Note: Descriptions are shown in the official language in which they were submitted.
:`
132~980
METHOD AND APPARATUS FOR CONTINUOUS SYNTHESIS
OF AQUEOUS ALUMINUM SULFATE SOLUTION
FROM ALUMINUM HYDROXIDE SLUDGE
BACKGROUND OF THE INVENT~ON
5 1. Field of the Invention:
This invention relates to a method for continuous synthesis
of an aqueous aluminum sulfate solu1ion from the aluminum sludge
separating, in a state containing aluminum hydroxide as a main
component thereof, from the effluent originating in the step of
10 treatment of alunminum for the production of Alumite (a product
Gf aluminum having a corrosion proof coating formed thereon by
anodization) operated by an aluminum-related enterprise and to
an apparatus for effecting the method.
2. Description of the Prior Art:
In the work of Alumite treatment, as a pretreatment therefor,
an aluminum blank is etched with a caustic soda solution to remove
scratches or other similar flaws inflicted on the surface .hereo.
and smoo.hen the surface. Otherwise in the work of reprocessing
(recoating) a rejectable aluminum product, this product is
treated with a caustic soda solution for the removal of an o:cide
coat therefrom. Further, the aluminum blank is subjected to a
treatment for anodic oxidation and a treatment for electrolytic
coloration in a sulfuric acid solution. During these treatments,
Al ions are dissolved out of the aluminum blan~c into the aforemen-
tioned solution and entrained thereby into an adjoining washingtank. The spent washing water is forwarded to the step for
disposal of waste water. While the waste water is undergoing a
neutrali~ing treatment, there occurs a white precipitate o
aluminum hydroxide.
.-, .,, -- 1
1328980
The waste liquid (slurry) consequently produced is
generally caused by addition of an acrylamide type macromolecular
flocculant to form a floc of high solidity. This floc is
dehydrated to form an aluminum sludge (hereinafter referred to
simply as ~sludge") and the sludge i3 subjected to the subsequent
treatment (to be discarded or recovered). This sludge is
generally composed of 83 to 87% of water, 8 to 12% of Al(OH)3,
and 6 to 3% of impurities (such as SiO2 and organic substances).
In the surface treatment of aluminum,an aqueous sulfuric
acid solution is used for the removal of the coat from aluminum
surface and the coat adhering to the surface of a jig. These-
works for the removai of coats give rise to waste sulfuric acid
as a waste of aging. This waste sulfuric acid is generally
composed of 75 to 90% of free H2O and 2 to 0.5% of Al2SO4)3.
From the standpoint of prevention of environmental pollution
and preservation of resources, the sludge and the waste sulfuric
acid risin~ from the works of aluminum surface treatment are
.generally utilized for the synthesis of aqueous aluminum sulfate
solution. The reaction involved in the synthesis of aluminum
sulfate, however, proceeds so ~uickly as to render control of
reaction velocity difficult. Further, since the aluminum sulfate
as a final product has the pH value thereof regulated to suit
the purpose for which the product is put to use, the final pH
value of the liquid for the synthesis must be adjusted so as to
equal the pH value of the aluminum sulfate. Since this adjustment
of the pH value is difficult, it has been held difficult to
produce the aqueous aluminum sulrate soluiion by the method of
continuous synthesis.
2 -
i328980
The synthesis or the aqueous aluminum sulfate solution from
the sludge has been heretofore carried out exclusively by the
so-called batchwise process, which generally comprises charging
a vessel for the synthesis with a fixed amount of waste sulfuric
acid and heating the contents of the vessel with z fixed amount
of the sludge supplied gradually thereto.
The aforementioned conventional method requires the vessel
for synthesis to be made of a material excellent in corrosion-
proofness because the contents of the vessel are composed mainly
of highly concentrated waste sulfuric acid during the initial
stage of the synthesis. For example, vessels of the type
provided with a glass lining are now in popular use for the
synthesis. The vessel of this material has the disadvantage
that it is expensive, entails difficult maintenance works of
lS repair and inspection, and tends to break easily. Moreover,
since the synthesis is made batchwise, the vessel used therefor
is inevitably large and the driving power is proportionately
large and the equipment as a whole is voluminous and occupies
a large floor area. Thus, the synthesis entails a high cost or
equipment and the vessel used therefor cannot be easily e~panded
for increase of capacity. Further, owing to the batchwise
production, the synthesis has the disadvantage that the operation
to be involved is complicated in procedure and inferior in
efficiency and the vessel for the synthesis which is heated by
means of a jacket is destined to suffer from gradual loss or
thermal efficiency due to the phenomenon of scaling and entail
a high running cost. ~ -
~ .
., ~
1328980
As one of the various kinds of waste occurring in the works
of aluminum surface treatment, a dilute aqusous sulfuric acid
solution arising, in a state containing aluminum sulfate (herein-
after referred to brie.ly as "sulruric alum"), during the
S recovery of sulfuric acid from the electrolyte constitutes itself
a problematic by-product besides the aforementioned sludge and
waste sulfuric acid. The aqueous sulfuric acid solution
containing this sulfuric alum has not occurred to any appreciable
extent to date and, therefore, has been sufficiently coped with
by the treatment of waste water. rn recent years, various
methods for the treatment of electrolytes such as the method
~; using ion-exchange resins for separation and the method using
~:
~; diffuse transmission membranes for separation have advanced.
Owing to the advance of these methods, the aqueous sulfuric acid
solution containing the sulfuric alum is rapidly gainins in
volume. In the operation of the method using an ion-exchange
¦ resin for separation, for example, part of the sulfuric acid-
containing electrolyte in the electrolytic cell for the treatment
of anodic oxidation or the treatment of AC coloration is
subjected to an ion-exchange treatment. While the sulfuric acid
recovered by this ion-exchange treatment is returned to the
electrolytic cell, this treatment gives rise to a treated water
containing part of the sulfuric acid and sulfuric alum. Also in
the operation of the method using a diffuse transmission
membrane, part of the electrolyte in the elec~rolytic cell is
treated with the diffuse transmission membrane. While the
sulfuric acid recovered by this treatment is returned to the
- 4
.
:
.~, .
1328980
electrolytic cell, the treatment produces a trea~ed water
containing part of the sulfuric acid and sulfuric alum. The
dilute aqueous sulfuric acid solution containing the sulfuric
alum generally contains 3 to 6% of H2S04 and 7 to 9% of A12(S04)3.
In recent years, as the installation of facilities for the
treatment of electrolytes has increased in ratio to keep pace
with the imporovement of quality of the aluminum surface
treatment, this dilute aqueous ~ulfuric acid solution has been
growing in volume. In the circumstances, the question as to how
the dilute aqueous sulfuric acid solution ought to be safely
disposed of has been driving the industry concerned to its wit's
end. An idea may possibly be conceived o' utilizing this
solution for the aforementioned batchwise synthesis of an aqueous
aluminum sulfate solution. The present applicant for patent
himself is partially utilizing the solution for the synthesis.
Since the aforementioned sulfuric alum-containing aqueous sulfuric
acid solution is a dilute liquid, the use of this solution in an
increased volume inevitably results in a decrease in the reaction
velocity. Further, since the synthesis is made batchwise, it
does not suit commercialization.
SUMMARY OF THE INVENTION
This invention, therefore, has been produced for the purpose
of overcoming the drawbacks suffered by the prior art as
described above. An object of the invention is to provide a
method for permitting continuous synthesis of an aqueous
aluminum sulfate solution by effective use of the sludge, waste
sulfuric acid, and sulfuric alum-containing aqueous aluminum
~, ~.'~'
~;. , - 5 -
.,...,..' .
.: :
... ~ ,
'.-': -
,
1328~80
sulfate solution arising in the works of aluminum surface treat-
ment and causing serious anxiety to the industry concerned
regarding safe disposal thereof and an apparatus to be used for
effecting the method.
Another object of this invention is to provide a method
for continuous synthesis of an aqueous aluminum sulfate solution,
which method excels in productivity, workability, safety, etc.,
operates with relatively inexpensive equipment, and affords the
- product in a high yield, and an apparatus for effecting the
method.
Yet another object of this invention is to provide a method
for effecting continuous synthesis of an aqueous aluminum sulfate
solution with high energy efficiency at a low running cost and
~! an apparatus for working the method.
i 15 To accomplish the objects described above according to the
present invention, there is provided a method for continuous
production of an aqueous aluminum sulfate solution from by-products
occurring in the work of aluminum surface treatment, which method
is characterized by the steps of feeding aluminum sludge
~0 arising in the work of aluminum surface treatment and consisting
mainly of aluminum hydroxide, waste sulfuric acid arising during
the removal of coat in the aforementioned work of surface treat~
ment, and an aluminum sulfate-containing aqueous sulfuric acid
solution arising during the recovery of sulfuric acid in the
aforementioned work of surface treatment to a reaction tank
consisting of a plurality of tanks, i.e. first through n'th
tanks, causing the fed substances to flow sequentially through
.. . .
-- 6 --
~ .
.. .
. :
;. :
. . ~ , .
.~ . . . . ..
, ~ ~
1328980
the first tank through the n'tn tank ~last tank) to undergo
reactions under temperature conditions in the range of 80C to
the boiling point of the reaction solution and, at the same time,
feeding part of the aforementioned aluminum sulfate-containing
aqueous sulfuric acid solution to at least one of the second
tank through the last tank, and adjusting the pH value of the
reaction solution in the last tank at a level in the range of
1.6 to 2.5.
In the method described above, the adjustment of the pH value
of the reaction solution constitutes itself one of the important
3 factors for efficient continuous synthesis aimed at. In accordance
with the present invention, therefore, there is provided an
apparatus for continuous production of the aqueous aluminum
sulfate solution, which apparatus is designed in order for the
` lS adjustment of the pH value to be effected properly. This apparatus
q is characterized by comprising:
a reaction tank consisting of first through n'th tanks
adapted to permit continuous reactions respectively of aluminum
sludge arising in the work of aluminum surface treatment and
consisting mainly of aluminum hydroxide, waste sulfuric acid
arising during the removal of coat in the aforementioned work of
surface treatment, and an aluminum sulfate-containing aqueous
sulfuric acid solu~ion arising during the recovery of sulfuric
acid in the aforementioned work of surface treatment,
a first feed pipe for feeding the aforementioned aluminum
sulfate-containing aqueous sulfuric acid solution to the aforemen-
tioned first tank,
'~,~
.~ ".!--
7 --
:`~
; . .:
, . ~ ~ .
.,.,: .
1328980
an n'th ~eed pipe (n for an integer of the value of at
least 2) for feeding the aforementioned aluminum sulfate-containing
aqueous sulfuric acid solution to at least one of the second ar.d
subsequent tanks,
5 a first pH indicator--con1roller-recorder disposed inside
the aforementioned first or second tank,
a first control value interlocked with the aforementioned
,irst pH indicator-controller-recorder and adapted to con~rol
the flow volume within the aforementioned first feed pipe,
an n'th indicator-controller-recorder (n for an integer of
the value of at least 2) disposed in at least one of the afore-
mentioned second and subsequent tanks, and
an n'th control valve (n for an integer having the value of
at least 2) interlocked with the aforementioned n'th indicator-
controller-recorder and adapted to control the flow volume within
the aforementioned n'th feed pipe.
Further in the a'orementioned method, the control o. the
. temperture o the reaction solution also constitutes itself
~ another important factor efficient continuous synthesis aimed at.
:~ 20 In accordance with the present invention, therefore, there is
; provided an apparatus for reaction temperature control designed
;3 to permit continuous production of the aqueous aluminum sulfate
solution from by-products occurring in the work o. aluminum
surface treatment, which apparatus is characterized by comprising:
the aforementioned reaction tank consistins o. first through
' n'th tanks,
:j ~ - 8 -
.. . . . .
'~.~ ' ' .
~ -, , . .: . . ..
13~8980
a first heat exchanger for effecting exchange OL heat between
the final aqueous aluminum sulfate solution emanating from the
aforementioned reaction tank and possessing a boiling point
approximating.the boiling point OL the reaction solution and the
- 5 aforementioned aluminum sulfate-containing aqueous sulfuric acid
solution,
, a first, steam pipe disposed inside the aforementioned first
tank,
. a first temperature indicator-controller-recorder disposed
inside the aforementioned first tank,
a first temperature control valve interlocked with the
aforementioned first temperature indicator-controller-recorder
: and adapted to control the flow volume of steam within the
aforementioned first steam pipe,
lS an n'th steam pipe (n Cor an integer OL the value of at
least 2) and an n'th temperature indicator-recorder (n for an
~'~ integer of the value of at least 2) disposed inside at least one
: of the aforementioned second and subsequent tanks, and
.. . .
:.~ an n'th temperature control valve (n for an integer o~ the
- - .
,, 20 value of at least 2) interlocked with the aforementioned n'th
temperature indicator-recorder and adapted to control manually
or automatically the flow volume of steam within the aforemen-
tioned n'th temperature indicator-recorder.
- BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a flow sheet schematically illustrating a typical
apparatus adapted to continuous synthesis of an aqueous aluminum
sulfate solution according with the present invention.
~: Fig. 2 is a partial flow sheet schematically illustrating
_ g _
~_, . . .
" ,
,.',' : - .
,~ , .
, .,
.. .
1328980
another embodiment of this invention, and
Fig. 3 is a partial longitudinal cross section another
reaction tank usable in the apparatus according with the
present invention.
DETAILED DESCRIPTION OF THE INVENTION
In the present invention, an aqueous aluminum sulfate
solution is continuously synthesized by causing aluminum sludge
occurring in the work of aluminum surface treatment, waste
sulfuric acid occurring during the removal of a coat in the
arorementioned work of surface treatment, and an aluminum
sulfate-containing aqueous sulfuric acid solution occurring during
the recovery of sulfuric acid in the aforementioned work of
surface treatment to be reacted sequentially in a plurality of
reaction tanks. The synthetic aluminum sulfate solution obtained
at the end of these reactions is subjected to filtration and
concentration to give rise to aluminum sulfate as a finished
product. The attributes of the finished product depend on the
final pH value of the synthetic aluminum sulfate solution and are
regulated in a fixed range suitable for a particular purpose for
which the finished product is used. In the present invention,
the sulfuric alum-containing dilute aqueous sulfuric acid solution
is utilized for the adjustment of the pH value of the reaction
solution.
The reaction for the synthesis of aluminum sulfate is
represented by-the following formula (1) and the reaction formula
for the sa~e of calculation is represented by the following formula
(2).
1 0
~ . , - . , - iO
: .. :,
., .
1328980
2AL(OH) 3 + 3H2SO4 ~A~2 (S0~,)3 + 6H20 (1)
. A~205 + 3H2S04 ~ A~2 (SO~ ) 3 * 3H20 (2)
The reaction velocity constant in the reaction mentioned
j above and the effect of pH on the reaction were studied using a
3 5 sludge, a waste sulfuric acid, and a sulfuric alum-containing
dilute aqueous sulfuric acid solution possessing respective
compositions indicated in Table 1 below.
Table 1
: Waste Sulfuric alum-containing
Composition Sludge sulfuric aqueous sulfuric acid
acid solution
Al(OH)3 9.5% _
~ H2O 86.8% ) 9.4~) 85.8
.~ Other subs~ances (such 3.7%
15 as micro-molecular
:. 'locculant)
H2SO4 _ 90.0% 5.3%
A12~5O4)3 1 _ 0.6% 8.7%
..
Since the reaction of the aforementioned three materials
20 was e::perimentally demonstrated to be regarded as a homogenous
second order reaction, the velocity constant of the reaction was
determine by a batch test. From the results of the test, it is
found that the velocity constant at 90 to 100C was 0.12 ~ 0.671
~/A12O3 mol sec. The results of various runs of the test
conducted using the three materials in varied mixing ratios
indicate that the proper value of the velocity constant ought to
,,--, - 11 -
. . .
,.. : , :
~:. :
:,, ,
`
- ~ 1328980
exceed at least 0.01 0/A12O3 mol-sec. In view of commercial
operation of the synthesis, the highest permissible limit of the
velocity constant is 2.0 ~/A12O3-mol sec.
If the pH value is unduly high, namely the amount of free
5 H2SO4 is undully small, the reaction velocity is too low for the
synthesis to be commercially feasible. In consideration of the
fact that the final pH value of the synthetic solution is subject
to control, therefore, it is most desirable in the case of
continuous reaction to control this continuous reaction in such a
10 manner that the pH value of the reaction solution in the first tank
in the compound of reaction tanks will be lowered to heighten the
reaction velocity and the pH values of the reaction solutions in
the subsequent tanks will be gradually increased sequentially to
adjust the final pH value of the synthetic solution eventually to
the prescribed level. As the result of an experiment conducted
by the inventors, it has been found that when the sludge, the
'~ waste sulfuric acid, and the sulfuric alum-containing dilute
aqueous sulfuric acid solution are continuously fed in a fixed
gravimetric ratio to a multi-stage (3 to 6 stages) reaction tank
~;; 20 having the component stages provided with respectively fixed
volumes commensurate with the intended volumes of treatment at
respecitvely fixed temperatures (in the range of 80C to the
boiling point of the reaction solution, preferably 90C to the
?l~ ~ boiling point), the synthetic aluminum sulfate solution possessing
a pH value (in the range of 1.6 to 2.5, preferably 1.8 to 2.0)
resulting from completion of the reaction is continuously obtained
from the final stage of the reaction tank. The sulfuric alum-
containing dilute sulfuric acid solution is fed in such a manner
,i,,
- 12 -
. .
: . - , .
.. . .
~`;
1328980
that the pH values of the reaction solutions in the component
tanks will be retained at respectively prescribed levels. For
the purpose of increasing the reac~ion velocity thereby producing
the aqueous aluminum sulfate solution in an increased yield, it
is desirable to control the pH value in the first reaction tank
in the range of 0.1 to 1Ø For commercial operation of the
synthesis, it suffices to control this pH value in the range of
0.1 to 2Ø
Now, the present invention will be described in detail below
with reference to working examples of the present invention
depicted in the accompanying drawings.
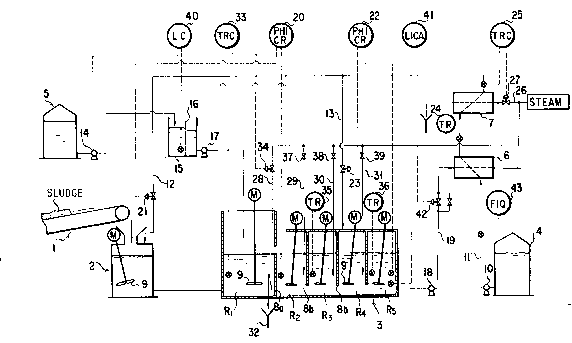
Fig. l is a flow sheet schematically illustrating a typical
apparatus adapted to effect continuous synthesis of an aqueous
alumi'num sulfate solution by the multi-stage tank type parallel-
flow reaction of the present invention. In the diagram, 1 standsfor a belt conveyor for forwarding sludge having aluminum hyd'roxide
as a main component thereof, 2 for a mixing tank, 3 for a reaction
tank consisting of first through n'th tanks, 4 for a tank for
holding a sulfuric alum-containing dilute aqueous sulfuric acid
2~ solution, and 5 for a waste sulfuric acid tank.
The reaction tank illustrated in Fig. 1 represents a case
satisfying n = 5, i.e. using five component tanks. The first tank
Rl and the second tank R2 are partitioned with a weir-like bulkhead
8a raised upright so as to permit overflow. The second tank R2
and the third tank R3, the third tank R3 and the fourth tank R4,
and the fourth tank R4 and the fifth tank R5 are severally
partitioned with respective bulkheads 8b suspended from above so
-- - - 13 -
~,. ... . .
.: ,
." . .. ..
,, .:
. '` '
:
1328980
as to form openings in the bottom parts thereof. These component
tanks are each provided with a stirrer 9 adapted to be rotated
by an electric motor M.
The sludge is fed as carried on the belt conveyor 1 to the
mixing tank 2. In the meantime, the sulfuric alum-containing
i dilute aqueous sulfuric acid solution kept at normal room temper-
ature in the dilute sulfuric acid solution tank 4 is forwarded
by a pump 10 via a feed line 11 to a first heat exchanger 6,
wherein the solution is heated to a temperature of about 65 to
, 10 70C through exchange of heat with the produced synthetic aluminum
' sulfate solution possessing a temperature of about 100C, and then
forwarded to a second heat exchanger 7, wherein the solution is
heated to a still higher temperature of about 95 to 100C through
exchagne of heat with steam possessing a temperature of boiling
point, and thereafter fed to the mixing tank 2 through a first
feed pipe 12. Part of the heated solution is fed to the fourth
-~ tank R4 preceding the last tank through a second feed pipe 13.
The mixture formed of the sludge with the sulfuric alum-containing
aqueous sulfuric acid solution inside the miY~ing tank 2 is now
. 20 in a state kept at an elevated temperature of about 70 to 80C,
mixed with the stirrer 9, and then forwarded to the first reaction
tank Rl. Meanwhile, the waste sulfuric acid held inside the waste
sulfuric acid tank 5 is forwarded by a pump 14 to a waste sulfuric
;` acid receiving tank 15, deprived of floating substances with a
screen 16 disposed inside the receiving tank 15, and then forwarded
:~ to the first reaction tank Rl by a pump 17.
, .
/~ .
~q ~ ~ i - 14 -
,", . . - .
1328980
- In the reaction tank 3, the reaction solution formed inside
the first tank Rl of the mixture of sludge, waste sulfuric acid,
and sulfuric alum-containing aqueous sulfuric acid solution is
caused to flow from the first tank Rl to the final fifth tank R5
as kept stirred by the stirrer 9 and undergo reactions represented
by the formula (1) mentioned above. As a result, the synthetic
aluminum sulfate solution is obtained at about 100C from the
fi th tank R5. This solution is forwarded by a pump 18 through
a produced solution line 19 and the first heat exchanger 6 to a
.iltration-purification unit (not shown).
Now, the control system will be described below. First, as
- regards the control of the pH value of the reaction solution, the
pH value of the reactLon solution in the first tank Rl and the
second tank R~ is fixed at a level of about 0.8. When the actual
feed amount o. the sludge is changed so as to fall far below the
theoretical feed amount, the feed amount of the waste sulfuric
~ acid is proportionately increased, with the result that the pH
,i value shifts from the fixed level, pH 0.8, toward the acidic side
(to pH 0.2, for example). In this case, therefore, the pH value
20 must be controlled. This adjustment of the pH value is effected
by a first pH indicator-controller-recorder (PHICR) 20 which is
disposed inside the second tank R2. When the reading of pH value
on the first pH indicator-controller-recorder is below 0.8
,~ ~strongly acidic side), a first control valve 21 disposed in the
i 25 first feed pipe 12 for the sulfuric alum-containing dilute aqueous
sulfuric acid solution and interlocked with the aforementioned
recorder 20 is closed. Conversely when the reading of pH value
r 1 5
. ,
,.
..
,
-
,-'.~ .
,: . . .
1328980
is above 0.8, the first control valve 21 is opened. Thus, the
pH value of the initial reaction solution is automatically
controlled to 0.8.by suitable control of the feed amount of the
sulfuric alum-containing dilute aqueous sulfuric acid solution.
The adjustment of the pH value in the first tank is normally
effected as described above. If the actual feed amount of the
sludge increases beyond the prescribed level as when the operation
of the apparatus is resumed after a stop or when the pH value in
the first tank is appreciably varled from the prescribed level by
a certain other factor, it naturally follows that the pH value
of the first tank Rl deviates from the prescribed level. For the
purpose of returning the pH value to within the fixed range, the
waste sulfuric acid having a lower pH value (strongly acidic side)
than the aforementioned sulfuric alum-containing dilute aqueous
sulfuric acid solution may be.used in the place of the dilute
aqueous solution just mentioned. When the reading of pH value on
the first pH indicator-controller-recorder 20 is below 0.8, the
supply of the waste sulfuric acid is discontinued by turning off
i the pump 17 of the waste sulfuric acid receiving tank lS. When
:~ 20 the reading of pH is above 0.8, the pump 17 1S opened and the
capacity thereof is increased (by opening the valve) to start
feeding the waste sulfuric acid to the first tank Rl. Thus, the
pH value is automatically controlled to 0.8. The suitable
increase or decrease of the feed amount of the waste sulfuric acid
possessing a low pH value is advantageous for pH adjustment
particularly where the pH value heavily deviates because the time
. required for the pH value to return to within the fixed range is
.
- 16 ~
~: . . . .
..
1328980
smaller than when the sulfuric alum-containing dilute aqueous
sulfuric acid solution is used. It will be readily understood by
persons skilled in the art that this pH adjustment can be accom-
plished by using both the sulfuric alum-containing dilute aqueous
sulfuric acidsolution and the waste sulfuric acid.
Optionally, the first pH indicator-controller-recorder 20
may be disposed inside the first tank R1 and adapted to detect
the pH value on the outlet side of the first tank Rl. As the
reaction proceeds, the pH value of the reaction solution increases
and shifts toward the weakly acidic side. When the pH value of
the synthetic aluminum sulfate solution within the fifth tank
(last tank) R5 falls on the strongly acidic side below the
,
prescribed level (1.6 to 2.5, preferably 1.8 to 2.0), the detector
of a~second pH indicator-controller-recorder (RPHCR) 22 disposed
inside the fifth tank R5 detects this deviation of the pH value
and closes a second control valve 23 disposed in the second feed
~;~ pipe 13 for the sulfuric alum-containing dilute aqueous sulfuric
:~
acid solution. When the pH value conversely rises above the
prescribed level, the aforementioned detector automatically opens
the second control valve 23. Tt is permissible to connect feed
pipes for the sulfuric alum-containing dilute aqueous sulfuric
acLd solution one each to the component tanks, disposing pH
indicator-cntroller-recorders one each in the component tanks,
` and effect fine adjustment of the pH values of the reaction
solution in the component tanks by the procedure described above.
In any event, since the adjustment of the pH value of the reaction
solution is effected by controlling the feed amount of the sulfuric
,, .
, ~ .
- 17 -
:;.- : :
"~
-.:- - . :. , -
.
~-: ,, ., ~ ,;,' ` , ~
.~ .. .
:: ~
132898~
alum-containing dilute aqueous sulfuric acid solution and/or that
of the waste sulfuric acid and further since the pH value of the
reaction solution is not less than 0.8 and the conversion in the
first tank is about 90~ and the pH value in the second tank cannot
fall below the aforementioned level, it suffices to use stainless
steel of the grade of about SUS ;16 JIL as the material for the
first tank and stainless steel of the grade of about SUS 316 L
as the material forthe second and subsequent tanks.
Then, as regards the control of the temperature of the
reaction solution, the temperature of the mixture of the sludge
and the sulfuric alum-containing dilute aqueous sulfuric acid
solution inside the mixing tank 2 is elevated to a level in the
range of 70 to 80C owing to the supply of the sulfuric alum-
containing dilute aqueous sulfuric acid solution which possesses
a temperature in the range of about 95 to 100C. The sulfuric
alum-containing dilute aqueous sulfuric acid solution kept at room
temperature inside the dilute sulfuric acid tank 4 is heated, as
described above, to a level of about 65 to 70C (as detected by
a temperature indicator-recorder (TR) 24) by means of the first
heat exchanger and then elevated further to a level of about 95
to 100C by means of the second heat exchanger 7. This temperature
is detected by a temperature indicator-controller-recorder (TRC)
25 disposed behind the second heat exchanger 7 in the feed line
for the sulfuric alum-containing dilute sulfuric acid solution.
When the temperature so detected is below the prescribed level,
a steam control valve 27 disposed in a steam feed pipe 2~and
interlocked with the aforementioned recorder 25 is opened.
. .
- - 18 -
- : .. ..
., . ,
' '
` 1328980
When the detected temperature is above the prescribed level, the
steam control valve 27 is narrowed. By thus controling the amount
of steam entering the second heat exchanger 7, the temperature
is automatically controlled to the prescribed level. To the
S reaction tanks are connected s~eam feed pipes. A first steam
pipe 28 is immersed in the form of a coil or a planar plate in
.he reaction solution held inside the first tank Rl and then led
to a waste discharge groove 32 so as to effect exchange of heat.
To the second through fourth tanks, a second steam pipe 29, a
third steam pipe 30, and a ~ourth steam pipe 31 are respectively
connected so as to e'fect introduction of steam. The temperature
; of the reaction solution insid~ the first tank Rl is controlled
by a first temperature indicator-controller-recorder (TRC) 33 and
a first temperature control valve 34 disposed in the aforementioned
~ lS .irst steam pipe 28 and interlocked with the aforementioned
'13~ recorder 33. When the temperature o' the reaction solution in the
~;~ first tank R~ is above the prescribed level, the first temperature
control valve 34 is narrowed. When this temperature is below the
prescribed level, the control valve 34 is opened. Thus, the
., .
temperature is automatically controlled to the prescribed level.
` To the third and fifth tanks R3 and R5 are respectively connected
~ a third and a fLfth temperature indicator-recorder (TR) 35 and 36.
`33~ Depending on the temperatures detected by these temperature
indicator-recorders 35 and 36, the control of temperature is
manually effected by opening or closing second through fourth
1 controi valves 37 through 39. Optionally, the temperature of the
' reaction solution may be automatically effected by disposing
-- 19 --
,.. . .. . . . . .
. , ; . - . .. .
~s ~
'
. :
1328980
temperature indicator-controller-recorders one each in the
component tanks and interlocking these recorders to the respective
temperature control valves in the steam pipes. Where the system
is designed so as to elevate the temperature by introducing
f 5 steam into the component tanks, the reaction solution is diluted
and consequently suffered to entail use of extra energy in the
subsequent work of purification. It is, therefore, desirable to
effec~ the elevation of the temperature of the reaction solution
in the first tank by the e::change of heat between the steam pipe
10 and the reaction solution as illustrated in the diagram.
Further in Fig. 1, the reference numeral 40 stands for a
liquid level indicator-controller (LC) in the waste sul~uric acid
receiving tank lS for preventing the motor o~ the pump 17 from
d idle rotation. It automatically actuates the pump 14 when the
15 li~uid level of waste sulfuric acid in the waste sulfuric acid
receiving tank lS falls below a fixed line mark and it stops the
! operation o, the pump when the liquid level rises above the mark.
The reference numeral 41 stands for a liquid level indicator-
t controller (LICA) provided with an alarm and adapted to control20 the liquid level of the reaction tank 3. It automatically narrows
a control valve 42 when the liquid level of the reaction solution
~ Ealls below the fixed mark and it opens the control valve 42 when
f' the liquid level rises above the mark. The reference numeral 43stands for a cumulative flow amount indicator (FIQ) adapted to
25 display the feed amount of the sulfur alum-containing dilute
aqueous sulfuric acid solution. Continuous synthesis of an
aqueous aluminum sulfate solution was carried out with the
apparatus of Fig. 1 under the following condition~.
~:
- 20 -
.. . .
.,. ~ - .. . .
- : ' ' ,
. ' . . . .
~ , ~ ... .
1328980
`''
` Conditions of synthesis
: Sludge:
. Feed amount - 1,429 kg/hour
:;~ Composition - Al(OH)39.5% by weight
-~` 5 Impurities 3.7% by weight
H~O86.8% by weight
. Waste sulfuric acid:
. Feed amount - 72.6 liters/hour
. Composition - Free H2SO490.0% by weight
. 10 A12(SO4)3 0.6% by weight
Specific gravity - 1.804 (25C)
Sulfuric alum-containing aqueous sulfuric acid solution
Feed amount - 3,112 liters/hour
Composition - Free H2SO45.3% by weight
Al~(SO4)3 8.9% by weight
H~O Balance
Specific gravity - 1.134 (12C)
Reaction temperature: 99 to 100C
~; : pH value of first tank: About 0.8
pH value of fifth tank: About 2.0
As the result, the aqueous aluminum sulfate solution could
be continuously synthesized at a conversion of about 98%. The
aqueous aluminum sulfate solution thus obtained was subjected
to a filtration test under the conditions, i.e. 300 ml as the
}~ 25 amount of synthetic solution used, 20C as the filtration
~; : temperature, filtration under a vacuum (without filtration aid)
as the manner of filtration, 95 cm2 as the available area of
- 21 -
, .
.
.
1328980
filtration, Filter Paper, #2, of Toyo Roshi Co., Ltd., and 120 Torr
as the filtration pressure (degree of vacuum). The test revealed no
problem as to the filtration property of the sclution.
Fig. 2 illustrates another typical apparatus for continuous
S synthesis of an aqueous aluminum sulfate solution of this
invention, depicting the only portions with respect to which this
apparatus differs from the apparatus of Fig. 1. The devices
(such as, for example, waste sulfuric acid tank and heat
`~ exchangers), the measuring instruments (such as, for example, pH
indicator-controller-recorders), and the feed lines (such as, for
e~ample, feed line for sulfuric alum-containing aqueous sulfuric
acid solution) which are not shown in Fig. 2 are identical to those
shown in Fig. 1. The apparatus illustrated in Fig. 1 is provided
with the mixing tank 2. This mixing tank is di~posed as illus-
trated simply because no reaction tank can be installed below thebelt conveyor where the existing apparatus is adopted in its
unmodified state. This installation of the mixing tank is not
always necessary. As illustrated in Fig. 2, the aforementioned
three raw materials can be directly fed to the first tank Rl of
the reaction tank 3 instead. In the apparatus of Fig. 2, the
sludge is forwarded by a pump through a sludge feed pipe 44. Of
~1 course, it can be forwarded by a belt conveyor as in the apparatus
of Fig. 1. In the reaction tank 3 illustrated in Fig. 2, the
component tanks Rl through R5 are partitioned alternately with
weir-like bulkheads 8a and bulkheads 8b suspended from above so
as to prevent otherwise possible short pass OI the reaction
: .
~ solution. For the purpose of more effectively preventing the short
.,
~ - 22 -
~. .~ , ,- : - , .,
. ,
-: . - ~ :
1328980
pass of the reaction solution, the component tanks may be provided
therein with a baffle plate 23 as illustrated in Fig. 3.
The control of the temperature of the reaction solution in
the first tank Rl is automatically effected, similarly to the
5 aforementioned apparatus of Fig. 1, by causing the first tempera-
ture indicator-controller-recorder 33 to control the first
temperature control valve 34 disposed in the first steam pipe 28.
By the same token, the temperatures of the reaction solution in
the second through 4th tanks R~ through R4 are automatically
~ 10 controlled by means of second through fourth temperature
indicator-controller-recorders (TRC) 45 through 47 disposed in
the respective component tanks and second through fourth tempera-
ture control valves 48 through 50 disposed respectively in the
second through fourth steam pipes 29 through 31. Further, the
lS temperatures of the component tanks are controlled by exchange of
heat between the portions of the reaction solution inside the
component tanks and the second through fourth steam pipes immersed
in the form of a coil or a planar sheet in the reaction solution.
The apparatus illustrated in Fig. 2 is provided with a
synthetic solution circulation line 52 which issues from the
discharge side of the pump 18 ror discharge of the synthetic
solution and returns into the first tank Rl. This circulation
line 52 is provided therein a manual valve 51. Owing to this
device, the aqueous aluminum sulfate solution can be returned to
the first tank Rl by opening the manual valve 51 when the second
pH indicator-controller-recorder (PHICR) 22 (Fig. 1) serving to
display the pH value of the synthetic solution displays an
- 23 -
:.~ ... .
:- .
132~980
abnormal value (outside the prescribed range). Of course, the
apparatus of Fig. 1 may be provided with the circulation line.
It is evident from the description given above, the following
effects and advantages are derived from the present invention.
a) Since the synthesis of the aqueous aluminum sulfate
solution is carried out continuously by the multi-stage tank type
parallel reaction, the capaclty for production is notably improved
as compared with the conventional synthesis by the batchwise
operation and, for a fixed volume of production, the apparatus
permits a generous reduction in overall size and floor area. The
apparatus, when necessary, may be installed indoors.
b) Since the adjustment of the pH values of the reaction
system is effected by the use of the sulfuric alum-containing
dilute aqueous sulfuric acid solution, the pH adjustment can be
attained easily and the free H2SO4 concentration in the first tank
~ ~ ,.
j is low. By controlling the pH values of the reaction solution in
the component tanks in the range of 0.8 to 2.5, therefore, the
reaction tanks made of stainless steel can be safely used. The
! reaction tanks, accordingly, can be given necessary maintenance
20 by proper welding, repair, and inspection. Thus, the cost of
equipment is low. Since the apparatus is continuously operated,
~ the pH value of the reaction solution can be continuously con-
s trolled without requiring the valves, pumps, stirrers, etc. to be
switched from time to time. The apparatus, therefore, can be
~ 25 easily automated. it is also effective in improving the work-
i ability and the safety.
,, .
- 24 -
,
~ 1328980
c) On the basis of the experimental data on the reaction
velocity constant, the capacity for production can be increased
with a minor modification of the apparatus.
d) The system of the present invention for reaction
temperature control entails virtually no loss of thermal
efficiency due to scaling because the heating is effected by the
use of the heat exchange units installed independently of the
~, reaction tanks. Further, the control of the temperature can be
,
,~ effected automatically. Owing to the ease of temperature control
10 coupled with the effect of continuous operation, the apparatus
enjoys high energy efficiency and low power consumption and a low
running cost as well.
The other advantages and effects of the present invention
will be apparent from the foregoing description of the invention.
: .
,~:~
:,~
,~
,~ .
.. . .
. .
's
i,s~
.,:,,
... .
~, .
',:
'`,
~ , .
., .
- 25 -
:
.' : '