Note: Descriptions are shown in the official language in which they were submitted.
1327767
ENHANCED IN-SITU ELECTROCHEMICAL DEGRADATION
OF ORGANIC CONTAMINANTS IN GROUNDWATER SYSTEMS
FIELD OF THE INVENTION
This invention relates to destruction of organic
contaminants in groundwater systems by way of
electrochemical action.
BACKGROUND OF THE INVENTION
The electrochemical oxidation-reduction of various
organic water contaminants, has been investigated. Such
work has been directed primarily at the treatment of
waste water effluent in a variety of flow-through
reactor designs. Usually a bed of electrically
chargeable material forms an electrode in the reactor,
to establish the necessary redox conditions for the
electrochemical degradation of the contaminant.
Sharifian, H. and Kirk, D. (1985) Electrochemical
Oxidation of Phenol, J. Electrochem._Soc. 133, 921,
discloses the electrochemical oxidation of phenol in a
flow through reactor having a packed bed of lead oxide
pellets. It was found that in this system, the
electrochemical oxidation of phenol produced
hydroguinone, benzoquinone and other products including
carbon dioxide. It was also found that the intermediate
produqts were further electrochemically degraded to
simpler carbon containing compounds with further
production of carbon dioxide gas. de Sucre, D.
Watkinson, A.(1981) "Anodic oxidation of phenol for
waste water treatment", Can. ~our. Chem. Eng. 59:52,
also addressed the flow through reactor design for
degradation of phenols. The system provides for the
removal of phenols from waste water effluent. The
system entails the use of a lead oxide anode in the
reactor.
It is also appreciated that electrical fields may
be used to remove matal ion contaminants from water
systems as disclosed in Runnells, D. and Larson, J.
(1986) "A laboratory study of electromigration as a
possible field technigue for removal of contaminants
from ground water", Groundwater Monitor. Rev. 6:85. The
$
- 1327767
investigations were directed to the electromigration of
copper contaminated solution. It was found that by
properly positioned electrodes, cspper ions readily
migrated to the cathode for recovery and removal from
the water system. It is suggested in this reference
that a grid of electrodes may be implanted in the ground
to achieve by electro-osmosis the removal of metal ion
contaminants in groundwater systems. However, no
thought has been given as to whether or not such a
system could be similarly used to achieve the electro
chemical oxidation-reduction of organic contaminants in
groundwater systems.
SUMMARY OF THE INVENTION
According to an aspect of this invention, a process
for electrochemically degrading organic contaminants in
groundwater comprises applying a voltage to a ground
aquifier through a grid work of a plurality of spaced
apart oppositely charged positive and negative rods
which are embedded in the ground. Organic contaminants
in the groundwater are sequentially degraded
electrochemically as the groundwater flows through the
grid work by applying sufficient voltage across the
plurality of oppositely charged rods to effect the
electrochemical degradation.
According to another aspect of the invention, the
rods of the ground work are spaced apart a distance
which develops for a given applied voltage sufficient
electrical charged between ad~acent rods to effect the
electrochemical degradation of the contaminants.
BRI~F DESCRIPTION OF THE PREFERRED EMBODIMENTS
Preferred embodiments of thQ invention are shown in
the drawings, wherein:
Figure 1 is a section of a flow through reactor
used in investigating the electrochemical oxidation of
phenol;
Figure 2 is a plot of the phenol degradation by
electrochemical action; and
Figure 3 is a plot of the trichloroethylene
degradation by electrochemical action.
1327767
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
By way of in-situ groundwater treatment, using
electrochemical methods, significant reductions in
organic contaminants can be achieved by implanting the
grid work of electrodes in ground aquafiers. The grid
work is established by the sequential serial lacation of
positive and negative electrodes, such that the flow of
water through the aquifier is across the grid work of
oppositely charged electrodes. Hence, a sequential
electrochemical and/or oxidation of organic contaminants
is achieved. For example, in the electro chemical
reduction of phenols, benzoquinone and maleic acid, the
benzoquinone and maleic acid are further
electrochemically oxidized to produce carbon dioxide,
which can be accomplished seguentially as the
groundwaters containing the organic contaminants flow
through the grid work of oppositely charged electrodes.
It has also been established that electroreduction of
trichloroethylene, another common groundwater
contaminant can be achieved.
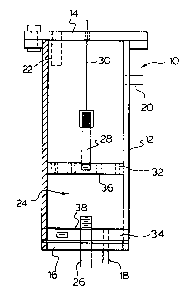
A flow through reactor shown in Figure 1 was
provided to investigate the electro-oxidation of
phenols. The reactor can be operated in either a
down-flow or up-flow mode. In the up-flow mode, a pump
i8 used to force the flow of liquid through the system.
The reactor 10 as shown in Figure 1, consists of a
cylindrical housing 12 and end caps 14 and 16. An
effluent/in~luent port 18 is provided at the base of the
reactor extending through the end cap 16. In the upper
region of the reactor is a constant head port 20 which
provides constant level o~ liquid in the reactor.
Material may be introduced in the over-flow and/or inlet
port 22, depending upon the direction of flow desired.
The reactor comprise~ a media of crushed graphite
24, which is in contact with a graphite electrode 26.
The electrode 26 i8 electrically connected, so as to be
positively charged. The anode 28 is negatively charged
by electrical lead 30 extending outwardly of the end cap
14. The carbon i8 contained in the reactor by way of
- 1327767
perforated plate 32, which allows the liquid to flow
through the carbon bed 24 in either direction.
Furthermore, it is appreciated that the electrodes 26
and 28 can be charged in an opposite manner so that
electrode 28 becomes the cathode in electrochemical
reduction, rather than oxidation.
The bed of graphite may be formed from
electrochemical grade graphite blocks. The blocks are
crushed in a mechanical jaw crusher to achieve a
textural range of .5mm to 2mm in diameter with a median
particle diameter of 1 cm. The granular graphite is
packed between two 100 mesh stainless steel disks, and
confined in place between the plexiglass~plate 34 and
the upper perforated plexiglass 32. The bed height may
be varied up to a maximum of reactor height of
approximately 29 cm. The diameter of the reactor is
approximately 14 cm. The stainless steel disks are
indicated as such at 36 and 38. The crushed graphite
was chosen in view of it being relatively inexpensive,
and possessing good electrochemical properties. The
graphite when crushed is ideal in providing a
flow-through reactor, because the granular graphite
increases the effect of the electrode surface area. For
purposes of phenol oxidation, the organics are oxidized
directly by electron transfer, and/or by reaction with
the surface oxides that are produced in a charged
transfer step from the constituents in the solution. A
source of DC voltage was used, and operated at
approximately 4 volts with a measured direct current in
the range of 1-4 amps.
An alternative system which was used to establish
electrochemical degradation of organics, is a system
which simulates porous ground media common to an
aquifier. Quartz sand of uniform texture was loaded
3S into a container of approximately 4 litres in volume.
The quartz sand is inert with respect to ionic exchange
and/or absorption reactions. The sand was saturated
with a model waste water, and optionally recycled with a
pump. Electrodes were implanted in the sand, which were
,~
1327767
oppositely charged. The preferred type of electrodes
are those made of titanium due to its resistance to
oxidation.
Phenol solutions were passed through the reactor of
Figure 1. The concentration of phenol in the effluent
was measured by Chem Metrics3calorimetric test kit.
Further analyses was by Total Organic Carbon (TOC)
analyses. The TOC analyses was performed in a Beckman~
915A Analyzer, which has separate organic and inorganic
channels. Analyses were also conducted on a gas
chromatograph Varia~ 3700, fitted with a 0.1% SP1000 on
a Carbopac~ C column and flame ionization detection.
With reference to the following Table 1, reaction
conditions of four continuous flow through runs are
quantified.
TABLE 1
Run
1 2 3 4
Feed Volume(L) 4.0 8.0 1 8.0
Initial Phenol(mg/L)122.5 122.5 440 1750.0
pH 6.0 6.0 6.0 5.0
Temperature () 24 25 25 25
Flow rate (ML/min)200 200 25 100
Measured Potential(V-DC) 4.0 4.0 4.0 4.0
Measured Current(Amp) 1.3 1.3 1.4 3.5
Duration (Min) 40 80 40 2 hrs
Effluent Content
(Phenol) mg/L (a) 88.0 93.0 246.0 625.0
(b) 92.0 87.5 238.0 650.0
(c) 90.5 90.5 225.0 575.0
(d) 89.5 91.0 215.0 600.0
Percent 27.3 26.6 57.0 65
Concentration reduction
The effluent was sampled at four different
intervals as indicated by a, b, c and d. This
information provided a better approximation of overall
efficiency of the unit depending on the various
parameters noted.
~. '
1327767
Additional runs similar to that outlined in Table
1 were conducted, the results of which are shown in
Figure 2. Runs number 1, 2, 3, 4 and 5 were analyzed
on the basis of Chem Metrics colometric test system.
Run 3 was analyzed on the basis of Total organic Carbon
reduction. Run 2 was conducted on the basis of filling
the reactor with sand rather than crushed carbon. The
greater than 90% oxidation demonstrates the
effectiveness in sandy soils.
The tests in the porous crushed carbon med~a were
conducted using trichloroethylene water contaminant.
The results are shown in Figure 3 as indicated by
percent reduction in trichloroethylene, based on gas
chromatograph analysis, and also based on total organic
carbon analysis.
The results clearly indicate that substantial
reductions in organic chemical content in water can be
achieved by electrochemical methods. Up to 93~
reduction in phenol concentration can be achieved in 36
hours using a 12 volt DC 6 amps power supply.
Similarly, significant reductions in trichloroethylene
are achieved by electroreductive dehalogenation.
Contaminated groundwater containing organics such as
phenol or trichloroethylene can therefore be treated by
electrochemical methods. By way of implanting, a
multiple electrode array can be set up such that in
knowing the direction of flow of groundwater through the
aquifier, the groundwater can pass through sequentially
positively and negatively charged electrodes. In so
doing, the organics are degraded principally to carbon
dioxide.
Although preferred embodiments of the invention are
shown herein in detail, it will be understood by skilled
in the art that variations may be made thereto without
departing from the spirit of the invention or the scope
of the appended claims.