Note: Descriptions are shown in the official language in which they were submitted.
~2~
-- 1 --
39
CHEMICAL PROCESS
This invention relates to a novel chemical process for
preparing aryl piperidine carbinols and to novel
intermediates used in that process. This application is
a divisional application of copending parent application
SN 515,598 of the same title, which was filed August 8,
1986 and includes claims to a process for the preparation
of aryl-piperidine carbinols.
British patent no. 1422263 and US paten~ no 4007196
disclose compounds of for~ula A
~ ~ ~ x A
CHIOR~
in which Rl represents hydrogen, trifluoro (Cl_4)
alkyl, alXyl or alkynyl, R2 represents an alkyl or
alkynyl group having 1-4 carbon atoms, or a phenyl
group optionally substituted by Cl_4 alkyl, alkylthio,
alkoxy, halogen, nitro, acyl.amino, methylsulfonyl or
methylenedioxy, or represents tetrahydronaphthyl, and X
represents hydrogen~ alkyl having 1-4 carbon atoms,
alkoxy, trifluoroalkyl, hydroxy, halogen, methylthio,
or aralkyloxy.
The compounds of formula A are disclosed as having
pharmacological properties that make them useful as
an~i-depressants.
i~
;:
,: ., ,: ' ' `: ' '
,. . '~
- 2 ~ 7 ~ ~ ~
One compound that has proved especially valuable is
paroxetine [R1 = H/ R2 = 5-(1,3-benzdioxylyl), X ~ 4-F]
which is in the (-)-trans configuration.
In the above-mentioned patents, the compounds of
formula A are prepared using an intermediate of formula
B
R'- ~ ~ X
CHloH B
in which Rl and X are as defined above.
The piperidine carbinols of formula B are prepared by
reduction of an ester of formula C
R' -- Y~
COOCH~ C
with a complex metal hydride reducing agent.
The compound of formula C is obtained by reacting
arecoline (when Rl = methyl) or arecoline homologues
with phenyl (or substituted phenyl) magnesium bromide.
~27~
-- 3 --
This procedure has the disadvantage that arecoline is a
~owerful irritant and that the ester of formula C is
obtained as a mixture of ClS and trans configuration
compounds.
We have now discovered a new process for preparation of
piperidine carbinols which advantageously avoids the
use of arecoline and selectively produces the
trans-isomer in a good overall yield.
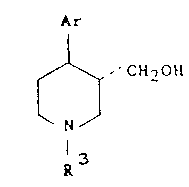
Accordingly, the present invention provides a process
for preparing a compound of formula I
Ar
~, CH~OH
N
1 3
in which Ar represents an aryl or substituted aryl
group and R3 represents hydrogen, an alkyl or aralkyl
group, by reduction of a compound of formula II
Ar
~ C02 K~
o N II
13
in which Ar and R3 are as defined for formula (I), and
R4 is an alkyl group.
. . ..
$ ~
-- 4 --
In formulae I and II, Ar may be I X where X is as
defined for formula A. Preferably X is fluorine or
hydrogen, R3 is hydrogen, methyl or benzyl, and R4 is
ethyl.
The reduction may be carried out conventionally using a
metal hydride, for exarnple using lithium alumini~n
hydride or aluminium hydride in an inert solvent such
as tetrahydrofuran or in a tetrahydrofuran/toluene
mixture.
The compounds of formula I are obtaine~ in the trans
configuration but as a mixture of enantiomers. The
compounds may be resolved into their enantiomeric forms
by conventional methods, such as by use of an optically
active acid, for example (+)-2'-nitrotartranilic acid
or (-)-di-p-toluoyltartaric acid.
The compounds of formula I may be used as intermediates
in the preparation of compounds of formula A making
use of the procedures set out in British Patent no
1422263 or US patent no 4007196.
For example, to prepare paroxetine, the compound of
formula I in which Ar = ~ F, and preferably R3= Me to
protect the nitrogen atom, in the (-)-trans -
configuration, is reacted with thionyl chloride or
benzenesulphonyl chloride and then with sodium
3,4-methylenedioxyphenoxide. Subsequently the N-methyl
group is replaced by reaction with phenyl chloroformate
followed by de-acylation with KOH to obtain R3= ~.
The present invention also provides the intermediates
of formula II as novel compounds. Preferred
substituents are as exemplified for formula I.
- 5 - ~ ~ 2 7~ ~
The compounds of formula II may be prepared by reaction of alkyl
amido malonates of formula III where R3 and R4 are ~s defined
previously
Co2R4
I
CE~2
I
CoNHR3 III
with appropriate cinnamic acid esters~ such as alkyl and aryl
esters for example a compound of formula IV
Ar
Co20R5 where Ar is as previously defined and oR5 is a
leaving group.
The reaction may be perormed as a condensation in a base/solvent
system, for example sodium hydride/dimethyl sulphoxide; potassium
tert. butoxide in e~hanol or tetrahydrofuran; or sodium methoxide
or ethoxide in ethyl acetate.
Advanta~eously the compound of formula II is prepared as a 'single
pot' reaction forming the cinnamate in situ from the appropriate
benzaldehyde. For example the appropriate alkyl amidomalonate is
added to a mixture of the benzaldehyde and sodium methoxide in
ethyl acetate.
Compounds of formula II in which R3= alkyl or aralkyl may also be
prepared by conventional alkylation of compounds of formula II in
which R3= H. For example, ester-imides o formula II in which R3=
H may be reacted with alkyl or aralkyl halides in the presence of
mild bases such as potassium carbonate.
The above described processes for producing the novel
intermediates of formula II also form part of this invention.
- -
....
.. . .
: . :
- 6 ~ L3 ~ ~
As used herein, the terms alkyl, aryl, aralkyl, alkoxy
and aralkyloxy include, but are not limited to, groups
in which the alkyl moiety, if present, is straight or
branched and contains from 1 to 6 carbon atoms, more
especially from 1 to 4 carbon a-toms, and the aryl
moiety, if present, is phenyl.
The following Examples illustrate the preparation of
novel intermediates of this invention (Examples 1 to 4)
and the novel process of this invention (Examples 5 to
g). Temperatures are in C.
:~ ... .
`$ ~
-- 7 --
Examole 1
.
(i)-trans-3-Ethoxycarbonyl-4-(4 -fluorophenyl)
piperidin-2,6-dione (El)
potassium t-butoxide (l.Olg) was added to a solution of
ethyl amidomalonate (1.38g) in tetrahydrofuran (38ml)
maintained at 33. After cooling to 25, ethyl
4-fluorocinnamate (1.50g) was added and the mixture
stirred overnight at room temperature. Brine was
added, the mixture extracted with ethyl acetate (3 x
60ml) and the organic solution dried and evaporated to
give the crude product. This was chromatographed on
silica gel, using dry ether as eluent, to give the
title compound (0.92g, 43~), m.p. 97-99.
H-n.m.r. (CDCl~)
h= 1.07 (t, J=8Hz, 3H)
2.67-2.90 (m, 2H)
3.50-3.84 (m, 2H)
4.00 (q, J=8Hz, 2H)
6.70-7.27 (m, 4H)
8.90 (br.s, lH)
Example 2
( )-trans-3-Ethoxycarbonyl-4-(4'-fluorophenyl)-~-methyl
piperidin-2,6-dione (E2)
(a) Sodium hydride (80~, 2.6g) was added slowly
to a stirred solution of ethyl N-methylamidomalonate
(11.9g) in dimethyl sulphoxide (50ml) under nitrogen.
After hydrogen evolution had ceased and the dark
solution had cooled to room temperature, ethyl
4-fluorocinnamate (15.3g) was added in one portion
., : ..
.~
~27~
along with further dimethyl sulphoxide (lOml) and the
whole stirred at room temperature for 19 hours. After
extraction with petroleum ether (3 x 16ml), the mixture
was stirred and neutralised by the addition of glacial
acetic acid (4.9ml) followed by the addition of ethyl
acetate (75ml) and water (35ml). ~le organic solution
was separated, washed with brine (30 ml), sodi~
bicarbonate solution (22ml) and brine and finally dried
over anhydrous sodium sulphate. Evaporation gave an
off-whi~e crystalline solid (22.7g), which was
triturated with isopropyl ether before filtering and
drying to give the title compound as a white
crystalline solid (20.0g, 86%), m.p. 91-96.
H-n.m.r. (CDCl~)
6 =1.10 (t, J=8Hz, 3H)
2.75-3.00 (m, 2H)
3.18 (s, 3H)
3.50-3.90 (m, 2H)
4.10 (q, J=8Hz, 2H)
6.80-7.30 (m, 4H)
(b) A solution of compound El (i)-trans-3-
ethoxycarbonyl-4-(4'fluorophenyl)piperidin-2,6-dione
(l.Og) in anhydrous dimethylformamide was stirred and
cooled to about 0 and methyl ioclide (0.679) and
anhydrous potassium carbonate (O.51g) added. The
mixture was stirred at 0-2 for 7.5 hours, after which
it was diluted with water and extracted with ethyl
acetate (lOOml). The extract was washed with water,
brine and dried over anhydrous sodium sulphate.
Evaporation gave the title compound as a pale yellow
oil, ca. 90% pure by n.m.r. spectroscopy.
~ :
" " ',
~3~7~
g
Example 3
~ trans-3-Ethoxycarbonyl-4-(4'-fluorophenyl)-
-
piperidin-2,6-dione (E3)
A solution of ethyl amidomalonate (17.5 gms, 1.0 eq at
70%) in ethyl acetate (50 ml) was added to a solution
of ethyl cinnamate (21.6 gms, O.lm at 90~) in ethyl
acetate (200 mls) containing sodium methoxide (7.6 gms)
over 0.5 hr at 20C. The slurry was stirred for 8
hours at 20C and 72 hours at 5C. 'rhe slurry was then
added to a mixture of water (200 ml) and acetic acid
(2.5 eq).
After separation of the rich ethyl acetate the solvent
was evaporated under reduced pressure and product
isolated via crystallisation from propan-2-ol by adding
heptane.
Yield = 5.2 gms
Product was a mixture of 20% methyl, 80~ ethyl esters
Structure confirmed by NMR and HPLC comparison with the
N-methyl equivalent~ (E2)
Example 4
(+)-trans 3-Ethoxycarbonyl-4-(4'-fluorophenyl)-N-methyl
-piperidin-2,6-dione (E4~
A solution of p-fluorobenzaldehyde (lOOg) in ethyl
acetate (lOOml) was added slowly to a mixture of sodium
methoxide (105g) in ethyl acetate (9OOml), maintaining
the temperature at 10-20C and stirring for a further
30 minutes at 15-25C. Then a solution of desiccated
: , ., . -.
'
" ; ' ' - .
~7~
-- 10 --
ethyl ~-methylamidom~lonate (139g) in ethyl acetate
(200ml) was added over 1 hour whilst maintaininy the
temperature at 15-25C and stirring for a further 1-2
hours. The resulting mixture was added to solution of
acetic acid (120g) in water (475ml) and stirred for 15
minutes. The lower aqueous layer was then separated
and discarded. The rich solvent was washed with
saturated brine ~250ml). The solvent was removed by
vacuum distillation and replaced with propan-2-ol then
cooled with stirring to obtain the crystalline title
compound. Water (600ml) was added over 30 minutes and
the mixture stirred for 1-2 hours at lS-25C. The
product was filtered and washed with water then
isopropyl ether before drying.
yield = 80-90%
Example 5
~ trans-4-(4'-Fluorophenyl)-3-hydrox~methyl-N-methyl-
piperidine (E5)
Compound E2 (+)-trans-3-ethoxycarbonyl-4-
(4'-fluorophenyl)-~-methylpiperidin-2,6-dione (18.0g)
in tetrahydrofuran (80ml) was added slowly to a
solution of lithium aluminium hydride (6.0g~ in
tetrahydrofuran (40ml) under nitrogen, keeping the
temperature below 40. ~hen addition was complete, the
reaction mixture was stirred at room temperature
overnight, then warmed to 50 for about 7 hours and
finally stirred overniyht at room temperature.
Decomposition was ~ffected by the careful addition of
water (30ml) followed by 10% aqueous sodium hydroxide
solution (6.0ml). The hydrolysed mixture was stirred
for 1~2 hours before filtering off the precipitated
.. : '' "',', ~"' '' ' '
,
.
r3~ ~
salts which were washed with ethyl acetate (50ml~. The
filtrate was dried over anhydrous sodium sulphate and
evaporated down to give a solid product which was
triturated with ether, filtered and dried, to give the
title compound (s.Og, 65%), m.p. 122-126.
lH_n.m.r (DMSO-d6)
6 = 1.56-1.92 (m, 5H)
2.15-2.29 ~m, 4H3
2.77-2.85 (d, lH)
2.88-2.99 (m, lH)
3.02-3.14 (m, 2EI)
3.38 (s, lH)
7.06-7.29 (m, 4H)
Resolution
The title compound E5 (8.6g) and
(~)-2'-nitrotartranilic acid (10.4g) were dissolved in
warm acetone (70ml) and water (1.9 ml) added. After
cooling and allowing to stand at 5 for 2 days, the
crystalline salt was filtered off, washed with acetone
and dried in vacuo (7.7g, 39%).
The salt (7.6g) was suspended in water and a slight
excess of dilute hydrochloric acid added to precipitate
the tartranilic acid which was filtered off and washed
with water. The filtrate was basified with sodium
carbonate soLution and extracted with ethyl acetate
(total 60ml) and the extracts washed with sodium
carbonate soLution, water and dried over anhydrous
sodium sulphate. Evaporation gave (-)-trans-4-
(4'-fluorophenyl)-3-hydroXymethyl-N-methylpiperidine as
a crystalline solid which was triturated with
.
, . ' ' " . . , , ., . ,;
~327~
- 12 -
ether/petrole~n ether (b.p. 60-80), filtered and
dried (3.1g, 95% recovery), m.p. 102-103, [a]D26
(c=5 in aceto~e) -37.
Example 6
(i)-trans-4~(4~-Fluorophenyl)-3-hydroxymethylpiperidine
Compound El (~)-trans-3-ethoxycarbonyl-4-
(4'-fluorophenyl)piperidin-2,6-dione (2.0g) was reduced
with lithium aluminium hydride (0.58g) in refluxing
tetrahydrofuran (50ml) for 4 hours. Work up as
described in Example 5 gave the title compound as a
solid (0.28g, 22%).
Example 7
~)-trans-4-(4' fluorophenyl)-3-hydroxymethyl-~-methyl
piperidine. (E7)
Compound E4 (34g) in toluene (150 ml) was added slowly
to a slurry of lithium aluminium hydride (lOg) in a
tetrahydrofuran (So ml)/toluene 1150 ml) mixture,
maintaining the temperature at 0-5C under nitrogen.
(Commercially available solutions of lithium aluminium
hydride in tetrahydrofuran/toluene have also been used
successfully)~ The mixture was stirred for a further
1-5 hours and then allowed to warm up to 15-25C
overnight. The excess lithium aluminium hydride was
destroyed by the carefully controlled addition of water
(10 ml), dilute 15% sodium hydroxide (10 mls) and water
(30 ml~. The solids were then removed by filtration
and reslurried in toluene (100 ml). The combined rich
toluene layers were concentrated to a low volume (75
ml) and poured into petroleum ether [100-120~] (25
ml). After stirring for 8-24 hours the product was
~ Q3
- 13 -
isolated by filtration, washed with petroleum ether (10
ml) and dried.
yield = 65-75
Example 8
~ trans-4-(4'Fluorophenyl)-3-hydroxymethyl-~methyl
piperidine
Compound E7 (5g) was dissolved in acetone (S0 ml) and
added to a solution of (-)-di-p-toluoyltartaric acid
(11.25g) in acetone (50 ml) at 15-25Co The mixture
was stirred for 1 hour at 15-25C~ then a further hour
at 0-10C~ The crystalline salt was filtered off,
washed with acetone and dried.
Yield = 40-45%
The salt (6g) was suspended in water and methylene
dichloride (MDC). The (-)-trans-4-(4'-fluorophenyl)-
3-hydroxymethyl-N~methylpiperidine was extracted into
the MDC using sodium hydroxide. Evaporation of the
rich MDC gave an oil which was redissolved in toluen~.
The toluene was removed by evaporation to give an oil.
The addition of heptane and trituration gave a
crystalline solid which was filtered off and dried.
26
Yield = 8S-89~ [~] = -35(c-l~ in methanol).
D
., : .: . , . . ` .... :
1 ~ 2 7 ~ ~ ~
- 14 -
Example 9
.
(i)-trans-4-(4'-Fluorophenyl)-3-hydroxymethyl-N-methyl
piperidine (E9)
Lithium aluminium hydride (3.24g; 0.085 mol) was added
with stirring to dry tetrahydrofuran (200ml) at 0C
under an atmosphere of nitrogen. Concentrated
sulphuric acid (2.16ml) was then added dropwise and the
mixture was stirred at 0C for 1 hour to giv~ a
solution of aluminium hydride. The imide (E2, lO.Og;
0.034 mol) was added as a solution in dry
tetrahydrofuran (199ml) and the mixture stirred at
0-5C for 4-5 hours, then at room temperature
overnight. Sodium hydroxide (16.2ml of a 40% aqueous
solution) was added, and the resultant precipitate was
filtered and washed. Filtration was repeated several
times to remove cloudiness then the filtrate and
washing were evaporated in vacuo, to give the product
as an orange oil (6.9g, 91%~ which could be
crystallised from ether, m.p. 122-3C.