kg/h of contaminations
at the stripper exit were obtained.
Example 12
A mixture of 61 ooo kg/h consisting of 55000 kg of methanol,
2000 kg of dimethylether, 3500 kg of water and 500 kg of con-
taminations were fed to a distillation column, equipped with
loo valve trays.
The column was operated at a reflux ratio of 1:7 and a
pressure of 6 to 8 bar. Feed was passed to the 35th tray
from the top.
~7
-1
,
;~22~
- 18 - UK 372/373
1996 kg/h of pure dimethylether were withdrawn at the 9th
tray from the top.
From the bottom a mixture was withdrawn, consisting of
55000 kg/h of methanol, 3500 kg/h of water and l9o kg/h
of higher boiling contaminations, consis~i~g mainly of
alcohols with a number of C-atoms ~1.
12 kg/h of waste gas were washed with methanol counter-
currently.
Example 13
4000 kg/h of a mixture consisting of 800 kg of dimethylether,
2825 kg of methanol, 300 kg of water and 75 kg of conta-
minations boiling between methanol and dimethylether, was
fed to a distillation column, equipped with 70 bubble trays
at the 48th tray from the top.
The reflux ratio was 1:2.
At the 4th tray from the top, 796 kg/h of pure dimethylether
and at the 40th tray, 77kg/h of contaminations were wi-thdrawn.
2825 kg/h of methanol and 300 kg/h of water were withdrawn
from the bottom of the column.
Example 14
2000 kg/h of a mixture consisting of 1750 kg of dimethylether,
loo kg of methanol, loo kg of water and 50 kg of contaminations
were fed to a distillation column, equipped with 45 bubble
trays at the 34th tray from the topO
The reflux ratio was l:o,5.
1745 kg/h of pure dimethylether were withdrawn at khe 3rd tray
from the top. 48 kg/h of contaminations were withdrawn at the
29th tray from the top.
loo kg/h of water, loo kg/h of methanol and 5 kg/h of higher
boiling contaminations were withdrawn from the bottom.
In examples lo - 14, a highly pure odourless dimethylether in
practically quantitative yield was obtained.
.~
~227~
- 19 - UK 372/373
Example 15
Example 13 was repeated, however dimethylether was withdrawn
at the 18th tray from the top.
Contaminations were withdrawn at the 45th tray from the top.
No pure, odourless dimethylether could be obtained.