Note: Descriptions are shown in the official language in which they were submitted.
01737-26/GW~-~/fs
13239~
The present invention relates to a process for the selective
extraction of actinides and Au, Pt, Ir and Pd metals from aqueous
solutions using techniques of liquid-liquid extraction in the
presence of an organic phase or techniques of chromatography in
the presence of a solid phase, comprising the operations of;
adjusting the acidity of said aqueous solution to obtain a value
of the distribution coefficient such as to enable said actinides
and/or metals to be transferred to said organic phase or solid
phase; subjecting said acidified aqueous solution to extraction or
chromatography by said organic or solid phase, r~spectively,
containing as extracting agents dialkysubstituted amides of
formula (I)
n - c - N
O R"
in which ~ is a straight or branched aliphatic chain with 8 or
less carbon atoms, and R' and R" are straight or branched
aliphatic chains in which the sum of the carbon atoms in R' + R"
is less than or equal to 8, and contacting said organic or solid
phase containing said actinides and/or metals with a fresh aqueous
solution to reextract said actinides and/or rnetals from said
organic or solid phase and transfer them to said ~resh aqueous
solution for further processing.
Although these amides are known in the literature, they have
been used mainly as plasti~ying agents.
The process according to the invention may be applied in a
range of applications, including conventional nuclear technologies
for the recovery, separation and purification of metals in
solutions, in particular for the separation of natural and/or
enriched uranium and/or plutonium and thorium from
.. . .
-:
,
~32398~
solutions of dissolved spent nuclear fuel.
Currently uranium and plutonium are separated
using liquid liquid extraction, but with tributyl-
phosphate (TBP) as selective extracting agent.
Known as the PUREX process, it offers the dis-
advantage, especially with highly active fuels, of
producing breakdown products of tributylphosphate
which can interfere with the process because they
are not soluble in water or they form slightly soluble
complexes with some metals which are difficult to
remove. These complexes distribute themselves in
a semisolid phase between the two phases, progressively
blocking the extraction process and necessitating
the replacement of the organic phase.
The process according to the invention prevents
the problems cited above since breakdown of the di-
substituted amides gives rise mainly to secondary
amines and carboxylic acids, according to the following
general formula:
2 0 RCO-NR ' R " -- - -- - > RCOOH ~ HNR ' R "
These compounds are not as strong complexing agents
as the breakdown products of tributylphosphate.
Furthermore, as a function of the length of the ali-
phatic chains on the carbon and nitrogen, -the product
carboxylic acid and secondary amine may be soluble
in water and so eliminated automatically from the
'` ,
, : ~ ' ' ':
~323~
- 3 -
organic phase during the extraction.
It should also be considered that while re-extrac
tion of the uranium in aqueous phase with tributyl-
phosphate offers some difficulties, with the amides
said extraction is simple and quantitative.
Therefore an object of this invention is a process
for the separation of uranium and plutonium comprising:
a first step for separation of uranium and plutonium
from the solutlon of dissolved spent nuclear fuel;
10a second step for treatment with a reducing agent
to convert the Pu (IV) to Pu (III); and
a third step of liquid-liquid extraction to extract
the uranium into the organic phase, wherein the
extracting agents used are dialkylsubstituted amides
of formula (I)
/ R'
R - C - N ~I)
O \ R''
where R, R' and R" are as defined above.
20NN'-di-n-butyloctanamide has been found particularly
suitable for separation of uranium from plutonium.
This new process has been called AMDEX.
It has also been found that the disubstituted
amides used in the process for separation of uranium
from plutonium show a high selectivity in the separa-
~ tion of hexavalent actinides with respect to tetra-
': ~ ,, '
:.; ' . ' . ", ' :' - - . ,
'' , ' :' ' ' ~'
1323~5
valent actinides, particularly as a function of the
acidity of the solution.
Therefore a further object of the present invention
is a process for the separation using liquid-liquid
S extraction techniques, of hexavalent actinides from
tetravalent actinides, wherein amides of formula
(I) are used as extracting agents in the organic
phase.
/ R'
R - C - N \ (I)
O R~
where R, R' and R" are as defined above.
The advankage of this process, applied in particular
to the separation of U(VI) from Th(IV) (called the
TAMDEX process) and to the separation of U~VI) from
Pu(IV) (called the.PAMIDEX process) lies in the fact
that no previous reduction of plutonium to valence
3 is required and in the high selectlvity of the
process itself.
For the separation of uranium and thorium, N,N'-
di-n-butyl-2-ethylhexanamide has been found advan-
tageous as the dialkylsubstituted amide; for that
of urani.um and plutonium, N,N'-dibutyl-3,3'-dimethyl-
butyramide is preferred.
It has also been found tha-t the disubsti.tuted
amides show interesti.ng properties also for the separa-
.: ~ . :-
` ~L32398~
tion of non-nuclear elements of high commerc.ial or
strategic interest, such as gold, platinum, irid.ium,
palladium.
Therefore another object of this invention is
a process for the liquid-liqu:id extraction and/or
for the separation or quantitative recovery of one
of the metals cited above (Au, Pt, Ir, Pd) from aqueous
solutions prepared by dissolving the metals or from
industrial waste solutions at low concentration,
wherein amides of formula (I) are used as selective
extracting agents:
R'
R - C - N (I)
O Ra
where R, R' and R" are as described above.
This process has been called AUMIDEX.
In this process as well the acidity plays an im-
portant role in determining selectivity.
As mentioned above, the amides of formula (I~
. / R'
R - C - N \ (I)
O R~
where R, R' and R" are as described above, used in
the process for separation of metals from aqueous
solution according to the invention were previously
used mainly as plastifying agents.
- ~
323~
_ fi _
Therefore another object of the present invention
~` is the use of amides of formula (I) as sequestrants
or extracting agents in separation chromatography
or in liquid-liquid extraction of all the metals
S cited above from acqueous solution.
The dialkylsubstituted amides used according to
the invention have a different structure as a function
of their use. Amides with completely straight chains,
` such as N,N'-dibutyloctanamide or N,N'-dibutylhexan-
amide are particularly suitable as alternatives to
, tributylphosphate in a PUREX type process with parti-
; tion of plutonium by means of reduction (AMDEX pro-
', cess). They are also suitable for extraction of
gold (AUMIDEX process).
15Dialkylsubstituted amides with a branch in the
alpha position with respect to the carbonyl, for
example N,N'-dibutyl-2-ethylhexanamide, are parti-
cularly suitable for the separation of hexavalent
. actinides fxom tetravalent actinides (TAMIDEX).
20Dialkyl amides with other types of branching,
for example N,N'-dibutyl-3,3'-dimethylbutyramide,
are particularly suitable for the separation of hexa-
valent uranium from tetravalent plutonium with no
reduction of the plutonium to valence three (PAMIDEX
process).
Extraction of these metals is more rapid and phase
. .. , . ~ , . . .
. . . . . .
. ~ . . ~ - . ~ .
3239~
separation is quicker than in analogous tests with
tributylphosphate.
I'he possibility of saturation of the metal in
the organic phase is highly dependent not only on
the experimental condltions but also on the degree
of branching of the aliphatic or aromatic chain.
The concentration of the amides, generally dissolved
in an aromatic solvent, depends on the quantity of
metal to be extracted.
Table 1 reports the principle properties of the
amides used.
Table_l
Properties of the_amides
~olubility
15 Amide B.P. nD2o d ~2 dode- mesi-
cane thylene
N,N'-di-n-
butyl- 100-112 1.452 0.861 <0.01 >1000 >1000
octanamide (760)
N,N'-di-n-
butyl-2- 111-112 1.451 0.861 <0.001 >1000 >1000
ethyl- (1)
butyramide
N,N'-di-n-
butyl-3,3'- 145-150 1.449 0.833 <0.001 >1000 >1000
methyl- (28)
butyramide
N,N'-di-
sec-butyl- 130-150 1.490 0.856 <0.01 >1000 >1000
hexanamide (50)
_ _ _ . _ _
Tables 2 and 3 below report several distribution
coefficients D for the liquid-liquid separation process
:, ' ' ~ . :
, ~
- . ,
,, - : , .
~ 13239~
in which the amides according to the invention are used.
Table 2
Distribution coefficients
Organic plase: di-n-butyloctanamide or di-n-butyl-hexanamide
(lM in mesithylene)
Aqueous phase: 4M HN03 + 4M HCl (1:3)
metal D(o/a)
Au10000
Fe 10
Sn
Hg, Zn, Ag 0.1
Cu, Pb 0.01
Table 3
Distribution coeficients for U(VI) ! Pu(IV) and PU(III)
and some flssion products
(lM amide in mesithylene; phase ratio - l)
Nitric acid in aqueous phase
3M 6M
2 0 Di-n-butyl-actanamide
D U(VI) 13 18
D Pu(IV) 30 50
D Pu(III) 0.]
D Zr 0.1-0.01
D Ru 0.0001
Di-sec-butyl-hexanamide
D U(VI) 10 14
.
'' ' ' ~ '
`~` t323~5
. ,
D Pu(IV) 9 24
Di-3,3'~di-methyl-butyl-butYramide
D U(VI) 5 11
D Pu(IV) 3 12
Di-n-butyl-2-ethyl-octanamide
D U(VI~ 5 11
D Pu(IV) 3 9
The liquid-liquid extraction processes for which
the dialkylsubstituted ami~es according to the invention
are proposed consist essentially in placing the aqueous
solution containing many metals, including those to
be separated, in intimate contact with an organic solution
immiscible with water, containing a substance (in this
case an amide) capable of 'binding' and so extracting
selectively and quantitatively from the aqueous phase
to the organic phase the metal or metals of interest,
separating them from the others.
The organic phase containing the metal(s) is then
placed in contact in the same way with a suitable aqueous
solution to re-extract all or some of the metals in
the organic phase and return them to an aqueous phase.
Slightly acidic or reducin~ solutions are normally used
which, by changing the valence of the metal, make it
less extractable. This is the case for the separation
of uranium from plutonium in the PUREX process, where
.~ .
3239~
-- 10 --
the tetravalent plutonium extracted with tributylphosphate
into the organic phase is reduced to trivalent plutonium,
which is not soluble and so returns to the aqueous phase.
In other cases competitive water-soluble extracting
agents are used.
The apparatus used for these extraction operations
consists of multistep contactors of various types, such
as: banks of mixer settlers, pulsed columns and
centrifugal contactors.
The amides proposed may also be used in columns (pure
or dissolved in solvent) dispersed on a finely divided
inert support. In this case the aqueous solution
containing the metals of interest is passed through
the column and the metals remain 'bound' to the material
in the column. They are then recovered by passing a
suitable aqueous solution through the column. This
process is no longer liquid-liquid extraction, but rather
extractive chromatography.
~XAMPL~S
The invention will be illustrated with reference
to the attached drawings, in which:
figure 1 shows an example of the AMDEX process;
figure 2 shows and example of the TAMIDEX process;
figure 3 shows an example of the PAMIDEX process;
and
figure 4 shows an example of the AUMIDEX process.
~3239~5
The following examples are exemplificative only and
do not in any way limit the scope of the invention.
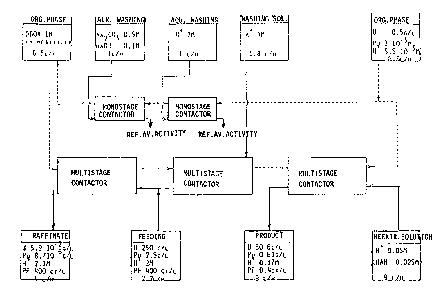
Example 1 _(AMDEX process)
With reference to figure 1, a lM solution of N,N'-
di-n-butylactanamid~ (DBOA) in mesithylene i9 passed
at a ~low rate of 6.5 liters/hour through a multistep
contactor in the opposite direction to an aqueous solution
containing 250 g/liter of uranium and 2.5 g/liter of
plutonium, which flows at a rate of 2j.2 liters/hour.
This 2M acid solution contains fission products as well.
After the extraction step, the fission products are
found in the extract in the same quantity, while the
concentration of uranium has fallen to 5.9 x 10~2 g/liter
and that of plutonium to 8.7 x 10-6 g/liter.
The organic solution is then treated with a reducing
agent to separate the Pu(III) Erom the uranium according
to the classic PUREX process, from which the AMDEX process
differs in its use of N,N'-di-n-butyloctanamide instead
of tributylphosphate.
Example 2 (TAMDEX process)
Figure 2 shows an example of a process for the
separation of U(VI) from Th(IV) using a lM solution
of N,N'-di-n-butyl-2-ethylhexanamide in mesithylene.
A solution containing 13.75 g/liter of uranium and 236.7
g/liter of thorium is separated chromatographically
to give an aqueous soluti'on containing approximately
, ,. , -
. : -
""` 1323~
- 12 -
120 g/liteL of thorium and ~ 0.015 g/liter of uranium
and an organic phase enriched in uranium. The final
extract contains more than 3.4 g/liter of uranium and
no detectable thorium.
Example 3_(PAMIDEX process)
Figure 3 shows a process for the separation of U(VI)
from Ph(IV). In this case N,N'-dibutyl~3,3'-dimethyl-
butyramide is used.
Example 4 (AUMIDEX process)
Figure 4 shows the broad outline of a process for
the separation and recovery of gold from solutions for
working and cleaning the metal or its minerals Starting
from a solution containing lOOOppm of gold and 2 g/liter
of iron, after varying the phase ratio and acidity,
a final solution is obtained with 37.4 mg/liter of gold
and only 0.02 mg/liter of iron. The amide used in this
process is N~N'-di-n-butyloctanamide~ In this case
it should be noted that favourable distribution
coefficient of gold (see table 2) (in general dissolved
in HCl/HNO3 mixtures) and the low concentration of the
metal allow the use of very low concentrations of
extracting agent~
,.: . ' . . , ~ :
: ,
;. ` ~ . . . :
.