Note: Descriptions are shown in the official language in which they were submitted.
` ` 132~1~3
.
TITLE
ROPE FOR TRACTION
~ Technical Field of the Invention
The present invention relates to a rope for
traction. More particularly, it relates to a rope for
. , traction of paragliders, water skies and the like which is
- excellent in light weight, safety to human bodies and
weatherability.
Background of the Invention
` Metallic ropes and wires have been used for
: traction of paragliders, water skies and the like.
However, because of their heavy weight the metallic ropes
sink in water and it is not easy to handle them. Further,
: they pose problems in that due to their reduced elongation
under tensile stre~s shocks upon traction directly affect
; the ob~ect being pulled which might be in~ured by a sudden
shock, and that because of their poor shock-absorbing
properties the ropes themselves might be in~ured and
broken.
- O~bect of the Invention
The invention is to solve the problems associated
. with the prior art and an ob~ect of the invention i3 to
:`
132~143
provide a rope for traction which is light, floatable on water,
easy to handle and excellent in safety to human bodies and
weatherability.
Summarv of the Invention
A rope for traction according to the invention has a
double structure of a core member coated with a sheath member, in
which the core member comprises a molecularly oriented shaped
article of an ultrahigh molecular welght copolymer, and the sheath
member comprises a braid. The ultrahigh molecular weight
copolymer is a copolymer of ethylene and alpha-olefin having 3 to
20 carbon atoms containing the alpha-olefin in an amount of 0.1 to
20 moles on average per 1000 carbon atoms of the copolymer and has
~ an intrinsic viscosity of at least 5 dl/g.
-~ The rope for traction according to the invention having
a double structure of a core member coated with a sheath member,
in which the core member comprises a strong and resilient
molecularly oriented shaped article of an ultrahigh molecular
weight olefin copolymer, and the sheath member comprises a braid,
is floatable on water, easy to handle and excellent in safety to
human bodies and weatherability.
The molecularly oriented shaped article has an increased
` elongation under load and an increased amount of energy until
break as well as improved shock resistance and creep resistance,
132~143
and in consequence, any sudden shock may be effectively absorbed
by the core member without attacking the object being pulled and
the rope is prevented from tearing.
Detailed DescriPtion of the Invention
:~ The invention will now be described in detail with
reference to the attached drawing in which:
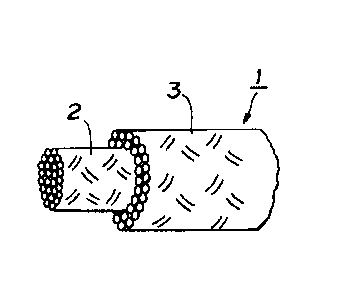
Fig. 1 is a partly cut out perspective view of a rope
according to the invention.
As shown in Fig. 1, the rope 1 for traction according to
the invention has a double structure comprising a core member 2
and a sheath member 3 enveloping the core member 2.
The core member 2 comprises a molecularly oriented
` shaped article of an ultrahigh molecular weight olefin copolymer.
The ultrahigh molecular weight olefin copolymer which can be used
herein includes ultrahigh molecular weight copolymers of ethylene
and at least one alpha-olefin having from 3 to 20 carbon atoms,
- preferably from 4 to 10 carbon atoms such as ultrahigh molecular
weight copolymer of ethylene and propylene, ultrahigh molecular
weight copolymer of ethylene and 1-butene, ultrahigh molecular
weight copolymer of ethylene and 4-methyl-1-pentene, ultrahigh
`~ molecular weight copolymer of ethylene and l-hexene, ultrahigh
molecular weight copolymer of ethylene and 1-octene and ultrahigh
molecular weight copolymer of ethylene and 1-decene. The alpha-
olefin mentioned above is contained in the copolymer in an amount
of from 0.1 to 20, preferably from 0.5 to 10, more preferably from
1 to 7 molecules on average per 1000 carbon atoms of the
copolymer.
'"`'"''
B
. .
:
, .~c j.,.
4 132~1~3
The ultrahigh molecular weight olefin copolymer used
herein has an intrinsic viscosi~y 1~] of at least 5 dl/g. By the
term "ultrahigh molecular weight" is meant that the polyolefin has
an intrinsic viscosity [~] of at least 5 dltg. Preferably, the
polyolefin used herein has an intrinsic viscosity of from 7 to 30
dl/g.
Such an ultrahigh molecular weight polyolefin can be
extruded and strongly drawn to provide a molecularly oriented
shaped article such as filaments and tapes which article is light
in weight, has increased elastic modulus and tensile strength, and
is excellent in water and saline resistance. As described above,
the ultrahigh molecular weight copolymer of ethylene and at least
one alpha-olefin is particularly preferred for the purpose of the
invention, since it can be extruded and strongly drawn at a draw
ratio of from 5 to 80, preferably from 10 to 50 to a molecularly
oriented shaped article having improved shock and creep
resistances in addition to the above-mentioned combination of
advantageous properties. Further, the molecularly oriented shaped
article made from the ultrahigh molecular weight copolymer of
ethylene and at least one alpha-olefin has an improved heat
resistance.
The core member 2 of the rope in accordance with the
invention comprises a molecularly oriented shaped article of an
ultrahigh molecular weight copolymer of ethylene and at least one
alpha-olefin having from 3 to 20 carbon atoms.
~,'
132~143
`~` Although the molecularly oriented shaped article
constituting the core member 2 is preferably a bundle of aligned
drawn filaments or a braid of drawn filaments, it may be in the
form of tapes or films drawn in at least one direction.
:
,
:.
: .
,''
'..'
, . .,~.
,
- 6 - 1 32~1 ~3
The molecularly oriented shaped article
constituting the core member 2 has a density of from 0.940
to o.g9o, and in particular from 0.960 to 0.985, a~
measured by a conventional density gradient tube method in
accordance with ASTM D 1505 at a temperature of 23 C.,
using carbon tetrachloride and toluene in the density
gradient tube.
The molecularly oriented ~haped article u~ed
herein has a dielectric constant (1 KHz, 23 C.) of
normally from 1.4 to 3.0, and preferably from 1.8 to 2.4,
and a dielectric 105s tangent (1 KHz, 80 C.) of from
0.050 to 0.008 %, and preferably from 0.040 to 0.010 %.
The measurements of the dielectric constant and the
dielectric loss tangent were carried out on molecularly
oriented filaments and tapes compactly aligned in the form
of films in accordance with ASTM D 150.
The degree of molecular orientation of the
moleculary oriented shaped article may be determined by X-
ray diffractometry, birefringence method or fluorescence
polarization method. In the case of molecularly oriented
filaments of the copolymer of ethylene and at least one
alpha-olefin, it is desirable from a view point of their
mechanical properties that they have a degree of molecular
orientation (F) of at lea~t 0.90, and preferably at least
0.~5, as determined from the half-value width. Such a
-:
.,:
.
`:
132~143
degree of molecular orientation is described in detail,
for example by Yukichi GO and Ki-ichiro KUBO, in Kogyo
Kagaku Zasshi (Journal of the Society of Industrial
Chemistry of Japan), 39, 992 ~193g), that i~, it i~
defined by the following formula:
Degree of Orientation (F) = (90 - 0.5 H )/90
wherein H is a half-value width (in ) of a
strength distribution curve along the Debye ring on the
~trongest paratroop on the equator.
;- The molecularly oriented shaped article used
herein has excellent mechanical properties. For example,
it has, in the form of drawn filaments, an elastic modulus
of at least 20 GPa, and in particular at least 30 GPa, and
a tensile strength of at least 1.2 GPa, and in particular
at least 1.5 GPa.
The molecularly oriented shaped article used
. herein has an impulse break down voltage of from 110 to
250 KV~mm, in particular from 150 to 220 KV/mm. The
..:
; impulse break down voltage was measured on the same
.~
samples as employed in the measurement of the dielectric
constant that was placed on a copper plate, the voltage of
which was increased by applying thereto impulses of
- negative polarity at a rate of 2 KV/3 steps by means of a
- ~ JIS-type bronze electrode having a diameter of 25 mm.
' i;~:
: .
'~
': '
,
1325143
The molecularly oriented shaped article made of
the above-mentioned ultrahigh molecular weight copolymer
of ethylene and at least one alpha-olefin i5 remarkably
excellent in shock resistance, breaking energy and creep
resistance.
The molecularly oriented shaped article ha3 a
breaking energy of at least 8 kg.m/g, in particular at
least 10 kg.m/g.
The molecularly oriented shaped article is
excellent in creep resistance. In an accelerated test for
creep at room temperature, that is, in a test for creep at
elevated temperature, it exhibits a remarkably reduced
creep. At a temperature of 70 C, and under a load of 30
% of the breaking load, the molecularly oriented shaped
article made of the above-mentioned ultrahigh molecular
weight copolymer of ethylene and at lea~t one alpha-olefin
exhibita a creep elongation (% elongation after gO sec.)
of not more than ~ %, and in particular not more than 5 ~,
and a creep speed between after periods of 90 sec. and 2~0
sec. ~ 9O 18~ taec 1) of not faster than 4 x 10 4sec 1,
and in particular not fa~ter than 5 x 10 5sec 1.
In addition to the above-described properties at
room temperature, the molecularly oriented shaped article
made of the copolymer of ethylene and at least one alpha-
olefin ha3 the following thermal properties.
,~'',
':
132514~
Namely, it has at least one crystal fusion peak
(Tp) at a temperature range at least by 20 C. higher than
the inherent crystal fusion temperature (Tm) of the
copolymer and the fusion heat based on the crystal fusion
peak tTp) is at least 15 %, preferably at least 20 %, and
more preferably at least 30 %, based on the total fusion
heat.
The inherent crystal fusion temperature (Tm) of
the above-mentioned copolymer can be determined by a so-
called second run in a differential scanning calorimeter,
in which the molecularly oriented shaped article of the
copolymer i~ once fused completely and cooled thereby
moderating the molecular orientation, and thereafter
heated again.
More particularly, with respect to the molecularly
., .:
oriented shaped article of the above-mentioned copolymer,
no crystal fusion peak appears in the above-mentioned
inherent crystal fusion temperature range of the
.- copolymer, or even if any peak is observed in this
temperature range, it appears only as tailing. The
crystal fusion peak (Tp) normally appearR in a te~perature
-- o
. ~: range of from Tm + 20 C. to Tm + 50 C., and in
... ~ particular in a temperature range of from Tm + 20 C. to
~ Tm + 100 C., and it appears frequently in the form of a
- plurality of peaks in the above-mentioned temperature
'',
,;
-- 10 --
1325143
range The crystal fusion peak (Tp) often appears in the
form of two separate peaks, that i5, a higher temperature
fusion peak (Tp1) in a temperature range of from Tm +
35 C. to Tm +100 C., and a lower temperature fusion peak
(Tp2) in a temperature range of from Tm + 20 C. to Tm +
35 C. Depending upon the conditions of the preparation
of the molecularly oriented shaped articles, the Tp1 or
TP2 comprises a plurality of peaks.
It is considered that the high crystal fusion
peaks (Tp1 and Tp2) prominently improve the heat
resi~tance of the molecularly oriented shaped article of
the ultrahigh molecular weight ethylene/-~ -olefin
copolymer and contribute to maintenance of the strength
retention ratio or elastic modulus retention ratio at a
high level after a heat hysteresis at an elevated
temperature.
It is preferred that the fusion heat ba~ed on the
higher temperature fusion peak (Tpl) in a temperature
- range of from Tm + 35 C. to Tm + 100 C. be at least
. j,.~
1.5%, especially at least 3.0 %, based on the total fusion
heat.
In the case where the fusion heat ba~ed on the
higher temperature fusion peak (Tp1) satisfies the above
requirement, even if the higher temperature fusion peak
'
1325143
(Tpl) does not apear as a projecting main peak, that is,
even if the peak (Tpl) is an assemblage of small peaks or
a broad peak, excellent creep resistance characteristics
can be obtained though it sometimes happens that the heat
resistance is somewhat degraded.
The melting point was measured using a
differential scanning calorimeter (Model DSC II supplied
by Perkin-Elmer Co.). About 3 mg of a sample was kept in
the restraint state by winding the sample on an aluminium
:
sheet having a size of 4 mm x 4 mm x 0.2 mm (thickness).
Then, the sample wound on the aluminum sheet was ~ealed in
an aluminum pan to be placed in a sample holder of a cell.
An aluminum sheet equal to that used for the sample was
sealed in an aluminum pan ordinarily kept vacant, to be
placed in a reference holder of the cell, whereby a heat
balance was kept. The cell was held at 30 C. for about 1
:j minute, and then, the temperature was elevated to 250 C.
at a rate of 10 C./min. and the measurement of the::
melting point of the sample at the first temperature
elevation was completed. Subsequently, the sample was
held at 250 C. for 10 minutes. Then, the temperature was
lowered at a rate of 20 C./min. and the sample was held
at 30 C. for 10 minutes. Then, the temperature was
elevated to 250 C. again at a rate of 10 C./min., and
the measurement of the melting point at the xecond
13251~3
temperature elevation (second run) was completed. The
maximum value of the fusion peak was designated a~ the
melting point. When the peak appeared as a shoulder,
tangential lines were drawn at the bending points on the
low temperature side and high temperature side ~ust close
to the shoulder, and the intersection point was designated
as the melting point.
A base line connecting points of 60 C. and
240 C. in the endothermic curve was drawn, and a vertical
line was drawn at a point higher by 20 C. than the
inherent crystal fusion temperature (Tm) of the copolymer
determined as the main fusion peak at the second
temperature elevation. A low temperature portion
~urrounded by these lines an area surrounded by the base
line, vertical line and endothermic curve including the
point of 60 C. was regarded as being based on the
inherent crystal fusion (Tm) and the high temperature
:
i portion an area surrounded by the base line, vertical line
and the endothermic curve including the point of
240 C.was regarded as being based on the crustal fusion
(Tp) manifesting the functions of the molecularly oriented
shaped article copolymer. The crystal fusion heats were
calculated from areas of these portions. According to the
above-mentioned method, the portion between vertical lines
at Tm + 20 C. and at Tm +35 C. was regarded as being
''
- 13 -
13251~
based on the fusion of TP2 and the high temperature
portion was regarded as being based on the fusion of Tpl,
and the heata based on the fusion of Tp1, and the heats
based on the fusion of Tp1 and the fusion of TP2 were
similarly calculated from the areas of these portions.
The molecularly orlented filaments of the above-
mentioned ultrahigh molecular weight copolymer of ethylene
and at least one alpha-olefin have a strength retention of
at least 95 %, and an elastic modulus retention of at
least 90 X, in particular at least 95 %, after a heat
hysteresis of 170 C., for a period of 5 minutes,
indicatlng the fact that they have a prominently superior
heat resistance which are not found in drawn filaments of
ultrahigh molecular weight polyethylene.
ProceQs for the preparation of a molecularly
oriented shaped article of an ultrahlgh
molecular weight polyolefin
Generally, a molecularly oriented shaped article
having high elastic modulus and high strength can be
prepared by extrudlng an ultrahlgh molecular weight
polyolefin into filaments, tape or the like and ~trongly
drawing the exrudate. Such a proces~ per Qe is known in
the art.
For examplo, Japane4e Patent ~lrrr~as~-n Lald-Open P~kc~
14 132~1~3 72932-17
Speciflcatlon No. 15408/81 dlscloses a process comprlslng splnnlng
a dllute solutlon of ultrahlgh molecular welght polyethylene and
drawlng the obtalned fllaments. Japanese Patent Lald-Open
Publlcatlon Speclflcatlon No. 130313/84 dlscloses a process com-
prlslng melt-kneadlng ultrahlgh molecular welght polyethylene wlth
a wax, extruding the kneaded mlxture, coollng and solldlfylng the
extrudate and drawlng the solldlfled extrudate. Furthermore,
Japanese Patent Lald-Open Publlcatlon Speclflcatlon No. 187614/84
dlscloses a process comprislng extrudlng the abovementloned melt-
kneaded mlxture, draftlng the extrudate, then coollng and solldl-
fylng the extrudate and drawlng the solldlfled extrudate.
;~ A process for the preparatlon of a molecularly orlented
shaped artlcle of a preferred ultrahlgh molecular welght poly-
olefln, that ls, an ultrahlgh molecular welght copolymer of ethy-
lene and at least one alpha-olefln, wlll now be descrlbed ln
detall.
PreParatlon of the CoPolYmer
The ultrahlgh molecular welght ethylene/a-olefln copoly-
mer ls obtalned, for example, by slurry-polymerlzlng ethylene and
at least one a-olefln havlng from 3 to 20, preferably from 4 to 10
carbon atoms ln an organlc solvent ln the presence of a Zlegler
~ catalyst.
'~
:,
. ~ .
i Xi
., ~
13251~3
Examples of the preferred ~(-olefin include, for
example, butene-1, pentene-1, 4-methylpentene-1, hexene-1,
heptene-l and octene-1. Of these, 4-methyl-pentene-1,
hexene-1 and octene-1 are particularly preferred. The~ -
olefin comonomer should be used in such an amount that the
1 -olefin content per 1000 carbon atoms in the polymer
chain is within the above-mentioned range. Moreover, the
ultrahigh molecular weight ethylene/~ -olefin copolymer
,..,'.s
should have a molecular weight corresponding to the above-
mentioned intrinsic viscosity
] -
;.~ (.
In the ultrahigh molecular weight ethylene/~ -
olefin copolymer used herein, the determination of the ~ -
olefin component is carried out using an infrared
- ~pectrophotometer (supplied by Nippon Bunko Kogyo~.
Namely, the ab~orbance at 13~8 cm 1, which indicates the
deformation vibration of the methyl group of the ~ -olefin
included in the ethylene chain, i~ meaqured, and the
measured value is converted to the number of the methyl
branche~ per 1000 carbon atoms using a calibration curve
prepared in advance using a model compound in a 1 C
- nuclear magnetic resonance spectroscopy.
'
Preparation of the molecularly oriented
~ shaped article
':
- 16 -
- 1325143
Upon preparation of the molecularly oriented
shaped article of the ultrahigh molecular weight ethylene/
-olefin copolymer, a diluent is incorporated in the
copolymer. A solvent for the ultrahigh molecular weight
ethylene copolymer or a wax having a compatibility with
the ultrahigh molecular weight ethylene copolymer is used
as the diluent.
A solvent having a boiling point higher,
especially by at least 20 C, than the melting point of
the above-mentioned copolymer is preferably used as the
solvent.
. As specific examples of the solvent, there can be
mentioned aliphatic hydrocarbon solvents such as n-nonane,
n-decane, n-undecane, n-dodecane, n-tetradecane, n-
octadecane, liquid paraffin and kerosine, aromatic
hydrocarbon solvents and hydrogenated products thereof
such as xylene, naphthalene, tetralin, butylbenzene, p-
cumene, cyclohexylbenzene, diethylbenzene, benzylbenzene,
:.
dodecylbenzene, bicylohexyl, decalin, methylnaphthalene
and ethylnaphthalene, halogenated hydrocarbon solvents
such as 1,1,2,2-tetrachloroethane, pentachloroethane,
hexachlorobenzene, 1,2,3-trichloropropane,
dichlorobenzene, 1,2,4-trichlorobenzene and bromobenzene,
and mineral oils such as paraffinic process oil,
naphthenic process oil and aromatic process oil.
- 17 -
132S1~3
Aliphatic hydrocarbon compounds and derivatives
thereof can be used as the wax.
A so-called paraffinic wax composed mainly of a
saturated aliphatic hydrocarbon compound having a
molecular weight lower than 2000, preferably lower than
lOoo, especially preferably lower than 800, is mentioned
~,.
as the aliphatic hydrocarbon compound. As speciflc
examples of the aliphatic hydrocarbon compound, there can
be mentioned n-alkanes having at least 22 carbon atoms,
such as docosane, tricosane, tetracosane and triacontane,
mixtures comprising an n-alkane as mentioned above as the
main component and a lower n-alkane, so-called paraffin
waxes and ethylene copolymer waxes, which are low-
molecular-weight polymers obtained by homopolymerizing
ethylene or copolymerizing ethylene with othero(-olefin,
waxes formed by reducing the molecular weight of
polyethylene such as medium-pressure, low-pressure or high-
pressure poleythylene by thermal degradation, and oxidized
waxes and maleic acid-modified waxes obtained by oxidizing
the foregoing waxes or modifying the foregoing waxes with
maleic acid.
As the aliphatic hydrocarbon compound derivative,
there can be mentioned, for example, fatty acids,
aliphatic alcohols, fatty acid amides, fatty acid eRters,
aliphatic mercaptans, aliphatic aldehydeR and aliphatic
- 18 -
132~14~
ketons having at least 8 carbon atoms, preferably from 12
to 50 carbon atoms, and a molecular weight of from 130 to
2000, preferably from 200 to 800, in which at lea~t one,
preferably 1 or 2, especially preferably one functional
group such as a carboxyl group, a hydroxyl group, a
carbamoyl group, an ester group, a mercapto group or a
carbonyl group is contained at the end or in the interior
of an aliphatic hydrocarbon group such as an alkyl group
or an alkenyl group.
As specific examples, there can be mentioned fatty
acids such as capric acid, lauric acid, myristic acid,
palmitic acid, stearic acid and oleic acid, aliphatic
alcohols such as lauryl alcohol, myristyl alcohol, cetyl
alcohol and stearyl alcohol, fatty acid amides such as
capric amide, lauric amide, palmitic amide and stearyl
amide, and fatty acid esters such as stearyl acetate.
The ultrahigh molecular weight ethylene
copolymer/diluent mixing ratio varies depending upon the
kind~ of the~e components, but it is generally preferred
that this mixing ratio be from 3/97 to 80/20, especially
from lS/75 to 60/40. If the amount of the diluent is too
small and below the above-mentioned range, the melt
viscosity becomes too high and melt kneading or melt
shaping is difficult, and surface roughening of the shaped
article is conspicuous and breaking is often cau~ed at the
'.~'
. ~ ~
1325143
drawing step. If the amount of the diluent is too large
and exceeds the above-mentioned range, melt-kneading
becomes difficult and the drawability of the shaped
article is insufficient.
It is generally preferred that melt kneading be
carried out at a temperature of from 150 to 300 C,
especially from 170 to 2~0 C. If melt kneading is
carried out at a lower temperature, the melt viscosity is
too high and melt shaping becomes difficult. If the
temperature is too high and exceeds the above-mentioned
range, the molecular weight of the ultrahigh molecular
weight ethylene copolymer is reduced by thermal
degradation and it becomes difficult to obtain a shaped
article having high elastic modulus and high strength.
Mixing can be performed by dry blending using a Henschel
mixer or a V-blender or by melt mixing using a single
screw or multiple-screw extruder.
Melt shaping of a dope comprising the copolymer
and diluent is generally performed by melt extrusion. For
example, filaments are obtained by melt extrusion of such
a dope through a spinneret. The filaments as extruded
through the spinneret may be drafted, that is, Qtretched
in the molten state. The draft ratio can be defined by
the following formula:
draft ratio - V/Vo
.,~
- 20 -
''
132~143
wherein Vo stands for the extrusion speed of the
molten resin in a die orifice, and V stands for the
winding speed of the cooled and solidified undrawn
filaments.
While the draft ratio depends on the temperature
of the mixture and the molecular weight of the ultrahigh
molecular weight ethylene copolymer, it may be at least 3,
preferably at least 6.
The so-obtained undrawn shaped article of the
ultrahigh molecular weight ethylene copolymer is sub~ected
to a drawing treatment. Of course, the degree of the
drawing treatment is such that molecular orientation in at
least one axial direction can be effectively imparted to
the copolymer.
It is generally preferred that drawing of the
shaped article of the ultrahlgh molecular weight ethylene
copolymer be carried out at a temperature of from 40 to
160 C, preferably from 80 to 145 C. Any of air, water
steam and liquid media can be used as the heating medium
for heating and maintaining the undrawn shaped article at
the above-mentioned temperature. If a solvent capable of
eluting and removing the above-mentioned diluent, which
has a boiling point higher than the melting point of the
composltion of the shaped article, for example, decalin,
æ
decane, kero~lne or the llke, i5 u~ed a~ the heating
.
.,
- 21 -
1325143
medium for the drawing operation, removal of the above-
mentioned diluent becomes possible, and drawing unevenness
can be eliminated at the drawing step and a high draw
ratio can be attained.
Of course, the means for removing the exces~ive
diluent from the ultrahigh molecular weight ethylene
copolymer i5 not limited to the above-mentioned method.
For example, there may be adopted a method in which the
undrawn shaped article is treated with a solvent such as
hexane, heptane, hot ethanol, chloroform or benzene and
then drawn, and a method in which the drawn shaped article
is treated with a solvent such as hexane, heptane, hot
ethanol, chloroform or benzene. According to these
methods, the exces~ive diluent can be effectively removed
and a drawn product having a high elastic modulus and a
high strength can be obtained.
The drawing operation may be carried out in one
stage or a plurality of stages. The draw ratio dependR on
the desired molecular orientation and the resulting
improvement of the fusion temperature, but in general,
satisfactory reRults are obtained if the drawing operation
is carried out at a draw ratio of from 5 to 80, e~pecially
from 10 to 50.
In general, drawing in a plurality of stages is
advantageous, and there is preferably adopted a method in
~ - 22 -
132~143
which at the first stage, the drawing operation is carried
out at a relatively low temperature of from 80 to 120 C
while extracting the diluent from the extruded shaped
article and at the second and subsequent stages, the
operation of drawing is conducted at a temperature of from
120 to 160 C, which is higher than the drawing
temperature adopted at the first stage.
Uniaxial drawing of filaments or tape is
accomplished by performing the drawing operation between
rollers differing in the peripheral speed.
The so-obtained molelcularly oriented ~haped
article can be heat-treated under a restraint condition,
if de~ired. This heat treatment is carried out at a
temperature of from 140 to 170 C, especially from 150 to
175 C, for a period of from l to 20 minute~, especially
from 3 to 10 minutes. By this heat treatment,
crystallization of the oriented crystalline portion is
further advanced, and the crystal fusion temperature is
shifted to the high temperature side, the strength and
ela~tic modulus are improved and the creep resistance at
elevated temperatures is improved.
The rope l for traction accordlng to the invention
has a double ~tructure of a core member 2 coated with a
sheath member 3, in which the core member 2 compri~es a
molecularly oriented shaped article such as filaments and
: .
132S143
23
tapes of an ultrahiqh molecular weight copolymer of ethylene and
at least one alpha-olefin, and the sheath member 3 comprises a
braid. The core member 2 preferably comprises a bundle of aligned
drawn filaments of the ultrahigh molecular weight polyolefin, or a
braid made thereof. The braid may be made of a single bundle of
drawn filaments. Alternatively, a number of bundles of drawn
filaments, for example, 3, 4, 6 or 8 bundles of drawn filaments
- may be twisted together and then braided to provide the core
member 2. Further, the core member may be a parallel lay rope.
- 10 Depending upon the intended use and object of the rope, the
diameter of the bundle of drawn filaments and the number of the
bundles to be twisted together may be selected.
The core member 2, in the form of a braid, of the rope
according to the lnvention preferably has a diameter ranging from
2 to 15 mm and a breaking energy of at least 3 kg.m/g, and ln
particular at least 4 kg.m/g. One of the advantages of the
molecularly orlented shaped artlcle used herein resides in the
fact that braiding reduction in available strength (spinning loss)
is low.
The sheath member 3 comprises a braid of yarns of
strands of conventional naturally occurrlng or synthetlc fibers.
Examples of usable fibers include, for example,
',
'
- 24 -
` 132~143
polyester fibers, acrylic fibers, cotton, polypropylene
fibers, polyethylene fibers, polyamide fibers, rayon
(viscose and cupra), linen and Vinylon fibers.
The number of yarns or strands for braiding the
sheath member 3 and the thickness of the sheath member 3
may be selected depending upon the intended use of the
rope. Normally, the thickness of the sheath member is
preferably of the order of form 0.1 to 2 mm.
The rope according to the invention can be
prepared by a double braiding method known per se. For
example, during or after formation of the core member 2 by
aligning or braiding a bundle of bundles of molecularly
oriented filaments of an ultrahigh molecular weight
polyolefin, the core member is wrapped around helically
with inter~ecting yarns of strands so that they may be
braided into an enveloping sheath member 3.
. ~
Effect of the Invention
The rope for traction according to the invention
has a double structure of a core member coated with a
sheath member, in which the core member comprlses a light
in weight, strong and resilient molecularly oriented
~haped article of an ultrahigh molecular weight
polyolefin, and the sheath member comprises a braid, and
in con~equence, it is floatable on water and easy to
. .
132~1~3
handle, and is excellent in safety to human bodies and
weatherability.
Example 1
(Polymerization for Preparation of Ultrahigh
Molecular Weight ~thylene/Butene-l Copolymer)
Slurry polymerization for forming an ultrahigh
molecular weight ethylene/butene-1 copolymer was carried
out in 1 Q of n-decane as a polymerization solvent in the
presence of a Ziegler catalyst. A monomer mixture gas
comprising ethylene and butene-1 at a molar ratio of
9~.2/2.35 was continuously supplied to a reactor so that
the pressure inside the reactor iY kept constant at
5 kg/cm2. The slurry polymerization carried out at a
; reaction temperature of ~0C was completed in 2 hours.
: The yield of a powdery ultrahigh molecular weight
ethylene/butene-1 copolymer was 160g, an intrinsic
: viscosity (at i35 C in decalin) of the copolymer was
8.2dl/g, and the butene-1 content determined by an
infraredspectrometer was 1.5 butene-1 molecules per 1000
carbon atoms
. IPreparation of Drawn and Oriented product of
. Ultrahigh Molecular Weight ~thylene/Butene-l
Copolymer)
A mixture comprising 20 parts by weight of the
powdery ultrahigh molecular weight ethylene/butene-1
- 26 -
1325143
copolymer obtained by the above-mentioned polymerization
and 80 parts by weight of a paraffin wax (melting
point = 69C, molecular weight = 490) was melt-spun under
the following conditions.
To 100 parts by weight of the above-mentioned
filament
mlxture was added 0.1 part by weight of 3,5-di-tert-butyl-
4-hydroxy-toluene as a process stabilizer. The mixture
was then melt-kneaded at a set temperature of 190 C.,
using a screw type extruder (screw diameter = 25 mm,
L/D = 25, manufactured and sold by Thermoplastics Co.).
Subsequently, the molten mixture was melt spun through a
spinning die having an orifice diameter of 2 mm, ~
splnnlng die being attached to the extruder. The extruded
melt was taken at a draft ratio of 36 times with an air
gap of 180 cm, cooled and solidlfied in the air to provide
an undrawn filament. The undrawn filament was then drawn
under the following conditions.
Two-staged drawing wa carried out using three
godet rolls. A heating medium employed in a first drawing
tank wa~ n-decane and the temperature wa~ 110C, and a
heating mediu~ employed in a ~econd drawing tank was
triethylene glycol and the temperature was 145C. The
effectlve length of each tank was 50 cm. The undrawn
filament was drawn with a first godet roll operated at a
2~t
- 2~ -
-~ 132~1~3
rotation speed of 0.5 m/min while adjusting a rotation
speed of a third godet roll to provide an oriented
filament of a desired draw ratio. A rotation speed of a
second godet roll was suitably selected so that a stable
drawing operation is made possible. Substantially all of
the paraffin wax initially added was extracted ~n n-decane
from the filament at a stage in which it is drawn. The
oriented filament was then washed with water and dried a
whole day and night at room temperature and under reduced
pressure, followed by determination of physical properties
thereof. Incidentally, the draw ratio was calculated from
the ratio of the rotation speed between the first and
third godet rolls.
(Measurement of Tensile Characteristics)
The oriented filament was tested for its elastic
modulus and tensile strength at room temperature (23C)
using a tensile tester (Model DCS-50M manufactured and
sold by Shimadzu Seisakusho). The sample was a bundle of
100 filaments each having a thickness of 10 denier.
In that case, a sample length employed between
clamps was 100 mm, and a rate of pulling employed was
lOOmm/min (a rate of strain of 100%/min). The elaRtic
modulus was an initial elastic modulus calculated from a
tangential gradient of the stress-strain curve. A
sectional area necessary for the calculation was obtained
- 28 -
13251~.~
by calculation from a weight of the sample, assuming the
density of the sample as 0.960g/cc.
(Tensile Elastic Modulus Retension and Strength
Retention after Heat Hysteresis)
A heat hysteresis test was carried out by allowing
a sample to stand still in a gear oven ~Perfect Oven
manufactured and sold by Tabai Seisakusho).
;" The sample having a length of about 3 m was wound
repeatedly on a stainless steel frame having a plurality
of blocks attached to both ends thereof, and both ends of
the ~ample were fixed. In that case, both ends of the
sample were fixed to such an extent that the sample does
not ~ag, and no positive tension was imposed on the
sample. Tensile characteristics of the sample after the
heat hysteresis test were determined according to the
procedure as described in the aforementioned measurement
of tensile characteristics.
; (Measurement of Creep Resistance)
The drawn and oriented filament was tested for its
creep resistance using a thermal stress distortion
measurement apparatus (Model TMA/SS10 manufactured and
sold by Seiko Denshi Kogyo), wherein the sample length was
1 cm, ambient temperature was 70C, and mea~urement was
;~ conducted under an accelerated condition by applying to
the sample a load corresponding to 30 % of a breaking load
`:
- 2~ -
13251 43
at room temperature. In order to evaluate a creep
quantltatively, the following two values were determined,
That i~, there were determlned values of a creep
elongation CRgo~X) at the time 90 seconds after
application of the load, and of an average creep speed
., --1
(sec ) between the time 90 second~ after application of
the load and the time lB0 seconds after the above-
mentioned time.
: Tensile characteristics of the bundle of drawn and
oriented filaments are shown ln Table 1.
~; Table 1
Sample Filament Draw Strength Elastic Elon- Degree of
denier/ ratiomo~ulu~ gation orien-
filaments tation
(GPa) ~GPa) (%) ~F)
....~
Sample 1000/ 22.3 2.4 60 5.50 0.975
-1 100
The lnherent crystal fuslon peak of the drawn and
oriented fllament (~ample-l) of the ultrahigh molecular
welght ethylene/butene-1 copolymer appeared at 126.~C,
and the proportion of fusion peak based on Tp to the total
- crystal fusion heat wa4 33.8X. The creep reslstance
~ i .
'
- 30 -
1325143
~.,
characteristics were such that CRgo was 3.1% and was
3.03 X 10 5 sec 1 After the heat hysteresis at 170C for
5 minutes, the elastic modulus retention ratio was 102.2%,
and the strength retention ratio was 102.5%, showing no
decrease in performance of the drawn and oriented
filament.
Furthermore, physical properties of this drawn and
oriented filaments were such that the amount of work
necessary for rupture of the filament was 10.3 kg~m/g, the
density was 0.973 g/cm3, the dielectric constant was 2.2,
the dissipation loss tangent was 0.024%, and the impulse
breakdown voltage was 180KV/mm.
A traction rope according to the present
invention, which comprises the following core and sheath
members was prepared.
Core member:
~ n eight bundle plaited rope having a thickness of
12,000 denier made of ultrahigh molecular weight
ethylene/butene-1 copolymer filaments (sample-1).
Sheath member:
A sheath member was obtained by plaiting polye~ter
spun yarns each having a thickness of 1,000.
Morphological and physical properties of the rope
thus prepared are shown in Table 2.
1325143
Table 2
__ I
Sampl~ Core Wall Unit Breaking Breaking Amount of energy
diameter thickness weight strength elongation required for bringing
of sheath about breakage
(mm) (mm) (g/m) (Ton) (%) (kg m/g)
___ . __ ___
- Sampl l
-lA 4.0 0.5 12 2.0 6.5 5.40
__ __ _ _
Example 2
(Polymerization for Preparation of Ultrahigh
Molecular Weight Ethylene/Octene-1 Copolymer)
Slurry polymerization of ethylen was carried out
in the presence of a Z~egler catalyst in 1 Q of n-decane
as a polymerization solvent. In that case, 125 ml of
octene-1 as a comonomer and 40 Nml of hydrogen as a
molecular weight-adjusting agent were collectively added,
prior to the intiation of the polymerization, to the
system, and the polymerization was then allowed to
proceed. Ethylene gas was continuously supplied to a
reactor so that the pressure inside the reactor is kept
constant at 5kg/cm2 and the polymerization was completed
in 2 hours at 70C. The yield of powdery ultrahigh
molecular weight ethylene/octene-l copolymer obtained was
....
- 32 -
132~143
178g, the Intrlnsic viscosity ~] ~at 135 C in decalin)
was 10.66dl/g, and the octene-1 content as determined by
an infrared spectrophotometer was 0.5 octene-1 molecule
per 1000 carbon atmos.
(Preparation and Physical properties of Drawn and
Oriented product of Ultrahigh molecular weight
~thylene/Octene-l Copolymer)
Drawn and oriented filaments were prepard from the
ethylene/octene-1 copolymer thus obtained in the sa~e
manner as described in ~xample 1. Tensile characteristics
of a bundle of the drawn and oriented filaments obtained
are shown in Table 3.
Table 3
Samplt Filament Draw Strength elastic ~lon- Degree of
V Denler/ ratlo mo~ul ~ gation orien-
Fllaments tation
(GPa) (GPa)(X) (F)
Sampl~ 1000/ 16.02.3 65 5.40 0.978
-2 100
.' ..
':
The inherent crystal fuslon peak of the drawn and
- orlented filanenta (sample-2) of the ultrahigh molecular
: ~, ..r
.
. ~:
. , ~ ~ .
- 33 -
1325143
weight ethylene/octene-1 copolymer appeared at 132.1 C,
and proportions of fusion heat based on Tp and Tp1 to the
total crystal fusion heat were 9~.~% and 5.0%,
respectively. The creep resistance characteristics of the
sample-2 were such that CRgo was 2.0% and ~ was 9.50 X 10
sec 1. After heat hysteresis at 170C for 5 minutes,
the ela~tic modulus retention was 108.2% and the strength
retention was 102.1%. Furthermore, physical properties of
the sample-2 were such that the amount of work necessary
for rupture of the filament was lO.lRg m/g, the density
was 0.971g/cm3, the dielectric constant was 2.2, the
dissipation losq tangent was 0.031%, and the impulse
breakdown voltage was 185KV/mm.
A traction rope according to the present invention
was obtained in the same manner as descri~ed in ~xample 1
using the drawn and oriented filaments (sample-2) of the
ultrahigh molecular weight ethylene/octene-1 copolymer
thus obtained. Morphological and physical properties of
the rope obtained are shown in Table 4.
'
- 34 -
1325143
Table 4
__ _ . _ _
Sample Core Wall Unit Breaking Breaking Amount of energy
diameter thicknesq weight strength elongation required for bringing
of sheath about breakage
~mm) ~mm) ~g/m) (Ton) (~) (kg~m/g)
_
;ample
-2A 4.0 0.5 12 2.0 6.3 5.20
Example 3
A powdery mixture comprising 20 parts by weight of
`; a ultrahigh molecular weight polyethylen(homopolymer)
having an intrinsic viscosity of t~]=7.42 dl/g as measured
at 135C in decalin and 80 parts by weight of a parrafin
wax having a melting point of 69C and a molecular weight
of 490 was melt-spun and drawn in the same manner as
described in Example 1 to obtain a drawn and oriented
filament. Tensile characteristics of a bundle of the
drawn and oriented filaments thus obtained are shown in
" Table 5.
';
.
- 35 -
132~143
Table 5
.
Sample Filament Draw Strength Elastic ~lon- Degree of
denler/ ratio modulu~ gation orien-
filaments tatlon
(GPa)(GPa) (X) (F)
Sampl~ lOOOt 23.12.590 4.10 0.9B0
-3 100
The inherent crystal fusion peak of the drawn and
oriented filaments (sample-3) of the ultrahigh molecular
- wel~ht poly~thylene wa~ 136.1C, and a proportion of
fusion heat based on Tp to the total crystal fusion heat
was 8.8X. Whereas, a proportion of fusion heat based on
Tp1 on the higher temperature side to the total cry~tal
fusion heat wais less than lX. The creep resistance
characteristics were such that CRgo was ll.9X and ~ wa~
1.07 X 10 3 sec 1. After heat hysteresis at 170C for 5
minutes, the elastic modulus retention was 80.4% and the
strength retention was 78.2X. Furthermore, physical
properties of the sample-3 were such that the amount of
work neces~ary for rupture of the filament was 10.2Kg m/g,
the density was 0.985g~cm3, the dielectric constant was
2.3, the diasipation loss tangent was 0.030%, and the
"~ .
,'
'.: i .
'.
.'
- 36 ~
1325143
impulse breakdown voltage was lB2KV/mm.
A traction rope of the present invention was
obtained in the same manner as described in ~xample 1
using the drawn and oriented filaments (sample-3) of the
ultrahlgh molecular weight polyethylene. Morphological
and physical properties of the rope obtained are shown in
Table 6. Table 6
Sampl~ Core Wall Unit Breaking ~reaking Amount of energy
dlameter thlcknesa welght strength elongation required for bringin
of sheath about rapture
~mm)~mm) (g/m) tTon) (%) ~g-m/g)
. _
Sampl~
- -3A 4.00.5 13 1.9 4.7 3.51
, .
Comparative ~xample 1
A traction rope was prepared using a400 denier
drawn filaments of Kebleri~29 ~aramid fiber produced by Du
Pont) as a core in place of the core member described in
~xample 1. Morphological and physlcal properties of the
rope thu~ obtalned are shown ln Table ~.
a~
'
'':`
. -37-
- 1325143
Table 7
Sample Core Wall Unit Breaking Breaking Amount of energy
diameter thicknes~ weight strength elongation required for bringing
of sheath about rapture
(mm) (mm)~g/m) (Ton) (%) (kg m/g)
___
Sampl~
-4A 3 8 0.5 12 1.9 3.6 2.80
~".
;
;