Note: Descriptions are shown in the official language in which they were submitted.
1 326755
Process for cDncentrating ~ulphuric acid
This invention relate~ to a proce6s for CencQn-
trating sulphuric acid to concentrations of up to about
80% H2S04 by multistage evaporation.
BACKG~OUND OF THE INVENTION
Dilute 5ulphuric acid with concentration~ sometime~
below 107/. H2S04 is obtained from many processes for the
production of inorganic and organic compounds and the
purification of exhau6t ga~ec containing S02. If this
- . acid is to ~e used again, it i~ generally necessary to
evaporate it to concentrations above 60X. H2S04 and
remove or de~troy the impurities.
For concentrating the0e ~ulphuric acids by evapora-
tion under energy Baving condition~ it has been propo~ed
to employ multistage proce~ses in which the vaporc
obtsin~d from one ~tage of evaporationare u~ed as fuel
for the next ~tage. The co~t of the apparatus required
`. for the eYaporation of sulphuric acid i~, however,
rælati~ely high owing to the corrosivene6~ of the acid.
It i~ for this raa~on and due to ~he fact ~hat the
boiling point Df the acid rise~ ~harply with increa~ing
H2504 concentra~ion, in other words the H20 partial
prussur~ Falls ~harply with in rea~ing H2SO~ concentra-
tio~ at the maximum evbporation temperature th~t can be
allGWed fDr the given material of the apparatu~, that
multi~tage proce~ses ha~e hitherto only bean employe~
~$
Le A 24 623
1 326755
for ra;sing ~oncantration~ to about 70% H2S04 (so-
called preconcantration)~ For reasons of co~t, the
~vaporators and pipes used for the~ processes are
preferably made of rubberized steel or plaEticfi and thc
heat exchanger~ are made of graphite. The operating
temperatures are therefore limited to a maximum of 100
to 1~0C ~o that the maximum final concentration that
can be obtained at an acceptable cost with thi6 method
of vapor condensation ;~ g0nerally 60 to 70% H~SC4,
depending on the temperaturez that can be reached.
Enamelled apparatu~ and heat exchanger~ made of
tantalum are so expen~ive that they are only usQd when
le~s expensive cunstructional material~ can no longer
be u~ed. This i~ the case when sulphuric acid at a
concentration of about 60 to 70% H2504 i~ evaporated to
- . a concantration of 80 ~o 92% H2S04. At this stage of
evaporation, temperat~res of 120 to 195C are required
owing to the low H20 partial pressure~ and the maximum
temperaSure require~ the use of tantalum as construc-
tional material for the heat exchanger on ac~ount of
it re~i~tance. Gla6s, anamel, cast ferro~ilicon and
Teflon are preferred material~ for apparatus, pipec and
pumps, Owing to thR high co t of the tantalum heat ex-
changer, heating i~ supplied ~y ~team at 5 to 15 bar~
i.e. the e~aporation of water is carr;ed out in one
~tage (~ea Winnacker, Kuchler, Chemi~che Tech~ologie~
30 Vol, 2~ 4th Ed., 1982, Carl-Hanser-Verlag Munchen Wien,
page~ 65-72).
Le A 24 623
~ 3~6755
3 23189-6606
THE INVENTION
I~ is an object of the present invention to provide an
economical process for the concentrating dilute sulphuric acid to
concentrations of up to about 80% without the disadvantages of the
process described above.
It has now surprisingly been found -that these
requirements can be fulfilled by a process in which the dilute
sulphuric acid is concentrated in a combination of circulation or
falling film evaporators and a horizontal evaporator.
According to the present invention there is provided a
process ~or increasing the concentra-tion of sulphuric acid to
about 80~ H2SO~ by multistage evaporation, wherein the evaporation
to concentrations of 40 to 65% H2SO4 is carried out in one or more
stages at reduced pressure and temperatures of not more than 120C
in circulation or falling film evaporators with graphite or Teflon
heat exchangers, and one evaporator stage is heate~ by the vapors
from a subsequent horizontal evaporator having a heat exchanger in
the form of a bundle of tantalum tubes in which the sulphuric acid
is evaporated to concentrations of 66 to 80% H2SO4 by evaporation
at a slightly reduced pressure, normal pressure or excess
pressure.
In the process according to the invention, evaporation
of the sulphuric acid is carried out in several stages.
Evaporation to 40 to 65~ H2SO4 is carried out in one or more
s~ages at reduced pressure and temperatures of not more than 120C
in circulation or falling film evaporators with graphite or Teflon
heat exchangers.
J~
1 326755
- 4
Sub~equent evaporation of the 40 to 65% sulphuric
acid to about 66 to 80% H2504 is carried out at slightly
reduced prassure or normal pres6ure or exce6s pre~ure
in a horizontal evaporator having[a heat exchanger in
the form of a bundle nf tantalum tubec~,
In thc proces~ according to the invention, evapora-
tion of the sulphuric acid in the horizontal evaporatori~ prefQrably carried out under cuch conditions that
aulphuric acid at a cQncentration of 66 to 80% ic
di~char~ed from thifi evaporator at temperatures of 160
to 195~C.
Th~ preferred constructional material for the hori-
zontal evaporator i6 glas~ or enamalled steel with
PTFE,
The hea~ ~xchan~ar of the horizontal a~aporator is
- preferably heated with ~team at 8 to 20 bar, The vapors
from the horizontal evaporator are prefsrably used as
fuel for a stage of evaporation in a circulation or
falli~g film evaporator, and the vapor~ from thi~ 4tage
~3y in turn be u~ed a~ fuel for a subsequent ~ta~e. The
optimum number of stage~ of circulation or falling film
evaporator~ i~ determined by economical con~iderations
taking intn account the overall performance of th~
plant, ths energy co~t~ and available cooling water.
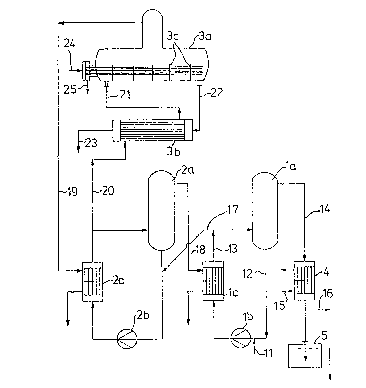
Brief d ecri~t_on of the drawinq
The Fi0ure show~ a multistage avaporation unit for
concentrating sulphuric acid.
Le A 24 623
~ - 5 _ t32~755
Detailcd dascriptisn o~_the drawing
Th~ proce~ according to th~ invention will now be
explainQd with reference the ~igure. Dilut~ ~ulphuric
acid is fed into ~vapor~tor stage (1). The drawing ~hows
a forced circulation evaporator consisting of ~he
evaporator (la~, the circulation pump (lb) and th2 heat
exchanger (lc). Dilute acid (11~ is fed into the
circulating acid (12~ and heated together with this ~cid
in the heat exchangRr tlc) by maans o~ the vapor~ (18)
from evaporator stage (2j. In the evaporator (la) water
is ~vapora~d from the heated circulsting acid S1~
according to the level to which ~he pre~sure is reducedO
The vapors (14) ars condensed in a conden6er (4) by
baing coolcd with water or ~ome oth~r cooling medium
- . (15~ and are then di~chsrged into the immer~ion ves~el
(5~. Condensation of ~he vapors may al~o be carried out
by direct con~ac~ with the cooling madium. Gases (16)
which ca~not be condensed are removed from the sy~tam
by a vacuum pump,
Partially evaporaLed ~ulphuric acit (17) flows from
evaporator ~tage ~) into evaporator sta~e (2) which
operates at a h;~her temperature and a highar pressure _
tharl ~tage 11). The vapor~ tl9) from the horizon~al
evaporator (3a) of evaporator stage (3) are used as ~uel
in thQ hea~ exchangQr (2c). Th~ 40 to 65% sulphuric acid
t20) ~hich is discharg~d from evaporator atage ~2) into
~aga l~ pre~rably preheated in a gla~s or PTFE
h0at exchanger ~3b) by the 66 to 80% ~ulphuric acid (22)
L~ A 24 62
- 6 - 1 ~267 55
which is discharged from e~apora~or (3a) at a tempera-
ture of 160 to 195C. Since ~he acid (21) is ~hen fed
in~o the horizontal evapora~or (3a) a~ approximately
boiling point, ~he ~urface of the ~an~alum h~at QX-
changer can be kep~ quite small. The preferred featur~
of installing vertical parti~ions (3c) along the length
of the hea~ exchanger ~ubes serve6 the sa~e purpose as
it subdividas the op2ration of this evaporator into
~ev2ral stages. The acid (23) cooled to 60 - lZ0C in
the heat exchang~r (3b~ may b~ further cooled by ~he
dilute acid (11) fed in~o the system or by cooling
water. Tha tantalum hea~ exchanger of ~he hDrizontal
evaporator (~a) i~ heated wi~h Gteam at 8 - 20 bar (24).
The energy conten~ o~ ~he steam condensate t25~ may ad-
vantageously by u~ilized for tha evaporation of sul-
_ phuric acid carried out by means of a flash e~aporation
in the fuel space of ~h~ heat exchanger (2c).
The advantage~ o~ tha process according to thainvention lie not only in th~ comparatively low C05t of
- ~he apparatus bu~ especially also in ~he fact tha~ owing
to the high dQw point of the vapors from the horizontal
svaporator, i~ is possible ~n carry out one more ~tage
of evaporation ~han in known preconcentration processes
under otharw;se identicsl condi~ions (in particular
iden~ical temperature of cooling water). The ~pecific
energy requirem~nt i~ th~reby reduced by 20 to ~0%. On8
particular advantage o~er known proce~a~ is that where-
Bi in the known prncesses th~ sooling water ~empara~uresmay b8 too high to enable multistage preconcentration
: 35
Le A 24 623
_ 7 ~ 6755
with utilization of the heat from the vapor~ to be
carried out economically unless the temperature of the
cooling water is lowered by special cooling apparatus,
which involves extra cost, in the process according to
this invention, the increase in the concentration of the
acid from 40 - 65% H2S04 to 66 - 80% H2504 in an evapo-
rator stage using a tantalum heat exchanger enables
evaporation to be carried out in at least two stageseven if the cooling water temperatures are extremely
high, say 30 to 35C. Due to the use of the multistage
horizontal evaporator at the stage of the highest sul-
phuric acid concentration in the process according to
~he invention, the amount of heating surface requîredat this stage is about 20 to 35% less than that required
in forced circulation evaporators so that the use of
~ high quality materials, in particular tantalum, is
economicall~ justified.
The advantages of th6 process accordin~ to the in-
vention compared with the known processes will now be
illustrated by the followin~ Examples which do not limit
the scope of the invention.
3~
L8 A 24 623
1 326755
~.
Sulphuric acid i~ to be concentr~ted from 30% H2S0
to 70% H2504 by evaporation. A comparison i6 made
between the characteristics of tha plants and their
energy requirements for evaporating 1 t H20/h by a known
proces~ (la) and by tha proces~ accordiny to the inven-
~ tion (lb).
1 a) Thz concen~ra~ion of sulphuric acid by evaporation
can only be carried out economically if limited tu two
stages. The ~aporator plant used is a two-stage forc~d
circulation ~acuum ~vaporator plant. ~0% sulphuric acid
is fed into 6tage 1 and concantrated tn 42% H2S04 by
evaporation at llQCo It flows into ~tage 2 where it i
conc~ntrated to 70% H2S04 by evaporation at 80C. The
hea~ exchanger of graphite tubes used in ~tage 1 is
- h~ated with ~team at 3.6 bar and the heat exchanger of
stage 2 is heated with the ~apors from 6tage 1. The
vapors from stage 2 are directly condensed with cooling
water. The 30% sulphuric acid fed in is prehsat~d from
~5C to 38C by tha 70% sulphuric a~id discharg~d frDm
t,h~ plant and the 70% acid i5 thereby cooled to 30C.
1 b) Concentration of the ~ulphuric acid by evaporation
i3 carried out in a three stage plant a~ shown in Figure
1, The acid t213 fed into tha horizontal evaporator ~3a)
has bean heated to 120C in the heat 0xchan~er t~b) ~y
tha ac;d ~22) dircharged from the horizontal ~vaporator
~0 and the acid ~22) i~ at the ~ame time soolad to 80 C.
The 70% sulphuric acicl ~2~ t,hen furthar cooled to
30C by the dil~te acid ~113 which iB thereb~ heated to
45C befora ~eing fed into stage (1). The vapors from
evaporator (1~) are rondensed by direct contact with
cooling water.
Le A 24 623
~ 9 ~ 1 32h755
The ~6% sulphuric acid from ~aga 1 is fed in~o
S stage 2 and discharged as 46% sulphuri~ acid into the
horizon~al evapora~or by way of the hea~ e~changar
(~b),
When the ~wo processe~ (la) and (lb) are compared
(Table 1), the process accordin~ to the inven~ion is
found to have the following advantages:
1. The energy consump~ion i5 only 77% in th~ process
lb a~cording to ~he invention compared wi~h the
known process la. ~hen ~he stQam condensate is usad
for the ganeration of 6team i~ is only 67%,
2. Owing to the higher daw point of ~he vapors ~o be
condensed ~la: 27C, lb: 31C) and the smaller
quanti~y o~ steam, the amount of cooling water re-
~- . quired when ths water ~emperature is ~he same is
only 42%.
3. The hea~ ex~hanga sur~aca and circulation of acid
ara Ie55 in (lb~, thereby par~ly compens~ting for
the ini~ial costs of ~he appara~u~ ~or stage ~.
The~e are in any cas~ low owing to the simplici~y
of construction and high specific evapor3~ion rate
of ~he hori~ontal evaporator u~ed ac~ording to ~he
invan~ion,
4. In the conv~ntional procas~ (la~, an evaporation
~omperature of up ~o 110C is raquired in ~he cir-
c~lation evaporator ~tage 1~ if tha cond~nsation
of vapor~ with ordinary cooling wa~er i3 ~o be
c~rried out economically. This eliminates many
Le A 24 623
1 326755
inexpen iva matsrials for the construction of the
pipas and eYaporator. In ~he proce ~ accord~ng to
the invention, a tamperature of only 85C i8 r~-
quired for a comparable acid concentration (~tage
2).
Example 2
6 t/h of 30% Sulphuric acid is to be concentrated
by evaporation to 96% H2504 and the organic impurities
are to be destroyed by oxida~ion with HN03 at about
330C. The vapor~ ~re to be condensed by indirect
cooling. The temperature of the available cooling water
i~ ~0C.
2a) Solution of problem ~ccording to the ~tate of the
art:
The sulphuric acid i5 concentrated to 70% H250~ by
_ evaporation at 100C in a ~ingle sta~e ~orced circula-
tion evaporator with a he~t exchanger compo~ed of
graphite tu~es. The 30% acid fed into the apparatu~ i8
preheated from ~0C to 46C b~ the 70% acid di6charged
from the apparatus. The 70% acid, which is thereby
cooled ~o 40C, i~ tran~Serred to the stage of high
grade concentration and oxidative purification by the
Pauling/Plinke method ~6ee Bodenbrenn~r fft al, DECHEMA-
Monogr. 86 ~1980) 1~7). ~efore the ~cid i~ fed into the
dephlegmator it is preheated by tho vapor~ from the hi~h
~r~de concen~ratio~. The 96% ~ulphuric acid ~1875 kylh)
~0 di~charg~d from the v~el is indirsctly cool~d to 40C
in a cooler with agitator by mean~ of cool;ny water.
Le A 24 623
- 11 - 1326755
The operating parameters for the preliminary
concentration and the high ~rade concentration are
summarized in Table 2. When the vapors are indirectly
cooled in stainless steel heat exchangQ tubes at the
6tage of preliminary concentration, a rise in the
temperature of cooling water from 30C to 40C i~ per-
mitted.
Multistage evaporation of water for preliminary
concentration in apparatus constructed from inexpensive
materials is possible only if the vapors are condensed
with a cooling medium at a lower temperature or the
vapors from the sacond stage undergo preliminary com
prassion by steam boosters,
2b) Solution of problem by the process according to the
invention: The preliminary concentrstion of sulphuric
- acid from 30% H2504 to 80% H~504 is carried out in tWD
~ages in a plant such as that shown in the Figure but
in which the circulation evaporator sy~tem is a single
stage ~ystem, in contrast to that allustrated~ The 80%
acid discharged from the horizontal evaporator is cooled
from 185C to 92C by the 42% acid which i6 to be fed
into this e~aporator, and the 42% acid ;~ ther~by heated
to 102C. Further cooling to 40~C i~ effocted by means _
of the 30Y acid which i5 thereby heated to 42C.
The comparison shows that the procQss accordin~ to
the invention ha~ the following ~dvantage~:
1. The ~nargy saving i~ 30%. ~hen sondsnsate o
heating ~team i~ u~ed for the generation of ~team
the Raving is 36'X.
Le A ~4 S23
- 12 ~ l 326~55
2. The requiremen~ for cooling wa~er is only 60%.
. The dimensions of the forced circulation evapara~or
sys~em are considerably smaller. The opera~ing
~emperature of 65C ins~ead of 100C enables inex-
pensive ma~erials to be ~sed for the appara~us,
e.g, rubberized containers and pipes.
~ _
Le A 24 623
- 13 - l 326755
o
I` o r~ ~o
~ ,` o U~ ~
o O O ~ ~ o
u) ~ o
I` - o ~
r~ O ~ r o
oo O ~r o
OD
N U~
C~ D O a~ o ~ ~ .
u~ ~ o .~
_ I~ ~r~ .-- ~ N
_ __ . __
_...__
O O O O O ~ O ~ O
Ln CO ~ f ` O ~ N N ~C)
N I` Lr ~)
~11
u, ~r
O O O ~ O ~ O ~D O O O ~;r
Ll') - o ~r o ~ !1~
, ~ .- ~In ~ ~ I` ~ ~ o
~r
O ~ ~ ~rC
Qu~ ~ ~ ~h ~ ~ ~
~ ~ C~ ~ ~ ~ ~ ~ ~ ~ ~ ~ O 1~ _
X ~ E ~ X E~ ii ~ A .Y ~
~r
O
U~
_.
0~
U~ ) C
~ o a
~ ~ O ~ O
d~ O
tJ1 ~ ~ ~
~ ~ ~ ~ O ~ ..
~ ~ cn
~ c t~
O ~ 3 0 O ~ ~ 3 u~ u~
Ul
~- aJ 0 a) ~ 0 h 0 0 X ~ tJ~ t5'
_~ ~ 4
G~ Q. O O u~
_1 E~i ~~ ~ Q, m ~1 ~ t~ Q5 ~1 ~ ~)
Q ~ la ~ O la ~ O to 11;
~ x ~ u u ~ ~o ~u o a1 a~
E~ ~ ~ ~ ~ O C~
Le A 24 623
. .
-~ - 1 4 - 1 32 67 55
OOOO~DIn o
u~ co ~ O a~
o
..
o o
CO oo
. , ~ ~~ o
n ~ ~ o . ~ In o ~ ~ u~ o o
I` ~r o u~ ~ ~ ~ ~ o ~ ~
. ~ ~ o , , o o~
o o ~ o ~ ~ ~ o o
O ~ ~O O er ~ ~r ~ o
o~ ~ ~ ~
~ .
_ _ .
_ .....
O O O ~9 0 0 el~
o a~
u~ rrl O ~ ~ ~
I` o ~ ~r
oo o o o u~ o o ~ ~u~ ~ o u~ ~r
o ~ o o 1~ ~ o o o
,_ o ~ ~
. ~ ~ ~ h ~ ~ ~ ~ S
.Y 3 `1 E ~ X ~ ~ ~ ~ ~ E
dP dP
~: :
:: :
~ h
g h o o Ç ~
`~ ~ ~ O c~ 3
h rl q) .
~ N r1
.,~
~ t~ O ~C ~ 0 U~ ~ ~ ~ O
t~l a~ O ~a hO (1~ X~) ~ tJ~ cn 1 ~
O ,~ a) ~ h ~
R E~ 1~1 rl E~ O rl 0 ~ O al
~ X ~ t~ o 4~ O~rl O
E~
Le A 24 623
-