Note: Descriptions are shown in the official language in which they were submitted.
1 326q30
1 61109-7316D
:
' .
CURABLE EPOXY RESIN COMPOSITIONS
'
FIELD OF THE INVENTION
. . .
This is a divisional of Canadian application, Serial No.
, 460,012 filed July 30, 1984 and relates to improved epoxy resin
. compositions. In addition, it relates to curable epoxy resin
compositions comprising reinforcing filaments and epoxy
prepolymers combined with aromatic polyamine curing agents.
q~
:1
. ~
~ ,. : . - -:, .
1 326930
:
BACRGROUND OF THE IN~ENTION
;~
Epoxy resin compositions are useful to encap-
sulate electronic components, and as structural adhes-
ives, and the like. Reinforced epoxy resin compositeshaving high strength to weightratios have found extensive use
in the aircraft and aerospace industries, and in other
applications where strength, corrosion resistance and
light weight are desirable. For instance, fiber resin
matrix materials have replaced aluminum and other metals
in primary and secondary structures of modern military
and commerical aircraft. Sporting equipment such as
tennis rackets and golf clubs have also adopted fiber
resin materials successfully.
Epoxy resin compositions and fiber modifica-
tions are abundant. Since the advent of fiber resin
matrix materials, much effort has been expended in
improving their properties and characterisitics, includ-
ing the development of many different curing systems.
Amine and polyamine curing agents have receivedwide acceptance, but the toxicity, low solubility, high
exotherm and variable curing rates seen with the most
commonly used amines, such as m-phenylenediamine, 4,4'-
diaminodiphenyl methane and 4,4'-diaminodiphenyl sulfone,
has made further improvement desirable. In particular,
for aircraft structural applications, epoxy resins cured
with available curing agents are either too brittle or
do not have sufficient strength and stiffness under hot/-
wet conditions. It is disclosed in U.X. Patent 1,182,377,
that certain aromatic polyamines are effective as curing
agents for a variety of polyepoxides, and the resulting
cured compositions are useful as films, moldings, coat-
' ~
:: . : . . :
~-: , . ~- .
. . , . .
~: . - .. . . . :
1 326930
- 3 -
O
ings and glass-reinforced laminates. There is no
indication in the properties presented in the U.R.
Patent that the curing agents exemplified therein will
produce the combination of toughness and strength under
S hot/wet conditions essential for use in the above-
mentioned structural applications.
, In U.S. 3,932,360, diamine cured polyurethane
products are described, in which the diamines are of
the formula, e.g.,
H2N~ O ~CH2)n ~NH2
wherein n is an integer from 2 to 12. This '360 pa~ent does
not deal with curing compounds having more than one
epoxide groups per molecule.
; In Gillhan et al, Organic Coatings and Applied
Polymer Science Proceedings, Vol. 46, p. 592-598,
March-April, 1982, polyepoxides cured with diamines of
the immediately preceding formula (n is 3), are
f 2S descri~ed.
.', .
The present development relates to curable
epoxy resin compositions. In one of its aspects, it
provides fiber resin matrixes comprising reinforcing
filaments in a heat-curable epoxy resin composition
comprising an epoxy prepolymer and a novel family of
~1 aromatic polyamine curing agents. No member of this
novel family of curing agents is specifically exemplified
I in the U.K. Patent. The invention provides neat resin
formulations having, after cure improved physical
~.j
~ f
~: . . - :
.: : ,
.. ~ ,, , - . .
:- -
1 326q30
4 75365-5D
propertles, e.g. hlgher elongatlon and satisfactory hot/wet
modulus. The epoxy composltlons of the present lnventlon, cured
, wlth fllaments, exhlblt lmproved lnterlamlnar toughness and
resldual compresslon strength after lmpact, while malntalnlng
compresslon strength under hot/wet condltlons.
' SUMMARY OF THE INVENTION
The present lnventlon seeks to provlde lmproved epoxy
; resln composltlons.
The present lnventlon also seeks to provide a flber
matrlx compositlon that affords satlsfactory compresslon strength
; over known matrlx formulations, especlally under hot/wet condl-
i tions, and lmproved compresslon strength after lmpact.
Accordingly, the present lnventlon relates to flber
i relnforced heat-curable epoxy resln composltlons comprlslng a
siliceous reinforcement fllament and
~1) an epoxy prepolymer or comblnatlon of prepolymers
~ having more than one epoxide group per molecule, an~
¦ ~ii) an amount effectlve to promote cure of an amlne-
functlonal curlng agent or comblnation of curlng agents selected
from those of the formula,
R NN~ O (CH2)z 0 C O_NHR
' ~`~
....
,~
:,. , ::
:. ~ :' ' : . , ' ., , . :
1 3 2 6 ~ 3 0 61109-7316D
wherein Rl is hydrogen or methyl, and z is an integer of from 2 to
12, with the proviso that when Rl is hydrogen, the amine-
functional curing agent is not present in greater stoichiometric
amounts than the epoxy prepolymer.
It is among the features of this aspect of the invention
to provide such compositions in filled and/or reinforced, e.g.,
glass fiber reinforced, embodiments which are useful as prepregs,
for example, to make laminates and other structual shapes in
accordance with procedures known in this art.
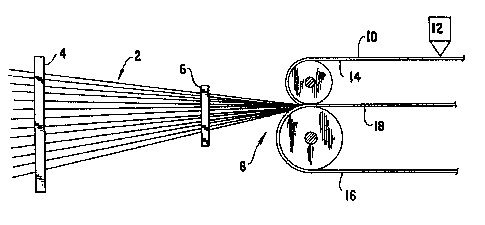
DESCRIPTION OF THE DRAWINGS
FIGURE 1 iS a schematic of one method for preparing a
fiber resin matrix prepreg tape of the present invention.
FIGURE 2 is an enlarged cross-sectional view of a strip
of the fiber resin matrix prepreg tape of the invention.
.
~i,
.
.
~,,
.. . .
1 326930
- 6 _
O
FIGURE 3 is a graphical representation compar-
ing hot/wet compressive strength versus dry impact
strength for composites according to this invention
with state-of-the-art composites.
,.,
DESCRIPTION OF THE PREFERRED EMBODIMENTS
:
In general, the resin compositions of this
invention are prepared by mixing the polyepoxide com-
pounds with the polyamines of the above-mentioned
formula in conventional quantitative ratios, e.g.,
- 1 epoxide equivalent to 0.3 to 3.0 NH- equivalents,
preferably 1.0 to 3.0 NH- equivalents, and especially
preferably 1.5 to 2.5 NH- equivalents, optionally with
heating, e.g., at a temperature in the range of 30 to
300~C., preferably at a temperature in the range of
80 to 180C., until a melt is obtained. The melt can
then be poured into a mold and reacted, for example,
;~ for 2 hours at 135C. and then for 3 hours at 180C.,
to form moldings showing outstanding mechanical and
electrical properties. The NH- equivalents is the
quantity of aromatic polyamine in grams in which 1 gram -
atom of hydrogen combined with amine nitrogen is present.
-~1 25 Fillers, pigments, dyes, reinforcements, suchas glass fibers or woven cloths, plasticizers, and mixtures
thereof, may be added to the epoxy resin - polyamine
composition before the reaction in order to modify
ultimate properties, in known ways. Applications can also
be made by trowelling, brush coating, immersion or dip-
coating, spraying and other convenient method. Catalysts,
such as boron trifluoride - organic amine adducts, and
the reaction product of toluene 2,4-diisocyanate and
dimethylamine can also be included, in quantities of
35 ~
"
. .
'
".. ,....... ,: :
. -, . . :
~;, .
... . .
. i~ . .
....
`~ 1 326q30
~ - 7- ~
O
, . . .
from e.g., 0.1 to 5% by weight based on the resin -
polyamine, to accelerate curing.
The fiber resin matrix compositions according
to the present invention can be prepared by embedding
filaments! e.g., glass fibers and/or non-siliceous
filaments in a curable resin composition to form a fiber
resin matrix which can be manipulated and cured to a
solid composite. ParticuIar selection of the filament
material, epoxy prepolymer and curing agent, as well
as including optional ingredients such as fillers, dyes,
catalysts, processing aids, etc.,can give a range of
curable compositions heretofore unknown in the art and
exhibiting improved physical properties over known
materials.
Glass filaments useful herein are well known.
, The non-siliceous filament component may be of any non-
glass, non-silicon dioxide-containing material which
improves the strength or other physical properties of
the curable epoxy resin component (described infra.).
Such filaments include, but are not limited to, filaments
comprised of carbon, graphite, silicon carbide, boron,
aramid, polyester, polyamide, rayon, polybenzimidazole,
polybenzothiazole, metal-coated such filaments, for
example nickel-coated and/or silver-coated graphite
fibers and filaments, or combinations of such filaments.
Fibers (woven or non-woven), tows or mats of such fila-
ments, or tapes (unwoven, flat bundles of the unidirec-
tional filaments) may be employed as desired. In appli-
cations dem~ing high stiffness to weight ratio or shear
strength, carbon fibers, graphite filaments, polyaramid
~ filaments or nickel-plated graphite filaments, as dis-
35 closed in assi~nee~s ~nPn~; n~ ~n~ n ~ n ~
.,' ,
~,
.~ ;' ' . . ~ . -: .
',',: . ,:
. . .
-81.326930
-- 423,551 are mDst preferred. ~-
-~ The epoxy resins suitable for the present inven-
~ tion are compounds having more than one epoxide group
; 5 per molecule available for reac~ion with the primary and
secondary polyamines of the present invention (described
infra.). Such epoxy prep~lymers include but are n~t lLmited to
polyglycidyl ethers of polyvalent phenols, for example
- pyrocatechol; resorcinol; hydroquinone; 4,4'-dihydroxy-
diphenyl methane; 4;4'-dihydroxy-3,3'-dimethyldiphenyl
- methane; 4,4'-dihydroxydiphenyl dimethyl methane; 4,4'-
~ dihydroxydiphenyl methyl methane; 4,4'-dihydroxydi-
~A phenyl cyclohexane; 4,4'-dihydroxy-3,3'-dimethyldi-
,",A~ phenyl propane; 4,4'-dihydroxydiphenyl sulphone; or
' 15 tris-~4-hydroxyphenyl) methane; polyglycidyl ethers of
the chlorination and bromination products of the above-
mentioned diphenols; polyglycidyl ethers of novolacs
(i.e., reaction products of monohydric or polyhydric
phenols with aldehydes, formaldehyde in particular,
~ 20 in the presence of acid catalysts); polyglycidyl ethers
i~ of diphenols obtained by esterifying 2 mols of the
sodium salt of an aromatic hydroxycarboxylic acid with
l l. of a dihalogenoalkane or dihalogen dialkyl
ether ~U.X. 1,017,612); and polyglycidyl ethers of poly-
phenols obtained by condensing phenols and long-chain
halogen paraffins containing at least 2 halogen atoms
, (U.X. l,024,288).
Other suitable compounds include polyepoxy
compounds based on aromatic amines and epichlorohydrin,
for example N,N'-diglycidyl-aniline; N,N'-dimethyl-N,N'-
diglycidyl-4,4'-diaminodiphenyl methane; N,N,N',N'-tetra-
~; glycidyl-4,4'-diaminodiphenyl methane; and N-diglycidyl-
, 4-aminophenyl glycidyl ether. Special mention is made
.~ ,.
.i, - . , . - .
.. . : -.
: I 1 326930
g
of N,N,N',N'-tetraglycidyl-1,3-propylene bis-4-
aminobenzoate.
Glycidyl esters and/or epoxycyclohexyl
: 5 esters of aromatic, aliphatic and cycloaliphatic poly-
carboxylic acids, for example phthalic acid diglycidyl
ester and adipic ester diglycidyl and glycidyl esters
of reaction products of 1 mol of an aromatic or cyclo-
aliphatic dicarboxylic acid anhydride and 1/2 mole of
a diol or l/n mol of a polyol with n hydroxyl groups,
or hexahydrophthalic acid diglycidyl esters, optionally
substituted by methyl groups, are also suitable.
Glycidyl ethers of polyhydric alcohols,
. 15 for example of 1,4-butanediol; 1,4-butenediol; glycerol;
~' l,l,l-trimethylol propane; pentaerythritol and poly-
ethylene glycols may also be used. Triglycidyl isocyan-
,; urate; and polyglycidyl thioethers of polyvalent thiols,
3 for example of bis mercaptomethylbenzene; and diglycidyl-
trimethylene sulphone, are also suitable.
Preferably the epoxy prepolymer component will
be selected from compounds having the idealized formula:
~G~o)
l c
,
.
. . , ~ ............ .
, .. ~ :
;: :
1 326~30
-10 -
o
and halogen and alkyl substituted derivatives of such
compounds, wherein c is 2, 3 or 4 and equal to the
valence of Q; Q is a divalent, trivalent or tetravalent
radical; G is -O-, NR'- or -N-; R is hydrogen or
alkyl; and d is 1 or 2 depending on the valence of G.
. The most preferred epoxy compounds will
include the following:
_(~N~(C~2~x~-N~)2
wherein x is an integer from 1 to 4, available commerci-
~ ally (where x=l) as Araldite ~ MY-720 (Ciba-Geigy);
,:1
i,~
', 30
.,
~ 35
..
' ~ ' :~ '' .
.~ '
.. :
1 326930
11
~ ~ ) 3
; - available commercially as XD7342*(Dow Chemical);
J
.
10 0~/\0-~ <~}/~ ~
CH3
;~.' available commercially as DER331*(Dow Chemical) or
.,3 EPON 828 (Shell);
.,,~,
1 ~5 ~ ~ ~
~ O
0~
~ 35 available commercially as EPON ~ 1n?7 ~C~A17~
;
.3
..
i ~;1 . . . . .
;: . . .
. . .
1 326930
12 -
X~ )YOy
~ CH2 f '~
n
,~ ,
,, wherein Y is 1 or 2, X is -O- or -N-,R3 is H or C~3
'l 15 and n is 2 to 8.
~,
Compounds in which X is -O- are available as
- a mixture under the tra~rk DEN-438 from Dow Chemical
Company.
Also preferred are triglycidyl ethers of
meta- and para-hydroxyaniline, e.g., represented by
t'he formula: '
O ~ O ~ N 4 ~ )
' These are available under the ~rademark ARALDITE~ 0500,
' 0510 from Ciba-Geigy.
The polyaminc curing agcnts arc of "t,hc fornula:
X{~ N)lR)
3 5
:~.
-; ~, . .. :-
.
:', . - . ~
^., - - -
1 326q30
- 13-
o
wherein a is 2 or 3, R is hydrogen alkyl or aryl, and
X is a divalent or trivalent organic hydrocarbon, hetero-
interrupted hydrocarbon, or substituted hydrocarbon
radical or -N- . They may beprepared from correspond-
ing starting materials, e.g., nitro compounds, byreduction, for example, according by methods described
i in U.K. Patent 1,182,377. In addition, commonly assigned
.:,
Canadian application No. 460,022, shows an elegant
method for N-methylation, using succinimide and formal-
dehyde with the primary amine, followed by reductive
:~ cleavage.
,,,'
~ Preferred curing agents are compounds accord-
,;'f 15 ing to the a~ove formula in which R is hydrogen, Cl-C3
alkyl, or phenyl and X is a divalent or trivalent
I radical of valence a, selected either from (1~ a divalent
group consisting of -(CH2)y~, wherein y is an integer
of from 2 to 12, -(CH2CH2OCH2CH2OCH2CH2)-,
~ > ~ C ~
-CH2~)-CHZ-~
.,
;l .
1 3S
.,
.:, .
.
, ............ . . .
,'.' ~ :
~::. . .. .
``.'. ' : ',~ . : '
''. : ~- ' ~ ' ~ .
~: .
,. . .
.
-
` 1 326930
14
~` .
..
-CH2 ~ CH2~ H2~C~cH2~ , or (2)
C 3
a trivalent group consisting of -N- and -(CH2)n-CH-
, (CH2)m- , wherein n and m are the same or different
.~, 10 integers from 1 to 4.
;~ More preferred curing agents are the following:
H2N- ~ -C-O-~CH2)z-O-C- ~ NH2, wherein
z is an integer of from 2.-.to 12, preferably 2 to 6,
~--C-O-~CN2)z~0~C~
! H2 NH2
. wherein z is an integer from 2 to 12, prefera~ly 2 to
6,
.,~ .
H2N- ~ ~-O-Y-O-C ~ NH2 ~ wherein
2 2 2 2 2 2
., .
' 35
.
.,. . , - .
:; :
..
:...................................... .
. ~ ,
~,
1 326930
~ 15 ~
., ~
~CH~ CH2-<~ CH2
i 5 CH3
C 2 ~ -CH2- 1_CH2_ , -O ; or
CH3
~, IN2-o-c-~NN2 CN2-O-C-~N 2
CN-O-C-~NN2 : CN-O-C-~Nd2
:~ CH2_o_C_(~NH2 CH2-0- -(~NH2
:~ 20
O-C~N21 : and
N ~-C O~CN2~Z <~I-CN3, wherein
. - ~
s~
. ... .
.ii ~ . .. . ~ . .. -
1 326930
. .
.:
-16 -
O
. .
. z is an integer cf from 2 to 12, preferably 2 to 6.
In the most preferred compounds, the primary
diamine will include one or more of a compound of the
formula:
.,
:.! R HN~e-O- (CH2) Z-O-c~N~Rl
. ,,
wherein Rl is hydrogen or Cl-C6 alkyl, e.g., methyl, and
z is an integer of from 2 to 12, preferably 2 to 6, and
most preferably 3. Also contemplated are the use of such
compounds in combination with other conventional poly-
amines such as methylene dianiline, phenylene diamine,
1 15 and the like.
"~
One method of forming the fiber matrix composition
, of the invention is illustrated in the drawings. As
seen in FIGURE 1, the basic fiber matrix material is
; 20 produced by delivering fiber 2 through conventional
eyeboards 4 and 6 to a pressure roller assembly 8. The
resin composition is coated in a layer 10 from a conven-
-.~, tional film coating applicator 12 onto a substrate
.' such as release paper 14 and passed through the pressure
~ 25 roller assembly 8. Release paper 16 is also delivered
') to the pressure roller assembly 8.
The pressure rollers 8 are set at a temperature
and pressure for imbedding the fibers 2 in the resin
. 30 layer 10 to form a fiber matrix composition 18. Practice has
taught that a temperature in the range of 190F. and
,' pressures of one thousand pounds over fifteen inch
centers are suitable for producing fiber resin prepreg
;;~ tape 18.
~ 35
... .
I 326930
_ 17
,.~
~. O
The fibers 2, the substrate 14 with resin
layer 10 and the release paper 16 are delivered to the
; pressure rollers 8 and passed therethrough at the
- rate of 5-20 feet/minute.
¦ The feed of fibe_ 2 and resin layer 10 to
the pressure rollers 8 is selected to produce a fiber
matrix of about twenty to sixty weiqht percent resin
and about eighty to forty weight percent fiber. For
example, one hundred twenty spools of 6K carbon fibers
are delivered within a twelve inch width to the
; pressure rollers 8 with a layer of resin 0.009 to 0.0013
pounds per square foot. The resulting fiber resin
matrix 18 results in a generally parallel array of
fibers, shown by FIGURE 2.
Fillers, pigments, dyes, curing catalysts and
other such conventional additives and processing aids
may be added to the fiber matrix compositions of the
invention before curing to influence the properties of
the final resin composite. In addition, polymeric
additives such as the butadiene-styrene-acrylonitrile
core-shell polymers and the like can be included for
their known effects on polymer properties.
,
,~ .
.. . . ... . . , -
. .
. , . .. - . . :
:~ : , - .. .. . .. , ~ .
t 326930
- 18-
O
The following examples will illustrate the
practice of the present invention and are provided by
way of demonstration and not by way of limitation.
EXAMPLES 1-5
3 The following procedure is used to prepare
Z and cure neat resin compositions: the epoxide pre-
polymer and the polyamine component are mixed at 135C.
, 10 for 10 minutes, and cooled to 100C, the catalyst, if
any, is mixed in, and the mixture is degassed for 10
minutes. The liquid resin is then poured into a mold
and cured for 2 hours at 135C and for 3 hours at 180C.
Properties are determined by the following procedures:
The flexural test is described in ASTM D-790, Method I.
Dynamic mechanical analysis was performed on a Dupont
981 Dynamic Mechanical Analyzer, and Tg was defined as
the temperature at which the loss tangent, tan ~, is a
maximum. ASTM D4065 test method covers this type of
Tg measurement. Conditioning before testing is
' described by the phrases "wet" and "dry". "Wet" refers
to conditioning for two weeks at 71C, immersing in
distilled water, prior to testing at 93C. "Dry" means
testing a sample, as prepared, at 2~ C. The formulations
tested and the results obtained are set forth in Table
I:
. ,
,,~
3 30
~ .
i
~ 35
!
:,~;....... '' ' ' :
.~: . ' ' . ': '
- 1~ 1 326930
o u~ O U~ ~ a'
~i Ul O a~
o o o
; ~r l o -- o ~ ~r
o o ,,
~; ~n ~ ~ ~ o
ct~ ~ I` Li U~
I ~
., i
~ I O O ~ ~ ~i
U.i O o
,;, W,
`' E I~ _i o ~D
~ *t~ ~ ~ O
: ' ~~ I O O _1 ~i
.'1 0 O O
! u~ Li ~ ~ _i
-' a O ,~ . o ~D
z I I ~ t) r~
~¢ ~ l -i o - io o t~i x t~i
u~
i 11'1 ~ ~ o `~1 1
~i ~ o ~ ~
~ ~l l ~ l l -- --i ~ l~ ~7
o ~ -i o -i o o ~ ~ t~i
e,
7r
O U~ i 'D O O
~ o l~ u~ ~
a ~ o o o_l ~r ~
u~
: ~ ~O l~ ~ . .
z ~ I
:, ~, _, _~ o o
~3
:~ ;,
l~ X - ~ ~ri
;, ~ u ~ ~ o ~v e ~ _'~ 3
., " e ~ ri ~_ O ~
lU ~ri J 04 0 !~ e ~ ~ s l I i Li I i
_i ~ r i ~O 3 ~a ~O ~ ~
:1 Wii & ~ ,.1~ & O O
mri 111 Ul ~ .ri N ~a
~I ~¢ J I r~ ~ e ~ ~ H ,~
,! E I ~r ~_ ~ri ri ~ ~ ~ 10 H Ui 11~
~j ~U X e ~ o ~ ~ , .ri O
;i ,~ o ~ ~ ~ e o ~J ~ tP ~, ~ Li
æ ~ ~ ~o J ~ -ri ~ .C I
H ! ri ri ~ ~U ;a P~ e J~ u~ u, ~ è u o
;j W H ~ o i O J ~i ri Cl~ 8 ~ ~ ~Hi i ql '10 ,~1~ --i O ~1
., ~, u., - e ~ ~ ~ ~ a-- ri 010 ~ ~ Li ~i ~ ` ~
P1 O Zri ~11 ri ~U I I ~ Ui ~ ~ O ~ ~1 0 ri b~
.~ 2 ~4~ .e ~~ e N - Ui U ~i 111 P~~ U~ Ui g E~
~ 2Z ;~ a .ri ~ Z ri ~ ri U O
X O ~ ri Ll 0 ~ .a Ql ~O ~ ~;
a E~i'~ Z 0: P~
., .
,.l . ,
.;
.'i. , ` " ' ' ` ': ., , ' , ' ~ ~J '.
"',' ~ , ' ~ ' "
:,',,' . ~ ' ' : :
1 326930
:.
- 20-
,~
O
The data demonstrate that when the composi-
tions of this invention are cured and tested, in com-
parison with a standard curing agent, para-diaminodi-
phenyl sulfone,flexural strength is increased, strain
is increased, and work-to-break is increased. Some
3 properties are decreased oniy slightly. In addition,
Tg is reduced by only an average 10%. The advantages
of the compositions of this invention are thus shown.
EXAMPLES 6-8
Three fiber resin matrix formulations were-
prepared from the following materials:
-!
component (a~ CELION~ 6X high strain
qraphite fiber
component (b)(i) ARALDITE~ MY720
EPON~ 1031 (see formulae,
suPra.)
~curing agent) (ii) trimethylene bis-(p-
. aminobenzoate)
' (optional curing agent) diaminodiphenyl sulfone (DDS)
? polymer modifier acrylonitrile-butadiene-styrene,
core-shell polymer
catalyst toluene-2,4-diisocyanate reaction
product with dimethyl amine
filler fumed colloidal silica ~Cab-O-
Sil* M-5 Cabot Corp.).
j *Tra~rk
.1, 1 '
?~
I-; ` ' ' .:
,~'' . , ' :,, ' :
.. . .
.. ,; , , , ~. ,
;,' ' : . ,,: `-: ~' ~ :, ~, :
l": ' ~ " . `' ' . . : . : '
1 ~26930
_ 21 _
: O
` Using an apparatus shown generally in Fig. 1,
prepreg tapes of the structure shown generally in Fig.
2, were prepared:
,j .
- 5 EXAMPLE 6 7 8
(28%) Resin mixture (parts bY weight)
N,N,N'N'-tetra(glycidyl-4,4'
diaminodiphenyl)methane 80 ao 80
Tetraglycidoxy tetraphenylethane 2020 20
10 Trimethylene bis-(para-
ami~obenzoate) 44 44 65
Diaminodiphenyl sulfone ~ 20
Polymer modifier* -- 5 --
1~ Catalyst
Fumed silica 6 6 6
,, , .. _
(72%) Filament(parts by weight)
(6K graphite fibers having a strain to
failure of about 1.5%)
:, . .
i3 * BLENDEX 311, Borg-Warner Co (Tradem~rk)
.; .
These samples were cured and compared against
~; commerical epoxy resin matrixes. rhe sheets of resin
involved were as follows:
Uni-Comp : 8 sheets [0]
Quasi-Comp : 16 sheets[(~45/0/90)2]s
i, Comp./Impact: 36 sheets.[(+45/0/90/0/90)2_
',! /+45/0/-90/l45]5
~1 .
,~i3
~ 35
.'
,................................ . .
:, , , . . ,
. ,~, - . . .
.. :.; , - ~ ., .,- :, . ,
... , . . , , -
., . ,, - .
1 326930
-2~
O
The compressive strength was measured on a modified
ASTM D695 specimen described in D.H. Woolsencraft et al,
Composites, Oct., 1981, pages 275-280. Both unidirec
~ tional and quasi isotropic laminates were tested by
! 5 this method. Compressive strength after impact was
measured as described in B.A. Byers, NASA Report No.
CR 159293, August, 1980. This property is tested by
subjecting a cured laminate specimen to 1500 in.-lb.
per inch of nominal thickness impact with a 0.62 diameter
- 10 spherical tip impacter while supported by a rigid base
~e.g., 3x5 in. steel cutout). The panel is then tested
in compression. The results are set forth in Table 2,
as follows:
, .
,,
.'
~ 25
;,
~ 30
q
~ 35
,:1
.,
.. - , .......................... .. . .
~,::`. . : , :
,:
, . . ..
:-;
- l ~
C -23- 1 326930
Z~
~1 ~
V7
~ ~ ~ U') O o U~ ~
~ ~¢ ~ I OD O
U~ H ~
p, ~ O
~. E~ o
O ~ U~
H ~1 ~ _I ~ _1 ~' 11~ I CO a~ I
~ a~ ~ I` co ~ I~ el' I 1~ ~ I
01
~ J~
P~ 0
l U er I
,~
,
,, 0
,~ ." ,~ a~
O _
1' W ¦ u~ ~o 0 0 ~1 0 ~ ~ ~ 3 h
' m5~ ~ ~ r~
,' ~ a~ ,1 ~ .C 0
:; ~ ~ ~
.1 1 ~ ~ I o I o I I ~ ~ ~ 0
:' o o
u~ ~
'' ~n ~n
P~ ~ CX
t,) ~a 3 ~ 3 ~ ~ .a ra 3 ~0 3
Q~ C E
a~ o
^, ~ E ~ j~ o E O u~
t~
4~
,~ ~
.,, ~
" ~ ' ' '` '.: ` ' ', ~ ` `
t 326930
_2~
Some of the foregoing data are represented
graphically also in FIG. 3. The data demonstrate that
reinforced compositions according to this invention
(Examples 6 and 7) have higher compression strength
after impact than two of the three commercial composi-
tions, and better hot/wet compression strength than one
of them.
:: EXAMPLES 9-11
,~
~ 10
: Using the general procedure of Exa~ple 1,
compositions were prepared and tested. The formulations
used, and the results obtained are set forth in Table 3.
.
:
;~
: 20
,
, ,
~, 25
.1
,~
, ~ .
.3
.~
't
.'~ ,
'. ' ' , . . ~ . ~, . .
. . - .
"".
::', ~ ' .
- 25- 1 326930
;
--~1 0 0 0 0
U~
H
~: o l o I co o I I o
H
U~
~ . ~
,, O o
H a~ ¦ O I CO O I --~ I Z
,. E-'
.' ~n o--( )=o
~0
O I ~ ~ ~
V ~ O N Y 1~ 1 U ~ U--U--~J
X 1 ~ 1
oa~ ~ ~ .~ _ -
.. 3 ~ d a. _ ~ ~ U l _)
'~ ~A ~ O ~ K ~--
~.! ~~ ~ I >~ Il~ C,) U~ ~ ~ t~ ¦
,~uJ ~tl o Q ~e ~ ~
~9 ~ 0 ~ ~ 0-~ ~ ~ ~ ~ 5:~ ~ o
rl ~ .a c,~_~
~ " o u
,, ~ ~ c~
o ~ ~ ~ (O
~ I ~ O ~
; E~ ~ . o o.,~ ~-rl ~ ~ ~ ~_
u~ Z U ~ e e e~ ~ ~r ~ , ~
d ~ ~ ~ O )=o
Z ~ ~ 0 0 ~ ., ~Z /
x o ~ al o 3 0 J~
Z a E~ m ~ _
: ,.
.,:. . : : :. : .
_26_ 1 326~30
.~,
. _ o
~ O ~D ~f. ~.f O r~
f.~
- O _I I o o ~, ~ In
,' _ o
i U~
g
: . H
~ Q n ~ J ",~ O
:' U~f f.
O
Z _I . N
~. ~ . ,,.~ .
C
:~ kf
. H
,1 0 ~ ~ 1~ f~
:, H Il~
E~-f . . f~ ~ f
,, U? f~¦ O O f.~ , ~
f~
;,,) O
:, L
~,,
O
- L
: 3
~ f ~ ~f ~
E~
H U~ ~
~ ~ ~f~.
U~ ~ J '*' Q.C~,,
1~
~ H f~ ,y ~, o
::f
~y
1 326q30
-27 _
EXAMPLES 12-13
~ _ _
By the general procedure of Examples 6-8,
the resins of Examples 9 and 10 were made into prepregs
with graphite fiber (CELION~ high strain graphite fiber).
The prepreg had a resin content of 28% and a reinforce-
ment content of 72%, by weight. Thirty six plies were
consolidated under heat and pressure into a unidirec-
tional laminate at 15~ F. for 1 hour and 350F. for
- 10 two hours. Compressi~e strength after impact was
measured 1500 in.-lb./in. thickness, with the following
results: Example 12, 34 ksi, and Example 13, 33 ksi.,
demonstrating excellent properties in this respect.
.
EXAMPLES 14-17
The general procedure of Example 1 was used
< to prepare and test compositions according to this
invention which also include, ~ethylene dianiline
i~ 20 bismaleimide. The formulations used and the results
~; obtained are set forth in Table No. 4.
~1
~ 25
:'~
;'',
~ 30
J
~ 35
.:
.... . . . .
1 326q30
_28_
TABLE 4: EPOXY COMPOSITIONS AND PROPERTIES
EXAMPLE 14 15 16 17
: COMPOSITION ~parts by weight)
N,N,N',N' tetraglycidyl-4,4'-
diamino ~iphenyl methane60 60 60 60
Diglycidyl ether of bisphenol-A 40 40 40 40
Trimethylene bis-~p-amino-
benzoate 50 50 50 50
: Methylenedianiline bis
; 10 maleimide * 5 10 15 20
PROPERTIES
Modulus, MSI dry 0.46 0.48 0.470.49
Strength, KSI dry 23.2 21.5 22.923.0
:. Strain, % dry 7.3 6.1 6.66.2
:~ 15 Work-to-break,
in-lbs./in.3 dry 1070 810 910840
Tg, C. dry 207 208 207206
._
20 ¢N ~C112~)
O O
~,, '.
I 30
.~
~,
~ 35
:j~
. , . :
?
, .: . ` ,
1 326930
-29-
EXAMPLES 18-21
The general procedure of Example 1 was used
to prepare and test compositions according to this
invention, substituting different epoxy resin prepoly-
mers:
( /I~)N N~O _~)
.
:'. ~ ~ O O
~ TGPC ~ ~ C- ~ CN2 ~ C ~ N ~ 0)7
"~ O
E RL- 4 2 9 9 --- ~ C 20C ~CN ~ C-O-CII z~CcO
2 5 ARALDI TE 0 5 1 0- - o~/\ O--(~ N ~ ) 2
''I
~ 30
,~
~ 35
.~
.:, .
.,., ~ . , .
1 326930
_30_
The formulations used and the results
obtained are set forth in Table No. 5:
" .
TABLE No. 5: EPOXY COMPOSITIONS AND PROPERTIES
5 EXAMPLE 18 19 20 21
COMPOSITION (Parts bY weight)
: N,N,N'N'-tetra~lycidyl 3,3'-
diamino-diphenyl sulfone 100
N,N,N'N'-tetraglycidyl tri-
10 methylene bis-(p-aminobenzoate) ~ 100 - -
Bis-(3,4-epoxy-6-methylcyclo-
hexylmethyl) adipate ~ ~ 100 100
:~ N,N-Diglycidyl-4-aminophenyl
glycidyl ether - - - 100
~rimethylene bis (p-
15 aminobenzoate) 51 38.5 37.4 62.8
PROPERTIES
Modulus, MSI dry 0.630.55 *NA 0.53
wet 0.350.18 NA 0.26
Strength, KSI dry 23.023.4 NA 21.3
Strain, % dry 4.0 5.5 NA 5.6
Work-to-break,
in.-lb./in 3 dry 485 770 NA 740
Tg, C. dry/wet 240/223 - NA /156
~ Not yet available
~!
`l 30
,,
,`.
. .
.~ .
:: ~ - , ` ` . , .
1 326930
. . .
_31 _
: EXAMPLES 22-24
:
The ~eneral procedure of Example 1 was used
to prepare and test compositions according to this
invention, substituting an N-methylated curing agent.
~ The formulations used and the results obtained are
- summarized in Table No. 6:
TABLE No. 6: EPOXY RESIN COMPOSITION AND PROPERTIES
EXAMPLE . 22 23 24
- COMPOSITION (equivalents)
'J
N,N,N'N'-tetraglycidyl-4,4'-
diamino diphenyl methane - 1.0 1.0 1.0
N,N'-dimethyl-~rimethylene
bis-(p-aminobenzoate) 1.0 0.8 0.6
PROPERTIES
Modulus, NSI dry 0.49 0.49 0.48
wet 0.19 0.22 0.25
;I Strength, KSI dry 22.8(y)* 21.7~y) 21.9(y)
~A 20 Strain, % dry 7.1(y) . 7.1(y) 7.5(y)
~1 Work-to-break,
,~ in-lb/in.3 dry >1698 ~1600 >1470
:~, Tg, C. dry/wet 158/120 165/- 163/-
:.
3 ~ (y) yield
,,
.~
:~,
.~3
` 30
,.,
:.
- 35
:s
'.' ,
:.
1 326930
- 32-
:, O
EXAMPLES 25-29
The general procedure of Example 1 was
repeated, increasing the ratio of amine equivalents
to epoxide equivalents. The formulations used, and the
results obtained are shown in Table ~o. 7:
.,
TABLE No. 7: INCREASING THE AMINE/EPOXY RATIO
EXAMPLE 25 26 27 28 29
COMPOSITION (equivalents)
. N,N,N',N'-tetraglycidyl-
4,4'-d~no di~henyl m~ne 1.0 1.0 1.0 1.0 1.0
Trimethylene bis(p-
aminobenzoate) 1.0 1.25 1.5 1.75 2.0
PROPERTIES
Modulus, MSI 0.49 0.48 0.51 0.53 0.54
Strength, KSI 19.0 18.8 20.4 22.7 23.1
Strain, ~ 4.3 4.5 4.9 5.5 5.4
Work-to-break,
in-lbs./in3 449 451 560 728 729
The beneficial effect provided by increasing
the ratio of amine equivalents to epoxide equivalents is
seen from these data.
'J~
. ' . .
;, '
~1 30
;~!i
;j
~,j " .
., . , ,~ . ~ .
:, .
.
.
1 326~30
- - 33-
EXAMPLES 30-35
The general procedures of Example 1 and Example
25-29 are repeated, including diaminodiphenyl sulfone
(DDS) as a co-curing agent and increasing the ratio of
the during agents to epoxide, as was done in Examples
25-29. The formulations used and the results obtained
are shown in Table No. 8:
TABLE No. 8: INCREASING THE AMINE/EPOXY RATIO
j EXAMPLE 30 31 32 33 34 35
'~ COMPOSITION (equivalents)
N,N,N''~N'-tetraglycidyl-
4,4'-d~m~nodi~yl me~ l.0 1.0 1.0 1.0 1.0 1.0
' Diaminodi~henyl sulfone 0.5 0.5 0.5 0.5 0-5 0-5
T~imethylene bis-~p-
aminobenzoate) 0.75 1.~ 1.25 1.50 1.75 2.0
PROPERTIES
~, .
Modulus, MIS 0.51 0.50 0.54 0.57 0.60 0.60
Strength, KSI 20~8 21.0 23.0 27.3 26.7 29.9
Strain, % 4.8 5.0 5.06.8 5.7 7.4
'~ Work to break,
in.-lb./in.3 545 592 6551156 915 1~76
'', The beneficial effect on properties resulting
from an increase in the ratio of amine equivalents to
epoxide equivalents again is demonstrated.
l~i
~ 30
.`i
:"
'~
'~i
:;
. ~
.
1 326930
-34 -
EXAMPLE 36
~isphenol A diglycidyl ether plus oligomers
(EPON~ 828, Shell Chemical Co.) was mixed with
trimethylene bi~p-aminobenzoate) at a ratio of 1.0
epoxy equivalents to 0.75 amine equivalents twt- ratio:
94.9 g. to 30.1 g.). The resin was coated onto
graphite fiber (CELION~ 6K high strain graphite fiber)
and cured into unidirectional 8 ply laminates by heat-
ing at 350F. for 2 hours. The interlaminar strain
energy release rate was 5.0 in.-lb.~in.2.
EXAMPLE 37
Bisphenol A diglycidyl ether and oligomers
(DER~ 331, Dow Chemical Co.) was mixed with N,N-dimethyl
` 15 trimethylene-bis(p-aminobenzoate) at a ratio of 1.0
¦ epoxy equivalents to 0.75 NH- amine eguivalents
(weight ratio: 75.9:52.3 g.). ~he resin was coated onto
graphite fabric (CELIONO 3K70, plain weave) and cured
to a 10 ply laminate by heating at 35~ F. for 2 hours.
20 Good quality laminates were produced.
EXAMPLE 38
,~,
3 A mixture comprising tris(4-glycidoxyphenyl)
~ 25 diglycidylmethane ~80 parts, Dow Chemical XD-7342),
i! bisphenol A diglycidylether ~20 parts, Dow Chemical
DER0 331), trimethylene bis(p-aminobenzoate), 38
parts, dicyandiamide, 2 parts, and the reaction product
of 2,4-toluene diisocyanate and dimethylamine, 2 parts,
all by weight, wa~ prepared and applied to CELION~
high strain graphite fibers and made into an 8 ply
~l unidirectional laminate. It had a compression
strength of 20.9 x 103 p8i at 7~ F.
3S
.. . .
., ~
. .
.,. . ~,, . ~ ~ .
, -~ - . ~ , : . .
1 326q30
_35
EX~MPLE 39
Tris-4 glycidoxyphenyl) methane
(Dow Chemical, XD-7342) was mixed with N,N'-dimethyl-
trimethylene bis(p-aminobenzoate) at a ratio of 1.0
epoxy equivalents to 0.75 amine equivalents (weight
ratio: 69.ag: 55.2g). The resin was coated onto graph-
ite fabric (CELION~ 3K70, plain weave) and cured into
a 10-ply laminate, by heating at 350F. for 2 hours.
Good quality laminates were produced.
, .
~: EXAMPLJ3 4 0
,,
An epoxylated novolac (Dow Chemical DEN~ 438)
; 15 was mixed with trimethylene bis-(p-aminobenzoate) at
A a ratio of 1.0 epoxy equivalent to 0.75 amine equiva-
lents (weight ratio: 78.9:26.1 g). The resin was
coated onto graphite fabric (CELION~ 3X70, plain weave)
and cured into a 10 ply laminate by heating at 35~ F.
20 for 2 hours. Good quality laminates were produced.
EXAMPLE 41
The procedure of Example 40 was repeated,
25 ~ub~titutinq for the polyamine, N,N'-dimethyl-
trimethylenebi~(p-aminobenzoate) (weight ratio : 72.7 g.
epoxy : 52.3 g. diamine). Good quality lamintes were
produced.
J, 30
.,1
,,,
::j
:,,
i 35
;~.
:~`
~,
:. , ... , ., ~
`
1 326930
-36 -
EXAMPLE 42
. .
Bisphenol A diglycidyl ether (DER~ 331, Dow
Chemical Co.). was mixed with 1,3-trimethylene
aminobenzoate) at a weight ratio of 94.9 epoxide: 30.1 g.
diamine . ~he resin was coated onto polyaramid satin
weave fabric (DuPont REVLARO2aSR) and cured into a six
ply laminate , by heating at 35~ F. for 2 hours.
s~ Good quality composites were obtained.
EXAMPLE 43
~;
The procedure of Example 42 was repeated,
substituting for the polyamine, N,N'-dimethyl
trimethylenebis-(p-aminobenzoate) (weight ratio 75.9 g.
q- epoxy : 52.3 g). Good quality composites were obtained.
EXAMPLE 44
,, .
The procedure of Example 42 was repeated,
$~ except that the the resin mixture was coated onto
nickel plated graphite fibers instead of polyaramid
cloth. The matrix composition was cured into 1~4" x
10~ x 1/8" composite rods by heating at 35~ F. for
two hours. Good quality composites were obtained.
.' , ' .
~,~
- .
.,.. , ... ,,, . . : ~ .
.~ . - . .: . . .: : . . . .
... , . . : . , , .. , .~, ~ ,
1 326930
37 ~
O
EXAMPLE 45
The procedure of Example 43 was repeated,
except that the resin mixture was coated onto nickel
plated graphite fibers instead of polyaramid cloth. The
matrix composition as cured into 1/4" x 10" x 1/8"
composite rods by heating at 35~ F. for two hours. Good
quality composites were obtained.
EXAMPLE 46
A resin compo ition is prepared by mixing the
following (by weight)
., .
(a) N,N,N',N'-tetraglycidyl-4,4'
diamino diphenyl methane 120 parts
(b) Polyether polyimide resin
(General Electric Ultem,
Example 11, above) lS parts
(c) trimethylene bis-p-amino-
benzoate) 48 parts
(d) Boron trifluoride-ethylamine
, complex (catalyst) 0.5 parts
,~
A prqpreg tape is prepared following the general
procedure of 6-8, with a 35 to 45 preferably 40% resin/
~, 55 to 65, preferably, 60% graphite loading. When this is
formed into laminates by the procedure of Examples 6-8,
excellent quality composites are produced. Preferred
range-~ of compositions are (a), 114-126 parts; (b),
14.25-15.75 parts; (c) 45.6-50.4 parts; and (d), 0.475-
, 0.525 parts.
:. ~
',1
,
,... . . . .
1 326q30
- 38 -
! It is seen that the present invention produces articles
of manufacture with beneficial properties, making them useful
in a variety of applications. Many variations will suggest
themselves to those skilled in this art in light of the fore-
going detailed description. All such obvious variations are
3 within the full intended scope of the appended claims.
.'i