Note: Descriptions are shown in the official language in which they were submitted.
1 328561
METHOD FOR PRODUCING METALLIC
TITANIUM AND APPARATUS THEREFOR
This invention relates to a method for producing
metallic titanium and an apparatus therefor, and more
particularly to a method and apparatus for producing
metallic titanium from titanium tetrachloride at a
reaction temperature above the melting point of
titanium.
In the known "Kroll" process, metallic titanlum i8
produced by the reduction of titanium tetrachloride by
metalllc magnesium.
In the Kroll process, the reduction is generally
carried out at a temperature below the melting point of
metallic titanium while keeping the reduction vessel at
normal pressure to produce spongy metallic titanium.
The spongy metallic titanium product is sub~ected to
vacuum separation or leaching to remove any excess
metallic magneslum and magnesium chloride (by-product)
remaining in the fine internal voids of the metallic
titanium product and 18 thus purifled. The purlfied
metalllc titanlum 18 then crushed and formed lnto a
shape sultable for meltlng. After meltlng, an lngot
of tltanium 18 obtalned.
As can be seen, the Kroll process 18 a batch type
process. Accordlngly, produclng the metalllc tltanlum
lngot accordlng to the Kroll process requlres at least
four dlscontlnuous or lndependent steps comprlsing a
reduction step, a vacuum separatlon step, a crushing
9tep and a melting step.
The Kroll process also has the following
dlsadvantages.
The spongy metalllc titanlum whlch is the reaction
- ,
.
1 32856~
-- 2
product is firmly adhered to a reduction vessel, 80
that much labour and time are required for removing the
deposited reaction product from the vessel.
Another disadvantage is that it is difficult to
remove the heat of reaction from the reaction system
during the reduction step sufficiently rapidly.
A further disadvantage is that the titanium is
produced at a sufficiently elevated temperature to
increase its activity. Accordingly, it is readily
contaminated with the material of the reaction vessel
wall.
Still another disadvantage is that the separation
step for purification of the titanium requires much
attention ln order to prevent contaminated of the
titanium with moisture, air and the like. Accordingly,
removal of the unreacted reactant and the by-product
must be carried out in a vacuum or argon atmosphere.
For the purpose of reducing metal halide with a
reducing metal agent without using the Kroll process,
other methods are proposed in each of whlch the
reduction i8 carried out at a reaction temperature
above the melting point of the metal to be produced
and the product is continuously removed from the
reaction vessel. The metal product 19 then obtalned
ln a molten state or ln the form of an lngot by coollng
the molten metal product for solldlflcatlon.
As an example, Japanese Patent Appllcatlon Laylng-
Open Publlcatlon No.35733/1981 dlscloses a method for
produclng metalllc tltanlum whlch comprlses the steps
of lntroduclng tltanlum chlorlde and a reduclng metal
agent both ln the vapour state lnto a reactlon vessel
to react both under condltlons 80 that a llquid
metallic titanium product is obtained together with the
.
:' ' . , - , ~
~: , . ~ . : . . .
; . . ~ ,~: - :
.
1 32856~
-- 3 --
chloride of the reducing metal agent in the form of a
vapour. The chloride by-product of the reducing metal
agent is separated from the titanium product for
recovery and the metallic titanium product is solidified
in a mould kept at a temperature below the melting
point of the metallic titanium product to obtain an
ingot which is removed from the reaction vessel.
Japanese Patent Publication No.19761/1971
discloses a method for producing metal comprising the
steps of introducing titanium tetrachloride vapour and
a liquid reducing metal agent into liquid metal in a
reaction vessel, heating a reaction zone to a
temperature above the melting point of titanium to
obtain a metallic titanium product and chloride by-
product of the reducing metal agent ln a moltenstate under a vapour pressure of the reducing metal
agent at the relevant temperature, separating the
product and by-product from each other using the
difference in their gravities, and separately removing
them from the reaction vessel.
Various similar methods have attempted to solve the
problems of the Kroll process by reducing the metal
halide with the reducing metal agent while keeping
the reaction temperature above the melting point of the
metal product to obtain the molten metallic product.
However, while these methods are disclosed in patent
literatures, they have not been commerciallzed on an
lndustrlal scale. The reason 18 belleved to be that lt
18 very dlfflcult to select a materlal for the reactlon
vessel whlch wlthsatands a sufflclently hi8h temperature
to produce actlve metal of a higher meltlng polnt such
as tltanlum, zlrconlum or the llke ln the reactlon
veosel and to keep it in a molten state.
!
'. ' ' :
,'' , . ' '.',
.,, ,
.. ' : ..
1 328561
More particularly, for example, the method
disclosed in Japanese Patent Publication No.19761/1971
is to reduce titanium tetrachloride with magnesium to
produce metallic titanium while keeping the temperature
in the reaction zone at about 1730C and the pressure
in the reaction vessel at about 5 atms corresponding to
a partial pressure of the magnesium chloride by-product
at that temperature to produce the metallic titanium
product and the magnesium chloride by-product in a
molten state. Thus, in the method the reaction zone
temperature is about 1730C and its pressure is about
5 atms which is substantially equal to the vapour
pressure of the magnesium chloride, produced in liquid
form. This results in the magnesium being boiled which
leads to a failure to keep the magnesium in an amount
sufficient to reduce titanium tetrachloride in the
reaction zone fully. This causes the reaction to take
place in the presence of insufficient magnesium which
often produces lower chlorides of titanium such as
titanium trichloride, titanium dichloride and the like.
Also, ln thls method, the reactants (titanium
tetrachloride in the form of a gas and magnesium in the
form of a liquld) are supplied through graphite plpes
to a molten layer of the reactlon product on a bottom
of the reaction vessel to carry out the reaction ln the
molten layer. Thls causes the open end of the graphite
pipes to be corroded by the active molten titanium
product. Also, the molten titanium product contacts
each of the reactants at a relatively low temperature
at the open end of the pipes, solidifying the reactants,
and so clogging the pipes. Furthermore, since the
reaction is a reduction taking place in the molten layer
of titanium, the titanium product is contaminated wlth
S
.
.~ ~. '. ',
1 328561
-- 5
unreacted reactants, the by-product and the like.
Moreover, the lack of magnesium in the reaction zone
leads to a decrease in reaction efficiency per a
reaction sectional area.
It is an ob;ect of the present invention to
provide a method and apparatus for producing metallic
titanium by the reduction of titanium tetrachloride
with a reducing metal agent which are capable of
continuously producing metallic titanlum at a lower
energy cost and on an industrlal scale.
According to one aspect of the invention, there is
provided a method for producing titanlum by the
reduction of titanium tetrachloride with a reducing
metal agent characterised by the steps of: maintaining
the temperature and pressure in a reaction zone in a
; reaction vessel above the melting point of the metallic
titanium to be produced and above the vapour pressure
of the reducing metal agent at that temperature;
supplying titanium tetrachloride and the reducing metal
agent to the reaction vessel to react to produce a
metallic titanium product and a chloride by-product of
the reducing metal agent while maintaining the product
and the by-product ln a molten state; separating the
metallic titanium product and the chloride by-product
of the reducing metal agent from each by making use of
the difference in their densities; collecing the
metallic tltanium product at the bottom of the reaction
vessel; and contlnuously drawlng out the metalllc
titanium product from the bottom of the reactlon vessel.
Preferably, the titanium product is solidified by
coollng as lt is withdrawn.
Preferably, a molten bath of chloride of the
reducing metal agent and optionally also of the
.
.
.,
:
.. :
: :
'~ ~ ' ' '
,. ~ .
1 ~28561
reducing metal agent is previously formed in the
reaction vessel so that the surface of the molten bath
constitutes the reaction zone and titanium tetrachloride
and the reducing metal agent are supplied to the
reaction region. Preferably the titanium tetrachloride
is supplied in liquid from the top of the reaction
vessel and the reducing metal agent is supplied either
in the same way or is injected into the bath.
Preferably, the chloride by-product of the
reducing metal agent ls discharged from the reactlon
vessel at a rate arranged to maintain the position of
the reaction zone substantlally constant. The method
may also include the steps of inserting a titanium
ingot into the bottom of the reaction vessel resulting
in the coalescence of the separated metallic titanium
metal product with the titanium ingot and drawing the
metallic titanium product out continuously together
with the titanium ingot at a rate corresponding to the
amount of the metallic tltanlum product being coalesced
with the titanium lngot.
Accordlng to another aspect of the lnvention,
there is provided an apparatus for producing metallic
tltanlum by the reductlon of tltanlum tetrachlorlde
with a reducing metal agent characterlsed by: a reactlon
vessel having reaction zone in which a temperature above
a melting point of the titanium product i8 defined and
which is kept at a pressure sufficient to prevent
boiling o~ the reducing metal a8ent and its chloride at
that temperature; a reducing metal agent feed pipe for
supplying the reducing metal agent in the form of a
liquid from the side or the top of the reaction vessel
to the reaction zone; a titanium tetrachloride feed
pipe for supplying titanium tetrachloride from the top
:
, ~ ' ~ ' '' ,
:. . .
~ ` t 3285~
-- 7
of the reaction vessel to the reaction zone; a discharge
pipe for discharging the chloride by-product of the
reducing metal agent from the side of the reaction
vessel; heating means arranged outside the reaction
vessel at a position corresponding to the reaction
zone; and a withdrawing section at the bottom of the
reaction vessel for continuously drawing out the
metallic titanium product.
One preferred embodiment of the invention includes
a reaction vessel made of a thick titanium plate in
which a reaction zone is defined and which is kept at
a pressure sufficient to prevent boiling of a reducing
metal agent and its chloride. A reducing metal agent
feed pipe supplies the reducing metal agent in the form
of liquid from the side or top of the reaction vessel
to the reaction zone, and a titanium tetrachloride feed
pipe supplies titanium tetrachloride from the top of
the reaction vessel to the reaction zone. A discharge
pipe for discharging a chloride by-product of the
reducing metal agent extends from the side of the
reaction vessel. Heating means are arranBed outside
the reaction vessel at a position corresponding to the
reaction zone for carrying out electromagnetic induction
heating, resistance heating or the like, and a mould
section i9 arranged at a bottom of the reaction vessel
for solidifying the molten metallic titanium product by
cooling and continuously drawing out lt from the
reaction vessel.
An alternative reaction vessel structure includes
a reaction vessel made of metal 8uch as copper or a
ceramic material such as alumina, zirconia or the like
in which a reaction zone is defined and which is kept
at a pressure sufficient to prevent boiling of the
~: ,
`
: ~ .
1 328561
-- 8
reducing metal agent and the chloride of the reducing
metal agent. The reaction vessel has a vertically
extending hollow shape and is open at the top and
bottom. The reaction vessel includes a cooling agent
circulating path for cooling the inner surface of the
reaction vessel and portions of its outer periphery at
a position corresponding to the reaction zone. The
vessel also includes a removal section with heating
means for heating a molten material which carries out
- 10 electromagnetic induction heating, resistance heating
or the like.
In the present invention, a suitable reaction
vessel provided with the heating means may comprise a
crucible, as disclosed in U.S. Patent No.3,755,091
which is adapted to melt titanium chips, titanium sponge
or the like for preparing a titanium ingot and is used
in an evacuated inert atmosphere. Such a crucible may
be incorporated in a pressure vessel for use as the
reactlon vessel in the present invention which includes
; 20 the reaction zone for reducing titanium tetrachloride
and the mould section for solidifying the metallic
titanium product by cooling and continuously removing
it therefrom.
The present inventors have conducted the following
' 25 reaction te9t in order to evaluate the reaction
efficlency for reducing titanium tetrachloride with
metallic magnesium according to the present invention.
; 30 REACTION TEST
A pressure in the reaction vessel was kept at 50
atms. The reaction vessel was charged with 845g
; metallic magnesium, which was heated to 1350C by
.,
, . ~ ~ , .
: . :
`
` '
1 328561
g
electromagnetic induction heating or resistance heating
to form a molten magnesium bath in the reaction vessel.
Immediately after the heating, 1340g liquid titanlum
tetrachloride was fed to the molten magnesium for 50
seconds at a feed rate of 1608g/min.
The temperature of the bath reached the melting
point of titanium in 15 seconds after the beginning of
the addition of titanium tetrachloride, thereby
producing liquid titanium. The yield of titanium was
99% and the reaction efficiency per unit sectlonal area
of the reaction vessel was 62.7kmol/hr.m2. For
comparison, the Kroll process was carried out and was
found to give a reaction efficiency per unit sectional
area of a reaction vessel of 1.3kmol/hr-m2.
The efficiency of reaction between titanium
tetrachloride and metallic magnesium in the gas phase
is calculated in an article entitled "Gas Phase
Reaction Test Report" by Prof. Takeuchi of Tohoku
University, Journal of Japan Institute of Metals, 23,
pp625-637 (1965), as follows:
In the reactlon test, the volume of a tltanlum
rlbbon for growing titanium on was 0.057m3 and the
deposition rate of tltanium to the titanium ribbon was
! 3.45kg/hr (72mol/hr). Accordingly, its volume efflclency
i8 72/0.057 ~ 1263mol/hr-m3 and lts reactlon efficiency
i per area 19 1.263kmol/hr-m2.
I It may not be strlctly falr slmply to compare the
; reactlon efflclency of the present lnvention to the
reaction efflciency calculated ln thls way because
- 30 reactlon condltlons such as temperature, a feed rate of
feedstocks and the llke were set dlfferently. However,
lt wlll be noted that the reactlon between the tltanlum
tetrachlorlde and meta111c =agnesluc ln the pre~ent
'.,
.
~.
1 328561
-- 10 --
invention exhibits a reaction efficiency at least 49.6
(62.7/1.263) times that of the above described gas
phase reaction and 48.2 (62.7~1.3) times as much as
that of the Kroll process. The fact that the present
invention exhibits such higher reaction efficiency is
believed to be due to the liquid metallic magnesium and
liquid titanium tetrachloride being supplied to the
reaction region kept there at a higher temperature and a
higher pressure.
A temperature of the reactlon zone is set above a
melting point of titanium. In order to precipitate
stably the metallic titanium product onto the bottom of
the reaction vessel while keep~ng it in a molten state,
it is desirable to keep the reaction vessel at a
temperature which is about 100-200C higher than the
melting point of titanium and to keep the pressure of
the reaction region at least above the vapour pressure
of the reducing metal agent at the reaction temperature
and preferably above the sum of the vapour pressures of
the reducing metal agent and its chloride.
More preferably, when titanium ~melting point of
1670C) ls to be produced using titanium tetrachloride
as the feedstock and magnesium as the reducing metal
agent, the bath in the reaction vessel is kept at a
temperature of at least 1670C and more preferably
1827C, and at a pressure above 42.6 atms, corresponding
to a partial pressure of magnesium and more preferably
above 48.6 atms corresponding to the total sum of the
partial pressure of magnesium (42.6 atms) and magnesium
chloride (5.98 atms) at the temperature of 1827C.
For reduction of titanium tetrachloride, the
reducing metal agent may be fed in a stoichiometric
amount. However, in order to carry out the reduction
. .
~ ' .
: ~
1 328561
-- 1 1
fully, it is desirable to feed a predetermined excess of
the reducing metal agent in the reaction region to
inhibit the production of lower titanium chlorides.
The invention may be carried into practice in
various ways and some embodiments will now be described
by way of example with reference to the accompanying
drawings, in which:
Pigure l is a vertical section through a first
embodiment according to the present invention;
Figure 2 is a view similar to Figure l showing a
second embodiment; and
Figure 3 i8 a partially cutaway perspective view
generally showing an example of a reaction vessel
incorporated in the apparatus shown in Pigure 2.
i 15 In the present invention, titanium tetrachloride
and a reducing metal agent are supplied in liquid
form to a reaction zone for reaction. Magnesium or
sodium may be used as the reducing metal agent.
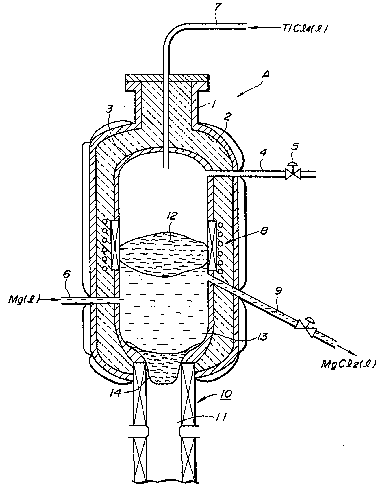
The apparatus shown in Pigure l includes a
reaction vessel structure A which also serves as a
pressure vessel. The reaction vessel structure A
lncludes an ou~er shell or outer wall l made of a steel
plate, an inner wall made of titanlum serving as a
reaction vessel 3 and a heat lnsulatlng materlal 2
between the outer shell 1 and the reactlon vessel 3.
' An inert gas (e.g., argon) ls lntroduced to the
'~ reactlon vessel 3 from a pressure ad~usting pipe 4
through a valve 5, 80 that the interior of the reactlon
vessel 3 is set and kept at a pressure sufficient to
prevent substantially any boillng of the magneslum and
- magneslum chlorlde, even when the temperature in a
reaction zone deflned ln the reaction vessel 3 rises
above the melting point of titanium. Por example, the
,
:`' . ~ ~ :
:
:' .
, .. . .
1 328561
- 12 -
reaction vessel 3 is kept at a pressure of about 50
atms when the temperature of the bath in the reaction
vessel 3 is 1827C. When the pressure in the reaction
vessel 3 is above or below the set value, an automatic
pressure adjusting valve (not shown) is operated to
keep the pressure at the set value automatically.
Liquid magnesium for use as the reducing metal
agent is supplied to the reaction zone through a
reducing metal agent feed pipe 6 extending through
the side wall of the reaction vessel structure A and
into the reaction vessel 3. Similarly, liquid titanium
tetrachloride is supplied to the reaction zone through
a titanium tetrachloride feed pipe 7 extending through
the top of the reaction vessel structure A and into the
vessel 3.
The reaction vessel 3 i8 provided at an intermediate
part of its outer periphery (in a vertical direction)
surrounding the reaction zone with a heater or heating
means 8 adapted to carry out electromagnetic induction
heating, re9istance heating or the like`to ad~ust the
temperature of the reaction region in the reaction
vessel 3 to a level above 1670C, corresponding to the
melting point of titanium. A discharge tube 9 is
connected to the reaction vessel 3 ad~acent to the
heating means 8, for dlscharglng magneslum chloride
by-product formed by the reduction.
A mould section 10 for solidifying the molten
metallic titanlum product is connected at the bottom of
the reaction vessel, for cooling and drawing out the
titanium product.
The production of metallic titanium using the
apparatus shown in Figure 1 will now be described.
Firstly, a titanium ingot 11 i8 inserted in the
: : ; . , .
1 328561
- 13 -
mould section 10 to close the bottom of the reaction
vessel 3 and then magnesium and magnesium chloride are
charged in small amounts into the reaction vessel 3.
The atmosphere in the reaction vessel 3 is replaced
with argon gas and then the heater 8 is operated to
melt the magnesium and magnesium chloride, resulting in
a molten bath of magnesium and magnesium chloride being
formed in the reaction vessel 3. The molten magensium
12 floats above the magnesium chloride due to the
difference in their densities, so that it may remain
separate from the magnesium chloride.
Subsequently, more argon gas is introduced into
the reaction vessel 3 to increase the pressure. Then,
liquid titanium tetrachloride is fed to the surface of
the molten magnesium 12 through the titanium
tetrachloride feed pipe 7 connected to the top of the
reaction vessel 3. Liquid magnesium is supplied to the
molten magnesium chloride layer through the magnesium
feed pipe 6 connected to the side of the reaction
vessel 3. Alternatively, the magnesium feed pipe 6 may
be connected to the top of the reaction vessel 3 so
that both the tltanium tetrachloride and the magneslum
may be supplied ln liquid from the top of the reaction
vessel 3 to the reactlon zone (as ln an apparatus of
Flgure 2 described herelnafter).
Tltanlum tetrachlorlde supplied to the surface of
the molten magneslum layer of the bath reacts as a
llquld wlth the llquld magneslum to produce tltanlum 14
and magneslum chlorlde 13. Alternatlvely, lt may react
as a vapour with magneslum vapour vapourlsed from the
molten magneslum layer of the bath of lndeed wlth
llquld magneslum.
The heat of reactlon and the effect of the heater
,
. .
" ' ~
~ 328561
- 14 _
8 cause the temperature of the molten bath in the
reaction vessel 3 to rise above the meltlng point of
titanium. However, the reaction vessel 3 is kept at a
pressure above a vapour pressure of magnesium at that
temperature, so the titanium product 14, the magnesium
chloride by-product 13 and the magnesium 12 are all
kept in a liquid state. Also, the molten bath is
vertically separated into three layer, namely magnesium
12, magnesium chloride 13 and titanium 14, in that
order, due to the difference ln their densities.
The molten metallic titanium product 14 precipitates
and sinks through the molten magnesium layer and the
i~ molten magnesium chloride layer to the bottom of the
reaction vessel 3 and reaches the top of the titanium
ingot 11 to coalesce with it as it is produced.
Correspondingly, the titanium ingot 11 is continuously
drawn out at a suitable rate, during which it is
solidified by cooling.
The magnesium chloride by-product 13 is discharged
through the discharge pipe 9 connected to the side of
the reaction vessel 3 at a discharge rate which is
ad~usted 80 that the molten bath in the reaction zone
18 kept constant in depth. The titanium ingot 11 is
drawn out at a rate corresponding to the amount of
titanium precipitated on the titanium ingot (or the
precipitation rate of the titanium) by means of rollers
(not shown). Accordingly, the position of the molten
titanium product above the titanium ingot 11 is kept
substantially constant.
The apparatus shown in Figures 2 and 3 is
constructed in substantially the same manner as that of
Figure 1 except for the construction of the reaction
vessel 3, the arrangement of the reducing agent feed
. -
.
,
.. . . .
.- . ,
:
.. . ~ -. . ~
1 32~56~
pipe 6 and the construction of the heating or heating
means 8.
More particularly, the reaction vessel 3 is formed
as a vertically extending cylindrical shape, the top
and bottom of which are open and is divided into two or
more segments 32 by means of vertical slits 3l in the
wall of the reaction vessel 3. In the illustrated
embodiment, it is divided into twelve segments 32.
Each of the segments 32 is formed of a material of good
thermal conductivity, for example, a metal such as
copper or the like. The slits 31 are filled in an
electrically insulating and heat resistant material to
insulate the segments 32 from one another electrically.
The segments 32 are each provided with an internal
cooling pipe 33 for supplying a cooling agent through
them to cool the wall of the reaction vessel 3 defining
the reaction zone therein. The cooling pipes 33 are
connected to one another and between a cooling agnet
inlet 34 and a cooling agent outlet 35 to form a path
for clrculating a coollng agent.
An upwardly extendlng duct 15 i9 connected to the
open top end of the reaction vessel the upper end of
which is connected to the exterior through a cylinder
section 16 and ln which the reducing agent feed plpe 6
18 located. The titanium tetrachlorlde feed plpe 7 is
posltloned wlthln the upper portlon of the reactlon
duct 15. Thus, liquid magnesium and llquld tltanium
tetrachlorlde are supplled through the feed pipes 6 and
7 to the reactlon zone. The reaction vessel 3 is
provlded at a bottom thereof with a mould section l9 at
the bottom, through which a tltanlum lngot ll ls
inserted into the reaction vessel 3.
The reaction vessel 3 constituted by the segments
.~
.
,
,;~
1 328561
- 16 -
32 has at its upper part on the outer periphery at a
position corresponding to the reaction zone in the
reaction vessel 3, an upper electromagnetic induction
heating coil 8a for raising a temperature of the
reaction zone above the melting point of titanium (or
1670C). On its lower part, the vessel 3 has a lower
electromagnetic induction heating coil 8b for melting
the top of the titanium ingot 11 and the magnesium
chloride adjacent the top to keep the top of the ingot
constantly ln a molten state during the reaction. Thus,
in the illustrated embodiment, the heating means 8
comprises the upper and lower electromagnetic induction
heating coils 8a and 8b.
As described above, the embodiment of Figures 2
and 3 is so constructed that the reaction vessel 3 i8
divided into a plurality of the cooled segments 32 and
the segments 32 are electrically insulated from one
; another by the slits 31. Such a construction
substantially prevents the generation of eddy currents
in each segment 32 due to electromagnetic induction
heating, thereby permitting the molten materials in the
reaction zone of the reaction vessel 3 and the top of
the titanium ingot to be sub~ected to induction heating
without heating the segments 32. The apparatus
includes a discharge pipe 9 for discharging the magnesium
chloride by-product which is connected to a substantially
central portion of a side of the reaction vessel, in
this case between the upper and lower electromagnetic
induction heating coils 8a and 8b.
In the illustrated embodiment, the reaction vessel
3 is made oE a metal material in view of economic
efficiency and maintenance. However, it may be formed
; of a ceramic material such as alumina, zirconia or the
:`
, ., ,,, ~, . . .
1 328561
- 17 -
like. In such a case, it would not be necessary to
divide the reaction vessel 3 into segments.
The operation of the apparatus shown in Figures 2
and 3 will now be described. Basically, operation of
the apparatus of Figures 2 and 3 is similar to that of
Figure 1.
First, a titanium ingot ll is inserted into the
mould section 10 to close the bottom of the reaction
vessel 3 and then magnesium and magnesium chloride are
charged in small amounts into the reaction vessel 3.
Then, the atmosphere in the reaction vessel'3 is
replaced with argon gas and the lower magnetic
induction heating coil 8b is operated to melt the top
of the titanlum ingot 11 while the upper magnetic
inductlon heating coil 8a is operated to melt the
magnesium and magnesium chloride charged into the
reaction zone,,resulting in a molten bath of
magnesium and magnesium chloride being formed in the
reaction vessel 3. Molten magnesium 12 floats, above
the magnesium chloride due to the difference in their
densities and the magnetic field by electromagnetic
induction, 80 that it remains separate from the
magnesium chlorlde. Part of the molten magnesium
chloride flows lnto the gap between the titanlum lngot
ll and the inner surface of the reactlon vessel 3 where
it solidifies by cooling, to glve pressure sealing and
electrical lnsulation actions.
Subsequently, more argon gas 19 lntroduced into
the reaction vessel 3 to lncrease the pressure, and
liquid magneslum and tltanlum tetrachloride are fed
through the magnesium feed plpe 6 and the tltanium
tetrachloride ~eed pipe 7 connected to the top of
the reactlon ves~el 3 to the surface of the QolteQ
.: :
:
~ ` , , , ' :- " ` :
1 328561
magnesium 12, forming an upper layer of the molten
bath or the reaction region. Alternatively, the
magnesium feed pipe 6 may be connected to the side of
the reaction vessel 3 as in the apparatus of Figure 1.
Titanium tetrachloride in the reaction zone or
at the surface of the molten magnesium layer of the
molten bath reacts in liquid form with the liquid
magnesium to produce titanium and magnesium chloride.
Alternatively, it may react as vapour wlth magnesium
vapour generated from the molten magnesium layer or
with liquid magneslum.
The heat of reaction and the effect of the heater
8 cause the temperature of the molten bath in the
reaction vessel 3 to rise above the melting point of
titanium. However, the reaction vessel 3 is kept at a
pressure above a vapour pressure of magnesium at that
temperature, 80 that the magnesium, the titanium
product and the magnesium chloride by-product are all
kept in a liquid state. Also, the molten bath is
vertically separated into three layers, namely,
magneslum 12, magnesium chloride 13 and tltanium 14,
in that order, due to the difference in their densities.
The molten metallic tltanium product precipitates
and sinks through the molten magnesium layer and the
molten magnesium chloride layer to the bottom of the
reaction vessel 3 and reaches the top 14 of the tltanium
lngot 11, where lt remalns ln the molten state and 18
sub~ected to stirring and mixing by the lower electro-
magnetic induction heating coil 8b. This results in
the molten titanlum product belng homogeneous.
The titanium product is coalesced with the top of
the titanium ingot 11 and the titanium ingot 11 is
continuously drawn out at a suitable rate, during which
~, -
. .
,
;
: . :
.: . . . ~, . .
1 328561
.
-- 19 --
.
the product is cooled and solidified bv the cooling
agent circulated in the cooling pipes 33 of the
segments 32.
The magnesium chloride by-product 13 is discharged
through the discharge pipe 9 connected to the side of
the reaction vessel 3 at a discharge rate which is
ad~usted so that the molten bath at the reaction zone
is kept at a constant level. At this time, a part of
the magnesium chloride flows into the gap between the
titanium ingot ll and the wall of the reaction vessel
and solidifies there to form an insulating layer which
serves to prevent contact between the ingot ll and the
reaction vessel. The insulating layer exhibits heat
insulating and pressure sealing actions. The insulating
layer may be partially broken by mechanical friction
when the titanium ingot 11 is downwardly drawn out,
however, when this happens, the magnesium chloride
rapidly flows from the molten magnesium chloride layer
into the broken portion of the insulating layer and
solidifies to re-form an insulating layer. Also, the
molten titanlum is heated by the lower electromagnetic
.,
induction heating ,coil 8b and tends to levitate at its
central portion. Accordingly, magnesium chloride
readily flows into the gap between the wall of the
reaction vessel and the tltanlum lngot 11 to facllltate
formation of the addltlonal lnsulatlng layer.
The tltanlum lngot 11 ls drawn out at a rate
correspondlng to the amount of tltanium preclpltated on
the tltanlum lngot by means of rollers (not shown).
Accordlngly, the posltlon of the molten titanlum product
above the titanium lngot ll ls kept substantlally
constant. A part of heat of reactlon ln the reaction
vessel ls removed upwards from the reactlon vessel 3 by
:,
.
,
;
- .,........ . ' ~ ' - ' ' ~ :
.
- : , ., "
1 328561
- 20 -
radiation and convection, however, a large part of the
heat is outwardly removed by the cooling agent
circulated in the circulation pipes 33 at the segments
32 constituting the reaction vessel 3.
Accordingly, the present invention is carried out
under conditions where the temperature of the reaction
zone is kept above the melting point of the metallic
titanium product and its pressure is kept at least at
the vapour pressure of the reducing metal agent at that
temperature, so that boiling of the reduclng metal
agent and its chloride may be substantially prevented
to keep them at a liquid state in the reaction vess.el,
resulting in the reduction being carried out
efficiently.
The present invention also allows the metallic
titanium to be produced in the form of a liquid. The
separation of the metallic titanium product and the
chloride by-product of the reducing metal agent is
simple, as is the recovery of the by-product, and the
titanium ingot may be directly removed, enabling the
whole production apparatus to be small-sized.
Furthermore, the present lnvention permits
producing of metallic titanium to be continuously
carried out, 80 that the separating, crushing and
melting steps requlred in the conventlonal Kroll process
may be eliminated, leading to a significant decrease in
manufacturing costs whlle providing titanium of a high
quality.
The above description has been made in connection
with producing of titanium. However, the present
invention can also be applied to the production of
metals such as zirconium, hafnium, niobium and their
alloys, silicon, and the like.
~ ;, ., : . : -
-~
.. . . .
~ ~ ; ! ' ; .
~ i ~ ` : . ,
1 328561
-- 21 --
The present invention will now be illustrated with
reference to the following non-limiting Examples.
EXAMPLE 1
The example was carried out using an apparatus
constructed in accordance with Figure 1.
A reaction vessel having an inner diameter of 20cm
was used and a titanium ingot having an inner diameter
of 10cm was inserted into the mould section of the
reaction vessel to close the bottom. 20kg magnesium
chloride and 4.6kg magnesium were charged into the
reaction vessel, which was then fully closed.
An atmoqphere in the reaction vessel was replaced
with argon, the magnesium chloride and magnesium were
heated to 1000C by electromagnetic induction heating
and the reaction vessel was pressurized to about
5Oatms.
Immediately after such conditions were established
titanium tetrachloride and liquid magnesium kept at
800C were supplied to the reaction vessel at feed
rates of 4.0Q/min (7.0kg/min) and 1.2Q/min (1.8kg/min),
respectively. This caused a temperature of the bath to
- rise rapldly to 1827C, and so the power for the
electromagnetic induction heating was decreased to keep
the temperature at 1827~50C.
~; Subsequently, the ingot was drawn out downwardly
at an average veloclty of 4.9cm/min. The operation was
continued for 3 hours, resulting in a tltanium ingot
belng produced ln an amount of 0.3 ton.
The magnesium chloride by-product produced during
the operation was continuously discharged from the
reactlon vessel at the appropriate rate to keep the
depth of the bath in the reaction vessel constant.
. .
.,
~ '. , ~ ''."' '`" ' ,
- ~ :
l 328561
The titanium ingot so produced was compared to
titanium sponge produced by the Kroll process. It was
found that the titanium ingot had a high purity and
quality as indicated in Table 1, in which the figures
are in wt% and the balance is titanium.
;. .
.
~ . :. , '
1 328561
-- 23 --
~ _ _ ~
._, ~ ~
_, n IL =
D _ _ __ . _
E~ u~ o M
z o Al
l ~ ', ', '' 3
I]) :l~ L. ,
.. . ~ ~.. .. ... .; - . .~
; . .. . , . ;, ~ ~ .
6 1
- 24 -
EXAMPLE 2
This example was carried out using an apparatus
constructed in accordance with Figures 2 and 3.
A reaction vessel having an inner diameter of 20cm
was used and a titanium ingot having an inner diameter
of 19.5cm was inserted into the mould section of the
reaction vessel to close the bottom. Then, 20kg
magnesium chloride and 4.6kg magnesium were charged
into the reaction vessel, which was then fully closed.
The at~osphere in the,reaction,vessel was replaced
; with argon and the top of the titanium ingot ànd the
reaction vessel were heated by electromagnetic
induction heating to heat magnesium chloride and
magnesium in the reaction zone to a temperature of
1000C. The magnesium chloride melted by the heating
flowed into the gap between a wall of the reaction
vessel and the titanium ingot to form an insulating
layer which also exhibited a pressure sealing action.
The reaction vessel was then pressurized to about
50 atms. Immedlately after such conditions were
attained, titanium tetrachloride and liquid magnesium
kept at 800C were supplied to the reaction vessel at
feed rates of 4.0Q/min (7.0kg/min) and 1.2Q/min
(1.8kg/min), respectively. This caused the temperature
of the bath to rlse rapldly to 1827C, and 80 the power
for the electromagnetlc inductlon heating waA decreased
to keep the temperature of 1827C~50C.
Subsequently, the ingot was drawn out downwardly
at an average velocity of 1.3cm/min. The operation was
continued for 2 hours, resulting in titanium ingot
belng manufactured ln an amount of 0.2 ton.
The magnesium chlorlde by-product produced durlng
the operatlon was continuously discharged from the
.
,.
.
. i
~ 32856~
- 25 -
reaction vessel at the appropriate rate to keep the
depth of the bath in the reaction vessel constant.
The titanium ingot so produced was compared to
titanium sponge produced by the Kroll process. It was
found that the tianium ingot had a high purity and
quality similar to that shown in Table 1.
. . .
, i . , . ~ .