Note: Descriptions are shown in the official language in which they were submitted.
1 326342
TITLE: PRODUCTION OF CARBON DIOXIDE BY COMBUSTION
OF LANDFILL GAS
BACK~OUND OF_THE INVENTION
The present invention is directed to an improved
process for the production of high concentration and high
purity carbon dioxide from landfill gas. In particular, the
present invention involves, as a central step of the process,
the combustion of all or part of the landfill gas in order to
increase the amount of carbon dioxide producible fro~ a given
landfill, produce valuable energy in the form of electricity,
steam, mechanical power, or the like, and to reduce the number
of impurities that must be removed from the carbon dioxide to
produce food grade carbon dioxide product.
Landfill gas or biogas is gas generated by anaerobic
digestion of organic residues and has a typical composition of
40 - 60% methane, 40% - 60% carbon dioxide, and a wide range of
impurities, including hydrocarbons, sulfur compounds and
chlorinated or fluorinated hydrocarbons. The impurities found
in landfill gas present a significant obstacle to the economic
production of food grade carbon dioxide from a landfill
source. In order to qualify as food grade carbon dioxide, the
carbon dioxide product should, in general, have less than about
five parts per million of total hydrocarbons, as equivalent
methane, and less than about 0.5 parts per million total
sulfur. Food grade carbon dioxide is required for carbonation
of beverages, such as beer and soda, and also for food freezing
applications.
LandEill gas has heretofore been recognized primarily
as a source of fuel. Various techniques have been proposed in
order to obtain the fuel value of the landfill gas. In one
~ ,
1 326342
approach, landfill gas is burned using air after only minor
cleanup is performed to remove the more detrimental compounds,
such as chlorinated hydrocarbons which produce hydrochloric
acid upon combustion. In a second approach, the majority of
the carbon dioxide is removed from the landfill gas and the
remaining high BTU content, methane rich stream is used for
fuel. Processes typically used to remove the carbon dioxide
also tend to remove a large fraction of the impurities in the
landfill gas and to mix these impurities with the carbon
dioxide. As a result, the carbon dioxide component of the
landfill gas is typically discarded. For example, Croskell et
al., in U.S. Patent No. 4,518,399, disclose a process for
recovering methane from landfill gas by utilizing a membrane
separation step and venting the carbon dioxide in the vicinity
of the landfill site, in order to maintain an oxygen free
environment. In a process disclosed in Portugese patent
830254E to deSousa Brito, the methane component of landfill gas
is concentrated by separating out carbon dioxide on an
adsorbent porous solid, preferably an alumino-silicate zeolite
molecular sieve having a high capacity for carbon dioxidç
adsorption and a miCropore diameter equal to or greater than 4
angstroms. The adsorbed carbon dioxide is incidentally
produced in high concentration, but will still contain
significant impurities.
Prior art methods proposed for carbon dioxide recovery
from landfill gas are not sufficiently cost competitive with
other sources of carbon dioxide. In particuIar, the prior art
separation of carbon dioxide with physical or chemical solvents
consumes energy, requires burdensome procedures, and typically
concentrates a large fraction of the landfill gas impurities in
the carbon dioxide rich stream. The carbon dioxide rich stream
1 326342
still requires significant impurity removal before food grade
product can be obtained. Therefore, there is a need for a cost
competitive method to produce high purity food grade carbon
dioxide from the biogas available in landfills.
Prior art processes for removing impurities from a
carbon dioxide containing gaseous mixture have included
subjecting the mixture to a combustion reaction. For example,
U.S. Patent No. 3,962,127 to woerner discloses removing
hydrocarbon contaminants, in the gaseous emissions from the
regeneration of a fixed bed dehydrogenation catalyst, by
contacting with a catalyst such as chromia-alumina in the
presence of air to convert a substantial amount of the
hydrocarbon contaminants to water and carbon dioxide.
U.S. Patent No . 4, 364, 915 to Proctor discloses a
process wherein a flue gas stream is admixed with a combustible
fuel such as methane and then passed to a combustion zone. The
purpose of the combustion is to lower the oxygen content of the
flue gas stream, which is then contacted with a lean carbon
dioxide adsorbing amine solution. The carbon dioxide is
thereafter recovered. The combustion step is preferably in the
presence of a catalyst at about 510C ~950F). The treated
flue gas contains less than 200 ppm oxygen.
The prior art described above, which utilizes a
combustion step to purify or otherwise treat a carbon dioxide
containing mixture, uses one of two general approaches: (1)
combustion with an auxillary fuel or (2) catalytic oxidation
without fuel. These approaches have the disadvantage, in the
latter case, of either employing expensive catalyst or
requiring frequent catalyst regeneration, and, in the former
1 326342
case, have the disadvantage of consuming valuable fuel
product. Moreover, the prior art has required elaborate and
complex arrangements, on the one hand, to avoid a high
temperature in the combustion zone and, on the other hand, to
ensure the desired oxidation of a range of impurities.
U.S. Patent No. 3,317,27~ to Ruhemann et al. discloses
a process of purifying carbon dioxide contaminated with a minor
proportion of impurities, including light hydrocarbons, by
first rectifying at a low temperature and an elevated pressure,
in order to remove the overhead fraction or light impurities,
and subsequently passing the bottom fraction or heavy
impurities to a catalytic oxidation zone. The process of
Ruhemann et al. is intended to ensure complete oxidation of the
hydrocarbon impurities while avoiding high flame temperatures.
Ruhemann et al. discloses application of his process to moist
impure carbon dioxide, from a natural source, having a methane
concentration of only about 1.7 percent.
U.S. Patent No. 4,460,395 to Nobles et al. discloses a
process of producing food-grade carbon dioxide from an impure
carbon dioxide stream containing light and heavy hydrocarbons
and light and heavy sulfur compounds. The feed gas is first
rectified with liquid carbon dioxide to remove heavy
hydrocarbons and heavy sulfur compounds, then passed to an
adsorber to effect the removal of the light sulfur compounds,
and next subjected to a combustion step to convert the light
hydrocarbons to water and carbon oxides.
The prior art also discloses, in general, recycling
the exhaust from a combustion zone. U.S. Patent No. 3,905,745
to Konda discloses reducing emission of harmful products from a
1 326342
combustion furnace wherein a mixture of oxygen, in an amount of
about 15 percent, and part of the exhaust gas, comprising a
recycle stream, is introduced into the combustion furnace, in
order to reduce the quantities of harmful constituents
otherwise emitted from the furnace. U.S. Patent No. 3,298,176
to Forsyth et al. discloses a similar process in which oxygen
is admixed with recycled exhaust gas, in order to prevent neat
oxygen from contacting the fuel oil introduced into the
combustion chamber of a power plant.
None of the aforementioned prior art processes
disclose an economic and simple method for producinq carbon
dioxide from landfill gas. Furthermore, the prior art has
failed to recognize the advantages of a combustion step in the
production of carbon dioxide from landfill gas.
OB~CTS OF THE INVENTIQN
Accordingly, an object of the present invention is to
produce carbon dioxide from landfill gas.
Another object of the invention is to increase the
amount of carbon dioxide that can be produced from a given
landfill.
Yet another object of the present invention is to
convert a wide range of impurities contained in the landfill
gas to a much smaller number of impurities and thereby
facilitate their removal.
Yet a further object of the present invention is to
produce high concentration and high purity carbon dioxide from
landfill gas.
r
1 326342
Yet a further object of the present invention is to
obtain useful energy by the combustion of methane and other
species originally in the landfill gas.
Yet another object of the present invention is to
assure removal of any bacteria originally in the landfill gas.
Yet a further object of the present invention is to
develop a cost competitive method of producing ~ood grade
carbon dioxide from landfill gas.
These and other objects of the present invention will
be more readily appreciated from a consideration of the
detailed description of exemplary embodiments thereof which
follow and the novel features of the present invention will be
particularly pointed out in conjunction with the claims
appended hereto.
SUMMARY OF THE INVENTION
According to the present invention, there is provided
a process for converting landfill gas to high concentration
carbon dioxide gas involving the combusting of the landfill gas
in admixture with oxygen or an oxygen rich gas in an internal
combustion engine, gas turbine, boiler or other energy
conversion device. In a first embodiment of the invention, a
portion of the combusted exhaust gas is recycled in order to
control the maximum temperature achieved during combustion. In
a second embodiment of the invention, the majority of the
methane in the landfill gas is separated and then the remaining
gas is combusted in admixture with oxygen or oxygen rich gas in
an internal combustion engine, gas turbine, boiler, or other
energy conversion device. In this second embodiment, the peak
1 326342
temperature achieved in the combustor is controlled by
appropriately selecting the amount of methane removed.
Several advantages are derived from the use of a
combustion step in applicants' process. The combustion step
accomplishes significant removal of impurities present in
landfill gas, and reduces the number of chemical species which
compose the impurities, thereby facilitating their subsequent
removal. The wide range of hydrocarbons, halogenated
hydrocarbons, and sulfur containing impurities in the landfill
gas are converted to primarily carbon dioxide, water, sulfur
dioxide, and hydrochloric acid.
The use of a combustion step according to the
invention furthermore increases the amount of carbon dioxide
which is produced in a given landfill. In the first embodiment
of the invention, combustion typically more than doubles the
amount of carbon dioxide that can be produced from a given
land~ill. In addition, the need to separate the methane and
carbon dioxide components of the landfill gas is eliminated.
In the second embodiment, in which some of the methane
is removed prior to combustion, the combustion increases the
amount of carbon dioxide that can be produced from a given
landfill by approximately 20%. The required separation is
therefore easier and less energy intensive than if all the
methane as well as the wide range of impurities were to be
removed without the benefit of a combustion step.
The combustion step has the further advantage of being
capable of being carried out using standard combustion
equipment. Finally, the combustion equipment used in the
present invention, such as an internal combustion engine driven
electric power generator or gas turbine driven electric power
generator, steam boiler, or the like can cogenerate
1 326342
electricity, steam, and/or mechanical energy and the overall
process can therefore supply rather than consume energy.
BRIEF DESCRIPTION_OF THE DRAWINGS
The invention will be more clearly understood by
reference to the following description of exemplary embodiments
thereof in conjunction with the following drawings in which:
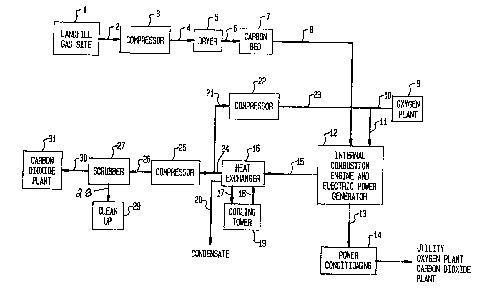
FIG. 1 is a schematic process flow diagram
illustrating a first embodiment of the present invention;
FIG. 2 is a schematic process flow diagram
illustrating a second embodiment of the present invention;
FIG. 3 iS a schematic flow diagram of one possible
configuration of a PSA unit, to perform the separation required
for the second embodiment of the present invention; and
FIG. 4 iS a timing diagram illustrating a full cycle
sequence of PSA operation corresponding to the configuration
shown in FIG. 3.
DETAILED DESCRIPTION OF THE INVENTION
Referring to FIG. 1, which illustrates a first
embodiment of the invention, a landfill gas site l serves as a
source of landfill gas comprising methane, carbon dioxide, and
various organic and inorganic impurities. The landfill gas
stream 2 is fed to a compressor 3 which provides sufficient
pressure to overcome the pressure drop through the subsequent
dryer 5, carbon bed 7, and interconnecting piping and still
provide sufficient feed pressure for the internal combustion
engine 12. Since the internal combustion engine 12 has been
typically designed to be operated using methane and air, the
feed pressure of the internal combustion en~ine is therefore
preferably selected such that the amount of power generated,
1 326342
which i~ dependent upon the amount of landfill gas with which
the cylinders of the engine are initially charged, is equal to
the amount of power that is generated when the engine is run
using methane and air. The compressed gas stream 4 is sent
to dryer 5 where the dew point is reduced to between -40~F and
-llO~F and preferably between -60F and -lOO-F. Alternatively, the
compressed gas stream 4 may be dried by passing throuqh a dessicant.
The amount of energy expended in drying stream 4 is a tradeoff to
increase the effectiveness of the carbon bed 7. The dried gas stream
6 is then cent to carbon bed 7, containing activated carbon. The
function of the carbon bed 7 is to reduce the concentration of
halogenated hydrocarbons to below 100 ppm, and preferably below
50 ppm, in order to minimize the amount of corrosive
hydrochloric acid produced in the engine. In addition to
halogenated hydrocarbons, some of the heavier hydrocarbons will
also be removed. The carbon bed 7 should be periodically
regenerated. This can be accomplished with a pressure swing
system or by emptying the bed periodically or by thermal
treatment with steam or hot gas. The treated stream 8 is then
sent to the fuel intake of the internal combustion engine 12.
Entering the air intake of the internal combustion engine 12 is
stream 11, which is an admixture of a high oxygen concentration
stream 10, and recycle stream 23, which is a recycled portion
of the internal combustion engine exhaust gas and consists
primarily of carbon dioxide. The ratio of the flow rates of
streams 8 and 10 is controlled, with respect to stoichiometric,
such that a slightly oxygen rich mixture is fed to the internal
combustion engine. The ratio of the flow rates of streams 23
and 10 is controlled such that the peak temperature achieved by
the engine is egual to or less than the peak temperature
achieved when combusting the near stoichiometric ratio of
1 326342
methane and air for which the engine was designed . The
internal combustion engine drives an electric power generator
and the electricity is used to provide power to an oxygen
plant, liquid carbon dioxide plant and/or the local utility.
An electrical line 13 leads to a power conditioning unit 14,
which controls the frequency and voltage of the generated
electricity. The exhaust gas from the internal combustion
engine in exhaust stream 15 contains primarily carbon dioxide
and water, in the form of steam, at elevated temperatures.
This stream must be cooled before a portion of it is returned
for recycle to the internal combustion engine 12. A water
cooled heat exchanger 16 may be used to cool the gas and
condense out the contained steam, thereby producing a
condensate stream 20 and a cooled gas stream 24. The water
used to provide cooling in the heat exchanger is sent via
conduit 17 to a cooling tower 19 and recirculated via conduit
18. Alternative options for cooling stream l$ include
replacing the heat exchanger with a waste heat boiler in order
to produce stçam. This steam may be optionally used to provide
energy to an ammonia adsorption refrigeration unit which in
turn could be used to provide refrigeration for the liquid
carbon dioxide plant. Other options for effective use of the
internal combustion engine exhaust heat would be evident to
those skilled in the art.
The cooled gas stream 24, which has a carbon dioxide
concentration greater than 95%, is partially split into stream
21. The amount of gas that comprises stream 21 is selected
such that the peak temperature in the internal combustion
engine 12 is about the same as the peak temperature achieved
when combusting the methane and air mixture for which these
~` 1 326342
commercially available engines are designed. The stream 21 is
fed to compressor 22 to increase the pressure such that stream
23 can be mixed with oxygen rich stream 10. The resulting
stream 11, as indicated above, is sent to the air intake of the
internal combustion engine.
The remaining portion of the cooled gas stream 2~ is
compressed by compressor or blower 25 before being sent via
line 26 to scrubber 27 for removal of sulfur dioxide and
hydrochloric acid. The concentration of sulfur dioxide in
stream 30 must be less than 0.5 ppm, and preferably less than
0.3 ppm: the concentration of hydrochloric acid in stream 30
must be less than 5 ppm, and preferably less than 0.7 ppm. The
spent scrubbing solution, stream 28, is neutralized in unit 29
and sent to sewer. The remaining gas stream 30 is sent to a
conventional, commercially available, liquid carbon dioxide
plant 31 for final purification and liquefaction.
It will be appreciated by those skilled in the art
that there are many possible modifications which can be made to
this embodiment of the present invention. For example, the
internal combustion engine could be replaced with a gas turbine
for driving the electric power generator. In general, for gas
flow rates typical of landfills, the gas turbine would have
lower efficiency in terms of electric power generation but
would produce more exhaust heat. As another example, the
internal combustion engine and electric generator could be
replaced by a boiler to generate high quality process steam.
A second embodiment of the present invention is
depicted in FIG. 2. Originating from landfill gas site 32, a
landfill gas stream 33 is sent to a compressor 34 which
1 326342
provides enough pressure for the gas to travel at ample rate to
a pressure swing adsorption (PSA) unit 43 and still have
adequate pressure to provide required pressure swing. The
compressed stream 35 is then ~ent to unit 36 to reduce hydrogen
sulfide concentration to below 4 ppm. The hydrogen sulfide
depleted stream 37 is sent to a dryer 38 where the dew point of
the gas is reduced to between -40F and -110F, and preferably
between -60F and -100F. The dried stream 39 is fed to a
carbon bed where halogenated hydrocarbons and most of the
hydrocarbons heavier than methane are removed. The partially
cleaned stream 41 is sent to a PSA separation unit 43. The
adsorbent beds of the PSA unit typically contain either carbon
.molecular sieve or a zeolite such as zeolite 13X. The PSA unit
produces a higher pressure stream 42 having a methane
concentration of 96% or greater and a lower pressure stream 44
having a methane concentration between 16 and 20%, and
preferably between 17 and 19%, with the remainder being
comprised predominantly of carbon dioxide. The concentration
.of the methane in the carbon dioxide rich mixture is preferably
selected such that the peak temperature that is attained in the
internal combustion engine 47 is equal to the peak temperature
that is attained when the engine is operated using the methane
and air mixture for which the engine is typically designed.
All or part Or the methane-rich stream 42 may be either combusted,
as for example in an internal combustion engine, otherwise used
as ~uel on-site, sold to nearby customers, or sold to a natural
gas pipeline company. The carbon dioxide rich stream 44 is
pressurized using compressor 45 and the compressed stream 46 is
sent to an internal combustion engine 47. The fuel containing
stream 46 and an oxygen containing stream 48 are used to charge the
1 326342
internal combustion engine. The ratio of oxygen to fuel should
be slightly oxygen rich with respect to stoichiometric. The
oxygen can be supplied by one or more liquid oxygen filled
storage tanks and vaporizer 49. Alternatively, the oxygen
could be generated on-site.
The internal combustion engine drives an electric
power generator and the electricity can be used to provide
power to an oxygen plant, liquid carbvn dioxide plant and/or
the local utility. A power conditioning unit 50 controls the
frequency and voltage of the generated electricity. The
exhaust gas from the internal combustion engine in exhaust
stream 51 contains primarily carbon dioxide and water, in the
form of steam, at elevated temperatures. A water cooled heat
exchanger 54 may be used to cool the gas and condense out the
contained steam, thereby producing a condensate and a cooled
gas stream 56. The water used to provide cooling in the heat
exchanger is sent via conduit S2 a to cooling tower 55 and
recirculated via conduit 53. Alternative options for cooling
stream 51 include replacing the heat exchanger with a waste
heat boiler in order to produce steam. This steam may be
optionally used to provide energy to an ammonia adsorption
refrigeration unit which in turn could be used to provide
refrigeration for the liquid carbon dioxide plant. Other
options for effective use of the internal combustion engine
exhaust heat would be evident to those skilled in the art.
The cooled gas stream 56 is compressed by blower 57
before being sent to scrubber 58 for removal of sulfur dioxide
and hydrochloric acid. The concentration of sulfur dioxide in
scrubbed stream 62 must be less than 0.5 ppm, and preferably
_~q :_
` ~ ~
1 3263~
less tha~ 0.3 ppm; the concentration of hydrochloric acid in
~tream 30 must be less than 5 p~m, and preferably less than 0.7
ppm. The spent scrubbing 601ution, stream 60, is neutralized
in unit 61 and sent to sewer. The scrubbed gas stream 62 is
cent to a conventional, commercially available, liguid carbon
dioxide plant 63 for final purification and liquefaction.
Such as in a cryogenic distillation column
It will be appreciated by those skilled in the art
that there are many possible modifications which can be made to
this embodiment of the invention. For example, the PSA unit
could be replaced with membrane modules. In this case the
methane rich stream would be the higher pressure non-permeate
and the carbon dioxide rich stream would be the lower pressure
permeate. As another example, the internal combustion engine
and electric generator could be replaced by a boiler to
generate high quality steam.
Comparing the above described first and second
ambodiments of the invention, the first embodiment generally
reguires higher capital and produces more carbon dioxide and
electric power while the second embodiment produces pipeline
quality methane in addition to the carbon dioxide. Depending
upon the local demand for carbon dioxide and electric power and
the current cost of capital, the selection of either the first
or second embodiment, or some combination of the two, can be
optimally employed.
EXAMPLE 1
This example illustrates a design based on the first
embodiment of the present invention. A landfill produces 2.56
million standard cubic feet per day (MMSCF/D) of landfill gas
1 326342
with a volumetric composition of 2 46% water, 53.47% methane,
43.75% carbon dioxide, 0.1% paraffinic hydrocarbons, 0.2%
aromatic and cyclic hydrocarbons, 0.01% hydrogen sulfide, and
0.01% other sulfur compounds. In addition, the paraffinic,
aromatic, and cyclic hydrocarbons contain about 0.03% (300 ppm)
chlorinated hydrocarbons. The gas is compressed to 75 psia and
the water content is reduced during compression to 0.49%. The
compressed stream is then dried to a dew point of -100F. The
dryer uses a two stage process which involves cooling the feed
to 50F followed hy desiccant drying. The dried stream is
subse~uently sent to an activated carbon bed which reduces the
chlorinated hydrocarbon concentration to 50 ppm. The partially
cleaned stream is then fed to the internal combustion engine at
a pressure of 50 psia. This landfill gas stream is combusted
in the internal combustion engine using an oxidizing stream,
which can be thought of as artificial air, that is made by
mixing oxygen, at a flow rate of 2.8 MMSCF/4, with a portion of
the recycled exhaust gas, at a flow rate of 4.7 MMSCF/D. This
mixture provides 2% excess oxygen, compared to stoichiometric,
to the internal combustion engine to assure complete methane
combustion. The mixture of oxygen, landfill gas, and recycled
exhaust gas combusted in the internal combustion engine
produces a flame temperature of 4510F. The internal
combustion engine drives an electric power generator which
produces 4620 kilowatts of electric power. Since the oxygen is
produced cryogenically, 5.85 tons per day of valuable crude
liquid argon is also produced.
1 326342
The 7.2 MMSCF/D of internal combustion engine exhaust
gas initially at 900~F is cooled to 100F in a heat exchanger.
The cooled gas is then divided into a 4.7 MMSCF/D stream which
is compressed to a nominal pressure of 50 psia and recycled to
the air intake of the internal combustion engine and a 2.5
MMSCF/D stream which is compressed to 25 psia using a blower.
The 25 psia stream is then sent to a scrubber where sulfur
dioxide concentration is reduced to 0.5 ppm and hydrochloric
acid concentration is reduced to 0.7 ppm. The scrubbed stream
is then fed to a conventional food grade liquid carbon dioxide
plant.
EXAMP~E 2
This example illustrates a design based on the second
embodiment of the present invention. A landfill produces 2.56
million standard cubic feet per day (MMSCF/D) of landfill gas
with a volumetric composition of 2.4~% water, 53.47% methane,
43.75% carbon dioxide, 0.1% paraffinic hydrocarbons, 0.2%
aromatic and cyclic hydrocarbons, 0.01% hydrogen sulfide, and
0.01% other sulfur compounds. In addition, the paraffinic,
aromatic, and cyclic hydrocarbons contain about 0.03% (300 ppm)
chlorinated hydrocarbons. The landfill gas is compressed to 97
psia before being sent to a hydrogen sulfide removal unit which
reduces hydrogen sulfide concentration to below 4 ppm. The
hydrogen sulfide removal step uses commercially available
technology. The stream is then dried to a dew point of -100F
before being sent to an activated carbon bed. The carbon bed
is once again used to reduce chlorinated hydrocarbon
concentration to 50 ppm. The dried and partially cleaned gas
1 326342
is then fed to a pressure swing adsorption (PSA) unit at a
pressure of 57 psia. FIG- 3 6hows a ~chematic of a PSA unit,
including valves numbered 01 through 10, check valves 11 and
12, adsorbent beds A and B, and high pressure product reservoir
13. A corresponding cycle sequence diagram showing valve
.positions and timing is shown in FIG. 4. The PSA unit operates
:between a feed pressure of 57 psia and a vacuum regeneration
pressure of 1.5 psia. The comPosition of the high pressure
product stream is 98% methane and 2% carbon dioxide; this
stream is compressed to 75 psia and is sent to a natural ga~
pipeline. A ~mall quantity of propane may be added to the methane-
rich steam prior to its introduction into the natural gas
pipeline. The composition of the combined atmospheric pressure
product and vacuum product strea~s is 81% carbon dioxide and
19% methane; this combined stream is compreæsed to 50 psia and
is combusted in an internal combustion engine using 2% excess
oxygen. The internal combustion engine drives an electric
power generator which produces 800 kilowatts of electric
power. The internal combustion engine exhaust gas initially at
900F is cooled to 100F in a heat exchanger. The cooled gas
is then compressed to 25 psia using a blower. The 25 psia
stream is then sent to a scrubber where sulfur dioxide
concentration is reduced to 0.5 ppm and hydrochloric acid
concentration is reduced to 0.7 ppm. The scrubbed stream is
then fed to a conventional food grade liquid carbon dioxide
plant.
It will be readily apparent to those skilled in the
art that the processes described in the first and second
embodiments can be modified depending on local conditions or
needs. Similarly, further energy saving measures can be
incorporated into the basic process. For example, the supply
of oxygen for the combustor, if in liquid form, can be used to
cool the exhaus,t from the combustor by a suitable arrangement
of flow lines.
~ '