Note: Descriptions are shown in the official language in which they were submitted.
~ 329 1 60
5-308/1~2l~/GBF
Process for the preparation of a macrocyclic compound
The present invention relates to a macrocyclic compound of formula I, to
a process for the preparation of said compound and to the use thereof for
controlling plant diseases. The invention further relates to microbicidal
compositions which contain this compound as active ingredient.
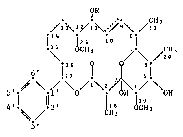
Specifically, the invention relates to a compound of formula I
~R
H3
15 CH3 ~ ~ ~20
5' ~ \ ~ \o/~\2 ~ / \ON (I)
4'~ 2' ~H8 CH3
3'
wherein R i8 methyl if there is a double bond in 9,.10-position
(~compound Ia~ or R is hydrogen i there i~ a single bond in
9,.10-positlon ~-compound Ib~ Throughout this specification, the
compound Ia will be designated as "Soraphen A" and the compound Ib as
, "Soraphen B". Pormula I encompasses in principle also the isomeric
i structures and mixtures thereof as well as any mixture of compounds Ia and Ib. Soraphen A i~ preferred~
Compounds of this class are novel and there are no references
ln the literature to such a structure
' . . .~, ~, .
:
, . . ' `
-
1 32q 1 60
-- 2 --
The compound of formula I is obtained by culturing 8 SorarlgiUm
(Polyan~ium~ cellulosum straln "So ce 26" by microbiological methods.
;~ Thls straln was deposited on March 5~ 1987, with the National Collection
`~ of Industrial and Marine Bacteria (NCIB), Torry Research Station,
~, Aberdeen, Scotland, ~K, under the number NCIB 12 411, in accordance with
the provisions of the Budapest Treaty on the International Recognition of
the Deposit of Microor~anisms for the Purposes of Patent Procedure.
~- Sorangium cellulosum belongs to the order of the Myxobacterales t suborder
-i~ Sorangineae, famlly of the Polyiqngiaceae.
The microorganism "So ce 26" was isolated in 1980 from a sample of goat
dung that had been collected on Djerba, Tunisia. "So ce 26" itself or
mutants or recombinants thereof which likewise produce compounds of
:~,
5~ formula I, constitute a further object of the present invention.
Sorangium "So ce 26" can be cultured by conventional biological methods,
e.g. in shake cultures or fermentorst with nutrient media. The
fermentation temperature i8 normally from 10 to 35C and ls p~eferably
ca. 30~-32C, and the pH is in the range from 6 to 8, preferably 7.4. The
process is carried out aerobically and under sterile conditions.
.,
.~
The composition of the nutrient medium can vary wlthin wide limits. A
carbon source and a nitrogen source as well as a source of inorganlc
elements comprlsing P, S, Mg, K and Ca must be present for the nutrients
to be essentially asslmilated.
The preferred carbon sources utilised in farmentation processes are
glucose, starch and cellulose. Additlonally, glycerol, acetic acid and
other sources may be used. Suitable nitrogen sources are NH4, N03 or al30
peptones. An organic compound used as nitrogen source cannot simulta-
~ neously be the sole carbon source and energy source for the fèrmentatlon.
Suitable mineral salts are chlorides, nitrates~ sulfate~, carbonates and
phosphate~ of the elements Na, K, NH4~ Mg and Ca. In addition, Fe, Cu~
Nn, Zn, Co and other elements may be present as trace elementa.
~J
: ~i
,',
;.1
;',' .
,~ . .
... . .
.~, .
.:`~ , .
~'' ' : '
_ 3 _ 1 3 2 9 1 6 0
The microorganism culture is added to the shak~ culture or fermentor inan amount of 0.1-7 % (vlv~, preferably 0.5-5 % ~vlv~. The reactlon time
at ca. 30C is about 2 to 7 days, more rarely up to 10 days. For large-
scale batches, smaller precultures are conveniently fermented initially.
The use of "So ce 26" i9 also possible in immobilised form, e.g. in the
form of carrier-fixed cells on alginate.
It will be clearly understood that the process of the present inventionis not limited to the use of the "So ce 26" strain NCIB 12 411, but
covers any other natural ~utant or recombinant, or one artificially
produced under laboratory conditions, which is able to produce a compound
of formula I. Those skilled in the art are readily conversant with
procedures for preparing mutants and recombinants of a microorganism.
Accordingly, the present invention relates to a process for the
preparatioD of the macrocyclic compounds of formula I by culturing the
microorganism Sorangium (Polyangium~ cellulosum "So ce 26" (NCIB 12 411)
or a mutant or recombinant thereof which is also able to produce the
compounds of formula I, in an aqueous culture ~edium. In a narrower
sense, the process comprises culturlng the microorganism Sorangium
(Polyangium) cellulosum "So ce 26" (NCIB 12 411) in a culture medium
containing at least one a~similable carbon source and one assimilable
nitrogen source as well as appropriate inorganic salts, in the
temperature range from 10 to 35C and in the presence or absence of an
adsorber resin, then extracting the culture broth or the isolated
adsorber resin with a suitable 601vent phase and, if desired, purifying
the residue ~o obtained by chromatography and/or recrystallisation.
Strain culture_and morphological description:
Straln cultures are kept on VYl2-agar ~0.5 % of baker's yeast by fresh
weight; 0.1 % of CaCl2; 1.5 % of agar; pH 7.2) or on filter paper over
ST21 agar ~0.1 % of KN03; 0.1 % of ~gS0~-7Hzo; 0.1 % of CaClz; 0,1 % of
K2HPO4; 0.01 % Of MDSO4~7~20; 0.02 % of FeCl3; 0.002 % of yeast extract;
standard trace element solut~on; 1 % of agar. The plates are incubated at
30C.
.' ' "
f~
~`
- 4 ~ 1329160
The strain forms large swarm colonies with many yellowish-orange to
black-brown fruiting bodies on filter paper over mineral salt agar or on
yeast agar (VY/2 agar). ~he fruiting bodies consist of small sporangioles
15-20 ~m in diameter, which lie tightly packed ln more or le98 large
heaps. The heaps are usually between 50 and 150 ~m in diameter. The
vegetative rods have the form ~ypical of Soran~ium: fairly co~pact and,
as viewed under a phase contrast microscope, dark cylindrical rods with
broadly rounded ends, on average 3-6 ~m long and ca. 1 ~m thick. The
strain grows in homogeneous cell suspension after relatively long
adaption to the growth in liquid media.
Biological characterisation of the strain "So ce 26" (NCIB 12 411)
glucose degradation : positive
cellulose degradation : positive
starch degradation : positive
NH4 as nitrogen source: positive
N03 as nitrogen source: positive
~,
The strain "So ce 26" produces two chemically closely related
compounds Ia and Ib, which inhibit the growth of numerous fungi. These
macrocyclic compounds can be isolated from the cell9 as well as from the
culture supernatant.
Lipophilic extractlon of the compound of formula I can be effected from
the aqueous fermentation broth with an organic solvent, e.g. a ketone
such a~ mathyl ethyl ketone or cyclohexanone; an alcohol of average chain
length such as isobutanol, pentanol or hexanol; a C1-C6alkyl ester of
acetlc acid ~ethyl acetate, propyl acetate, propyl acetate, butyl
acetatet isobutyl acetate and the like~; a hydrocarbon or a chlorinated
hydrocarbon such as hexane, toluene, dichloromethane, 1,2-dichloroethane,
chlorobenzene, dichlorobenzene and the like.
_j
The compound I can be readily extracted from the filtered cell cake with
; an alcohol ~e.g. meth=nol, ethanol~ acetone, methyl ethyl ketone~.
.
.
,~
. ~
~:
,.:
.'.................................... .~
: .
- 5 - 1329160
The compound of formula I can be iYolated from the respective extract by
concentratlon and/or precipitation and separated into the two
components Ia and Ib by fractional crystallisation or other separation
methods (e.g. column chromatography~.
The fermentation for the preparation of the compound of formula I is
preferably carried out in the presence of an adsorber resin on whlch the
desired product is adsorbed. Suitable adsorber resins are~ in particular,
neutral organic polymaric compounds, preferably nonionic hydrophobic
adYorber resins which are 6uitable for lipophilic extraction. The
compound of formula I i8 bound to the adsorber resin almost
`~ quantitatively. Examples of Yuch reslns are semipolar acrylate resinY 5
nonpolar polystyrene/divinylbenzene resins and, preferably, crosslinked
polystyrene. Such resins are added in amounts of 0.1 to 5 % (vlv~
; preferably 0.5 to 2 % (v/v), of the fermentation volume. Activated carbon
iH al~o suitable.
A technically very advantageous variant for obtaining the compound of
~ formula I consists in a fermentation of "So ce 26" (NCIB 12 411) under`~ ~ r. ~ the conditions indicated above and in the presence of a polystyrene resin
(e.g. XAD-1180, Rohm and Haas) which is conveniently in filterable form
(grains or granules~. Vpon completion of the fermentation~ the resin is
isolated by filtration, washed with water, and treated with methanol or
ethanol. The alcoholic extract is concentrated by svaporation~ the
compound of Is (Soraphen A), which crystallises after addition of diethyl
ether, i8 iYolated by filtration and from the filtrate the compound Ib
.jl
(Soraphen B~ is obtained and, if desired, purified by chromatogrsphy.
:'
The invention relate~ to the compound of formula I in the pure form or in
crysta}lised form. The invention also relates to biomasses, crude
eXtractY or adsorber resins resulting from the fermentation which contain
the compound of formula I and which ran be used as obtained or in fur-ther
formulated form for controlling plant disease~. Blomasses can also be
further used or mark~ted as ground or pressed solidY (cake~.
.', ''~
~f~de~ r k
.,
r~
., :
:,
., ' ' ' ' '~ ' " :
\,
- 6 - 1 3291 60
Surprisingly, it has been found that the compounds of formula I have, for
practical purposes~ a very advantageous biocidal 6pectrum again~t
phytopathogenic microorganisms, especislly agaiDst fungi. They have very
advantageou6 curative, systemic and~ in partlcular, preventive
properties, and are used for protecting numerous cultivated plants. The
compound6 of formula I can be u6ed to inhlbit or destroy the pests which
occur on plan~s or part6 of plants (fruit, blo6soms, leaves, 6tems,
tubers, rootsJ in different crops of useful plants, while at the same
time the parts of plants which grow later are also protected from attack
by phytopathogenic microorganisms.
A6 microbicide3, the compounds of formula I are effective against the
phytopathogenic fungi belonging to the following cla66es: Fungi
imperfecti (e.g. in particular ~otrytis and also Pyricularia,
}lelminthosporium, Fusarium, Septorla, Cercospora, and Alternaria~;
Basidiomycetes (e.g. Rhizocotonin, Hemilela, Puccinia). They are also
effective against the class of the Ascomycetes ~e.g. Venturia and
Erysiphe, and also Podosphaera, Monilinia and Uncinula), and of the
Oomycetes (e.g. Phytophthora~ Plasmopara). The compounds of formula I can
al60 be used as dres6ing agents for protecting seeds (fruit, tubers.
grains) and plant cuttings again6t fungus infections as well as again~t
phytopathogenic fungi which occur in the soil.
The invention also relates to compositions which contain the compounds of
formula I as active components, in particular plant-protecti~e
composition6 and to the use thereof ln the field of agriculture or
related fields. The invention further relates to a method of treating
plants~ which comprises applying thereto the compound6 of formula I or
the novel compositions which contain them.
Target crop6 to be protected within the scope of the present invention
comprise e.g. the followlng ~pecies of plants:
cereals (wheat, barley. rye, oats. rice, maize5 sorghum and related
species?, beet (~ugar beet and fodder beet), pomes, drupe6 and soft fruit
~apples, pears, plums. peaches, almonds, cherries~ strawberries,
raspberries and blackberries), leguminous plant6 (beans, lentils, peas,
~oybean6~, oil plants ~Fape. mustard. poppy, olives, sunflowers, coconut,
'
' :
':
.
.
~: f~,
1 329 1 60
-- 7 --
:,
castor oil plants, cocoa beans, groundnuts)~ cucumber plants ~cucumber,
marrow3, melons), fibre plants (cotton, flax, hemp, Jute), citrus fruit
(oranges. lemons, grapefruit~ mandarins), vegetables ~spinach~ lettuce,
asparagus, cabbages, carrots, onions, tomatoes, potatoes, paprika),
lauraceae (avocados, cinnamon~ camphor), or plants such as tobaccot nuts~
coffee, pineapples, sugar cane, tea, pepper, vines, hops, bananas and
; natural rubber plants, as well as ornamentals (composites~. ~his
recitation constitutsfi no limltation.
The compounds of formula 1 are normally applied in the form oE
compositions and ~an be applied to the cro,o area or plant to be treated,
simultaneously or in succession, with further compounds. These compounds
can be both fertilisers or micronutrient donors or other preparations
that influence plant ~rowth. They can also be selective herbicides,
insecticides, fungicides, bacteric~des, nematicides, mollusicides or
mixtures of several of these preparations, if desired toge~her with
r,~ further carriers, surfactants or application promoting ad~uvants
i~ customarily employed in the art of formulation.
.
Suitable carriers and adJuvants can be solid or liquid and correspond to
the substances ordinarily employed in formulation technology, e.g.
natural or regenerated mineral substances, solvents, dispersants, wetting
agents, tackifiers, thickeners, binders or fertilisers.
;`
A preferred method of applying a compound of formula I, or an agro-
chemical composition which contains at least one of said compounds, is
foliar application. The number of applications and the rate of
application depend on the risk of infestation by the corresponding
pathogen. However, the compouDd of formula I can also
penetrate the plant through the roots via the soil (systemir action~ by
impregnating the locus of the plant with a li~uid formulation, or by
i applying the compounds in solid form to the soil, e.g. in granular form
(soil application~. This granular formulation or a correspondlng powder
may also be the dried biomafis from the fermentor or the adsorber rasin
sieved off from the fermentation broth and contalning the comoounds of
formula I. The compounds of formula I may also be applied to
.~ .
.
.,:
' ! ,
; ~ .
~. .. . .
.
'. . ~ :.
... . '
1 32q 1 60
seeds ~coating) by impregnating the seeds either with a liquid
formulatio~ containing a compound of formula I, or coating them with a
solld formulation.
The compounds of formula I are used ln unmodified form or, preferably,
together wlth the ad~uvants conventionally employed in the art of
formulation, and are therefore formulated ln known manner to emulsifiable
concentrates, coatable pastes, directly sprayable or dilutable solutions,
dilute emulsion~ wettable powders, soluble powders, dusts~ granulates,
and also encapsulations in e.g. polymer substances. As with the nature of
the compositions, the methods of application, such as spraying,
atomislng, dusting, scattering, coating or pouring, are chosen in
accordance with the intended objectives and the prevailing circumstances.
Advantageous rate~ of application are normally from 10 g to 2 kg of
active ingredient (a.1.) per hectare~ preferably from 50 g to
500 g a.i./ha.
The formulations, i.e. the compositions containing the compound ~active
ingredient) of formula I and, where appropriate, a solld or li~uid
ad~uvant, are prepared in known manner.
~,
Sultable solvents are: aromatic and aliphatic hydrocarbons, e.g. xylene
mixtures, cyclohexane or paraffins, also alcohols and glycols and their
ethers and esters, such as ethanol, ethylene glycol, ethylene glycol
monomethyl or monoethyl ether and acetates; ketones such as cyclo-
hexanone, strongly polar solvents such as N-methyl-2-pyrrolldone,
dimethyl sulfoxlde or dimethyl formamide, as well as vegetable oils or
epoxidised vegetable oils such as epoxidised coconut oil, sunflower oil
or soybean oil; or water.
The solid carriers used e.g. for dusts and dispersible powders, are
normslly natural mineral ~illers such as calcite~ talcum, kaolin~
montmorillonite or attapulgite. In order to improve the physical
properties it is also posslble to add highly dispersed silicic acid or
highly dispersed absorben~ polymers. Suitable granulated adsorptive
carrlers are porous types, for example pumice~ broken brick~ sepiolite or
bentonite; and suitable nonsorbent csrriers are materials such as calcite
,
~ ~ .
.
' ' ' ' ' ' ' " " ' , ,
,
:
9 1329160
.,
or sand. In addition, a great number of pregranulated materials of
~, inorganic or organic nature can be used, e.g. especially dolomite or
pulverised plant residues.
' Depending on the nature of the compound of formula I ~o be formulated,
suitable surface-active compounds are non-ionlc, cationic and/or anionic
surfactants having good emulsifying, dispersing and wettlng properties.
~ The term "surfactanta" will also be understood as comprising mixtures of
¦ surfactants.
., .
; Suitable anionic surfactants can be both water-soluble soaps and water-
soluble synthetic surface-active compounds.
,!
More frequently, however, so-called synthetic surfactants ara used~
especially fatty ~ulfonates, fatty sulfates, sulfonated benzimidazole
derivatives or alkylsulfonates.
Nonionic surfactants are polyglycol ethez derivstives of aliphatic or
; cycloaliphatic alcohols, or saturated or unsaturated fatty acids and
alkylphenols, said derivatives containing 3 to 30 glycol ether groups and
8 to 20 carbon atoms in the ~aliphatic~ hydrocarbon moiety and 6 to
18 carbon atoms in the alkyl molety of the alkylphenols.
i~
~l Further examples of nonionic surfactants are nonylphenolpolyethoxy-
ethanols, castor oil polyglycol ethers, polyprupylene~ polyethylene oxide
sdducts~ tributylphenoxypolyethyleneethanol~ polyethylene glycol and
octylphenoxypolyethoxyethanol. Fatty acid esters of polyoxyethylene
sorbitan, e.g. polyoxyethylene sorbitan trioleate, are also suitable
`I nonionic surfactant3.
, ! j
Further surfactants customarily employed in formulation technology are
known to those skilled in the art or may be found in the relevant
, literature.
The agrochemiral compositions usually contain 0.1 to 95 % by weight of a
~, compound of formula I, 99.9 to 5 % by weight of a solid or liquid
adjuvant~ and 0 to 25 % by weight of a surfactant.
.,
:
~ . :
~, -
- lO - 1 329 1 60
Whereas commercial products wlll preferably be formulated as concen-
trates, the end user will normally employ dilute formulations.
?.
The compositions may also contaln further auxiliaries ~uch as
stabilisers, antifoams, viscosity regulators, binders, tackifiers as well
as fertilisers or other active ingredients for obtaining special effects.
,
The invention i~ illustrated in more detail by the following
non-limitative Examples.
1. Preparatory Examples
Example Pl: Preparation of the compound of formula I
A 70 litre fermentor (available from Giovanola, Monthey, Switzcrland) is
charged with 60 litres of medium ~0.1 % of peptone; 0.5 % of glucose;
0.05 % of CaCl2-2H20; 0.05 % of MgSO4~7H20; pH 7.4~. This medium i9
inoculated wlth 10 litres of a preculture prepared in the same medium in
a shaking flask (160 rpm, 30C~. Fermentation is then carried out at 32C
,~.
and at a stirring speed of 500 rpm and with aeration at a rate of 0.5 Nm3
-; per hour. The fermentation takes about 7 days. The compound of formula I
is produced during the logarithmic phase right through to the &tationary
~i~ growth phase. It becomes enrichad partly in the cells, partly in the
culture supernatant.
,~i
I The fermentation broth i9 then extracted with 5 x 2 litres of et}lyl
acetate for 5 minutes each time. The combined extracts are washed twice
with water and concentrated under vacuum. The residual dark oil i9
`~ ~ chromatographed over silica gel ~Lichroprep~S160~ Merck~ 25-40 ~m.
~ Eluant: dichloromethaneJacetone in gradients of 97J3 to 80/20~.
.
i The fraction~ containing compound Ia (Soraphen A) are concentrated once
more and chromatographed a second time over silica gel ~Lichroprep Si607
51 Merck~ 15-15 ~m. Eluant: dichloromethane/acetone Y713~. The resultant
Soraphen A crystallises from diethyl ether. Yield: 220 mg.
. 1 '~
rr~ d~ c
.i~ ,
.j
- 11 1 32q 1 60
The fractions containing compound Ib (Soraphen B) are concentrated once
more and chromatographed over the same type of silica gel ~15-25 ~m) with
dichloromethane/acetone as eluant (gradient: 90JlO to 85Jl5). Fine
purification is effected by means of a third chromatography on cross-
linked dextran gel such as Sephadex~LH-20 (Pharmacia) using methanol as
eluant. Yield: lO0 mg.
Example P2: Preparation of the compound of formula I in the presence of
an adsorber resin
The process is carried out in a f0rmentation volume of 300 litres with
the addition of 0.5 % ~v/v) of adsorber resin XAD-1180 (Rohm and Haas~.
The process conditions for this enlarged batch conform in other respect~
to those of Example Pl.
When thq fermentation is complete the polymer carrier 1B sieved off,
rinsed in a glass column, washed with 3 bed volumes oE water and eluted
with 2 bed volume~ o~ methanol. The eluate is concentrated to dryness
under vacuum and the residue is chromatographed over silica gel
(Llchroprep Si60 25-40 ~m, Merck, eluant: dichloromethanelacetone 97J3 in
gradients up to 80120~.
.
a) The fraction containing Soraphen A is concentrat0d and chromatographed
over sllica gel (Lichroprep Si60, 15-25 ~m, Merck. Eluant: dichloro-
methaneJacetone 97J3).
Alternatively, after treatment of the polymer carrier with methanol the
methanolic solution can be cautiously concentrated without
chromatography~ whereupon the desired Soraphen A cry~tallises out after
addition of diethyl ether.
The product can be recrystallised from diethyl ether. Yield: 1 g of
Soraphen A.
;, .
~r~de~ r k
~i
.
.
'
~ . .
,.
- 12 - 1329160
..
b~ The fraction co~taining Soraphen B is again chromatographed over
silica gel ~Lichroprep Si60~ 15-25 ~m, Merck; eluant gradient: dichloro-
metl1ane/acetone 90J10 to 85/15). ~ine purification i9 effected by
chromatography over Sephadex LH-20 (Pharmacia~ with methanol as eluant.
Yield: 0.6 g.
Chemical characteri~ation of Soraphen A (compound Ia~
Empirical formula:
C2gH440g. Mg: 520.
Colsurless crystals from diethyl ether. m.p. 101C.
1~]25 ~ -131 (MeOH: c=l)
UV (MeOH) ~ (log ~ = 317 sh (Z.03); 267 sh (2.47); 263 (2.59~; 257
- max
(2.68); 251 (2.66); 245 sh (2.66); 228 sh
(2.84): 215 (3.72~; 207 (3.94~
IR (KBr): 975, 992, 1021, 1045, 1071. 1102, 1152, 1187~ 1230, 1255, 1272,
1380, 1451t 1708 (sh~, 1729, 2854 (sh), 2923 cm 1,
MS FAB (neg. ions~ ~ 519 (M-H~
EI (70ev) - 520
high resolution: theory: 520,3036
found: 520,3020
TLC (TLC aluminium foil 60F2s4, Merck; eluant: dichloro-
_
methane/acetone 90llO) ~ ~ 0.5 (detection by spraying with anisal-
dehydelsulfuric acid reagent and heatlng to 120C~
HPLC (Column: 4x250 mm Nucleosil 100-7 C1g, Macherey Nagel;
flow 1.5 ml/min; eluant: methanollwater 70130 in 20 min linear to
100lO) Rt = 11.8 min.
Chemical characterisation of Soraphen B (compound Ib)
Empirical formula: !
C2gH~40~. Mg: 508.
[~]25 ~ _57 (MeOH; c=1)
~V ~eOH~ ~ (log ~ ~ 263 sh ~2.98); 256 sh (3.11); 247 sh (3.36~;
238 ~3.42); 215 sh ~3.77~; 207 (3.98~
,
.
,
,
- . ~
.
1 32q 1 60
- 13 -
` IR (KBr): 713, 973~ 994, 1050 ~sh), 1071 (sh~. 1098~ 1154~ 1183,
-.- 1232 (sh), 1274~ 1378, 1459~ 1723,. 2933, 2952,. 3377,. 3413,
3451 cm 1.
MS FAB (neg. ions) - 507 (M-H~
; TLC (TLC aluminium foil 60F2s4, Merck; eluant: dichloro-
methane/acetone 90110) RF ~ 0-5 (detection by spraying with anisal-
dehyde/sulfuric acid reagent and heating to 120C)
HPLC (Column: 4x250 mm Nucleosil 100-7 Cl.B, Macherey Nagel;
flow 1.5 ml/mln; eluant: methanollwater 70l30 in 20 min linear
, to 100/0) Rt ~ 9 0 min.
. ,
,i The NMR data of both compound~ are as follows:
:j .
.
,1
~ ! :
,i
~, .
.~ .
.,
!
?
.,
?
...
, ' ~ .
.
: ,:
1 329 1 60
- 14 -
j Table 1
IH-NMR dats (CDCl3; ~ in ppm)
., _
SORAPHEN
. A B
:'
. 1
~ 3.14 3.08
3 _ _
4 3.18 3.12
4.04 4.04
6 1.94 1.82
7 3.83 3.92
8 2.51 1.88
9 6.19 1)
5.48 1.80/1.402)
11 3.69 3.793)
12 3.43 3.353)
13A/B 1.69/1.26 1.59/1.35 )
14A/B 1.37/1.17 )
15A/B 1.48 1~
16A/B 1.69/2.10 )
17 5.86 5.72
18 1.12 1.10
19 3.38 3.41
1.05 1.01
21 1.03 0.84
22 3.29 -
23 3.4~ 3.36
2'/6'
3'/5' 7.27-7.38 7.25-7.36
3-OH 4.43 4.68
5-OH 3.56
: ~ultiplets 1.25-1.8 ppm
3): ~stign~ent i~ ~ach c~ exchsngeable
:
~,
' ~ ~ ' : ' ' -
;;' , i ,' ' ., ' '~', ` :'
.
1 32q 1 60
- 15 -
. .
Table 2
C-NMR data (CDCl 3 ~ in ppm)
.
SORAPHEN
A _ _ B
1 170.8 171.6
2 46.3 45.7
3 99.5 99.5
4 76.3 76.4
5 68.9 69.31)
6 35.6 35.1
7 42.4 71.3
8 35.3 32.5
9 139.6 27.92?
10122.8 27.32)
1185.0 68.91)
1282.8 69.32
1330.4 25.4
1423.3 24.72)
1525.7 22.82)
1635.7 34.6
1774.4 75.7
1811.5 11.6
1957.2 57.43)
2010.2 10.2
2112.4 14.1
2256.1 -
2358.0 57.23)
1'141.1 140.7
2';6~ 126.2 126.4
3';5' 128.5 128.4
4' 128.0 128.0
, .,
1); 2); 3): assignment in each case exchangeable
!
The following structural formulae Ia' and Ib' are assum~d for Soraphen A
compound Ia) and for Soraphen B (compound Ib~ on the basis of X-ray
~truct~i_1 an=ly~ nd th~ abov~ J~t~:
. ,j .
~'
::,
.,
;,. :; :
',,: , .
:
,~
.
.'
- 16 - ~3~ql60
: 22
~' OCH3
~ 3
CH3 12~
23 ~ \ ~CH3
1 i (Ia'~
\ 0/ \ /-0~,~ OH
CH3
/ CH3
-
i HO
/ ~\ /-\ /-\ ~.CH3
:~ !OCH3
t R , T tIb')
\ 0/ \ / 0~ / OH
~ OCH3
., ~ / CH3
.,;~
The invention also encompasse6 in particular the compounds Ia' and Ib',
their preparation and the use thereof as plant microbicldes.
. .,
Activity spectrum of compounds Ia and Ib in the plate diffusion te~t
! The concentration of the compounds i8 1 ~Ig/test flakes and the volume of
~i~ sgar i8 10 ml per plate (petri dishes of 9 cm dlameter). The same values
"~
vere determined for Ia and Ib.
~ Test organism Diameter of inhibitin~ zone .Lmm~
:`',
Debaryomyces hansenil 29
Candida albicans 25
, _
Hans~nula anomala 15
Nadsonia fulvescens --
emAtospora coryll 35
',
, .
.,~ ` . .
~ ~ '
1329160
- 17 -
Rhodotorula glutinis 21
Saccaromyces cerevisiae 20
Schizosaccharomyces pombe --
Torulopsis glabrata --
Alternaria solani 27
. .
Botrytis cinerea 30
Mucor hiemalis 30
Pythium debaryanum 45
Rhizopus arrhisus 10
The MIC ~minimum inhlbitory concentration) values are also identical for
both compounds Ia and Ib.
;, ~
Minimum inhibitory concentrations
,
Organismus MNKI~lml
, I ,
Nematospora coryli 0.05
Candida albicans 0.06
~ _ .
Rhodotorula glutinis 1.0
~` Saccharomyces cerevisiae 2.0
Torulopsis ~labrata 3.0
`A Nadsonia fulvescena 3.0
`~cor hiemali~ 0.03
~i The inhibition of Candida is still reversible even after treatment for
i --
24 hours. The proliferation of Nematospora coryll ceasea 90 minutes
after additlon of the compound I. At the same tlme the DNA and protein
synthesis are discontinued.
Serum binding: Beef serum has no lnfluence on the efficacy of
compound I.
.. . .
~i
:~
., .
~.,.
~ - 18 - ~ 3 2 9 ~ 6 0
,
2. Formulation Examples for the compound of formula I
(throughout, percentages are by weight)
2.l Emulsifiable concentrates a~ b~ c~
., _
compound of formula I 25 % 40 % 50 %
calcium dodecylbenzenesulfonate 5 % 8 % 6 %
castor oil polyethylene glycol ether
(36 mol of ethylene oxide~ 5 %
tributylphenol polyethylene glycol ether
(30 moles of ethylene oxide) - 12 % 4 %
cyclohexanone - l5 % 20 %
xylene mixture 65 % 25 % 20 %
Emulsions of any required concentration can be produced from such
concentrates by dilution with water.
:. .
2.2 Solutions a~ b) c~ d~
compound of formula I 80 % lO % 5 % 95 %
ethylene glycol monomethyl ether 20 %
polyethylene glycol (mol.wt. 400~ - 70 %
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oil - - l % 5 %
petroleum distillate (boiling range
160-190C) - - 94 %
These solutions are suitable for application in the form of microdrops.
2.3 Granulates a~ b~
compound of formula I 5 % lO %
kaolin 94 %
highly dispersed ~ilicic acid l %
. .
attapulgite - go %
The active ingredient is dissolved in methylene chloride, the solution i~
! sprayed onto the carrier, and the solvent is subsequently evaporated off
in vacuo.
''
,,
:, .
.; .
.
,, :
,. . .
,' ' '~ ' ' ' ' ~
1329160
-- 19 --
2.4 Dusts a) b~
compound of formula I 2 % 5 %
highly di3persed silicic acid 1 % 5 %
talcum 97 %
kaolin 90 %
Ready-for-use dust6 are obtained by intimately mixing the carrier~ with
the active ingredient. By the further addition of the three carriers
these dusts can be ground to ready-for-use dusts containing 0.001 % of
active ingredient.
A
Formulation Examples for aolid active ingredients of formula I
(tllroughout, percentages are by wei~ht)
2.5 Wettable powders a) b) c)
compound of formula I 25 % 50 % 75 %
sodium lignosulfonate 5 % 5 %
~odium lauryl sulfate 3 % - 5 %
sodium diisobutylnaphthalenesulfonate - 6 % 10 %
octylphenol polye-thylene glycol etber
(7-8 mol of ethylene oxide) - 2 %
highly dispersed ~ilicic acid 5 % 10 % 10 %
kaolin 62 % 27 %
The active ingredient is thoroughly mixed with the adjuvants and the
mixture i8 thoroughly ground in a 3uitable mill, affording wettable
powders which can be diluted with water to give suspeDsions of the
desired concentration.
~ .
2.6 Coated granulate
, compound of formula I 3 %
. polyethylene glycol (mol.wt. 200~ 3 %
kaolin 94 %
j The finely ground actlve ingredient is uniformly applied~ in a mixer, to
i the kaolin moi~taned with polyethlene glycol. Non-dusty coated granulates are obtained in thi~ mannar.
-
".
,.. ,: , - . ~: .
:' , ' '
- 20 - ~329~60
2.7 Suspension concentrate
compound of formula I 40 %
ethylene glycol 10 %
nonylphenol polyethylene glycol
(15 moles of ethylene oxide) 6 %
sodium lignosulfonate 10 %
carboxymethylcellulose 1 %
37 % aqueous formaldehyde solution 0.2 %
silicone oil in the form of a 75 ~0
aqueous emulsion 0.8 ~0
water 32 %
The finely ground actlve ingredient is intlmately mixed with the
adiuvants, giving a suspension concentrate from which suspensions of any
desired concentration can be obtained by dilution with water.
"
2.8 Biomass concentrate
The biomass resultlng from the fermentation is dried, ground,. and mixed
wlth ethylene glycol monomethyl ether in the weight ratio of 80:20. This
concentrate can be diluted in all proportions with water to give spray
suspensions.
3. Biological Exa~ples
~Throughout, "tsst compound" and "active ingredient" will b0 understood
8S meaning both Soraphen A and Soraphen B)
Exa:ple 3.1: Action against Puccinia g.raminis on wheat
a~ Residual-protective action
Wheat plants are treated 6 days after sowing with a spray mixture
~0.02 % a.i.~ prepared from a wettable powder formulation of the test
co~pound. After 24 hours the treated plants are infected with a
uredospore suspension of the fungus. The infected plants are incubated
for 48 hours at 95-100 ~o relative humidity and about 20C and then stood
in a greenhouse at about 22C. ~valuatloD of rust pustule development is
made 12 days after infection.
~. . : ; .
.''. , ' '' :
:,;~ : , : .
:- . , ,
'' . ' , '
: , ~ . . : .
- 21 _ 1329160
b) Systemic actlon
Wheat plants are treated S days after sowing with a spray mixture
~0.002 % a.1.~ based on the volume o~ the soil) prepared from a wettable
powder formulation of the test compound. After 48 hours the treated
plants are infected with a uredospore suspension of the fungus. The
plants are then incubated for 48 hours at 95-100 % relative humidity and
about 20C and then stood in a grcenhouse at about 22C. Evaluation of
rust pustule development is made 12 days after infection.
/
The test compound inhibited fungu3 infestation completely in both tests.
On the other hand, Puccinia infestation was 100 % on untreated and
infected control plants.
'
Example 3.2: Action against Phytophthora infestans on tomato plants
a?Residual protective action
After a cultivation period of 3 weeks, tomato plants are sprayed with a
spray mixture ~0.02 % a.i.~ prepared from a wettable powder formulation
of the test compound. After 24 hours tha treated plants are infected with
a sporangia suspension of the fungus. Evaluation of fungus infestation i~
made is made after the plants have been incubated for 5 days at YO-100%
relative humidity and 20C.
b) Systemic action
A spray mi~ture ~0.006% a.i.~, based on the volume of the soil~ prepared
from a wettable powder formulation of the test compound is poured on
tomato plants after a cultivation period of 3 weeks. Care ls taken that
~ ,
the spray mixture does not come in contact with the growing parts of the
plants. After 4B hours the plants are infected with a sporangia
`~ suspension of the fungus. Evaluation of fungus infestation is made after
the plants have been incubated for 5 days at 90-100% relative humidity
, and 20C-
~o f-ng~- 1nte~tat1on =as ob:el-ed wh~n e~luat1ne both test..
(
, :
~' .
,~
.
~ - 22 1 32q 1 60
Example 3.3: Action against Plas~opara viticola on vines
Residual protective action
Vine seedlin~s in the 4-5 leaf stage are sprayed with a spray mixture
(0.006 % a.i.) prepared from a wettable powder formulation of the test
compound. After 24 hours the treated plants are infected wlth a sporangla
suspension of the fungus. Fungus infestation is evaluated after
incubation for 6 days at 95-100 % relatlve humldity and 20C.
,,
The plants treated wlth the compound of formula I were free from
infestation, whereas fungus infestation was 100 % on untreated, infected
control plants.
Example 3.4: Actlon against Cercospora arachldicola on groundnut plants
Residual protective action
Groundnut plants 10-15 cm in height are sprayed with a spray mixture
(0.006 % a.i.) prepared from a wettable powder formulation of the test
compound, and infected 48 hours later with a conidia suspenslon of the
fungus. The infected plants are incubated for 72 hours at about 21C and
high humidity and then stood ln a greenhouse until the typlcal leaf
specks occur. Evaluation of the fungicidal action ls made 12 days after
infectlon and i~ based on the number and size of the specks.
.' '
The plants treated ~ith the compound of formula I were free from
infestation. Even after applicatlon of a spray concentration of 0.002 %,
the plants treated with Soraphen A exhibited no lnfestation at the
, conclusion of evaluatlon, whereas Cercospora infestation was 100 % on
untrested and infected control plants.
, . . .
Example 3.5: Action against Venturia inaequalis on apple shoots
`;~ Residual protective action
Apple cuttings with 10-20 cm long fresh shoot~ are sprayed wlth a spray
, mixture (0.02 % a.1.~ prepared from a wettable powder formulation of thetest compound. The plant~ are lnfected 24 hours later with a conidia
suspension of the fungus. The plants are then lncubated for S daya at
; 1
`l .
i,
i
i: , ~.. ; . .
., : - ~ .
'..................................... ~ , :
:, ~ . .,
r - ,
- 23 ~ 1 3 2 9 1 6
~; 90-100 % relative humidity and stood in a greenhouse for a further
10 days at 20-24C. Scab infe~tation is evaluated 15 days after
infection.
.
The cuttings treated with the test compound were free from infestation.
;'
~xample 3.6: Action against Botrytis cinerea on apples
Artificially damaged apples are treated by dropping a spray mixture
prepared from a wettable powder formulation of the test compound
(0.02 % a.i.) onto the injury sites. The treated fruit is then inoculated
with a spore suspension of the fungus and incubated for 1 week at high
~ humidity and about 20C. Evaluation is made by counting the number of
; in~ury sites attacked by rot and deducing the fungicidal action of thetest compound therefrom. The test compound inhibited fungus infestation
completely.
~xample 3.7: Action against Erysiphe graminis on barley
a~ Residual protective action
Barley plants about 8 cm in height are sprayed with a spray mixture
(0.006 % a.i.~ prepared from a wettable powder formulation of the test
compound. The treated plants are dusted with conidia of the fungus after
J 3 to 4 hours. The infected barley plants are stood in a greenhouse at
about 22C. The fungus attack is evaluated after 10 days.
~, b? Systemic action
A spray mixture (0.002 % a.i.~ based on the volume of the soil) prepared
from a wettable powder formulation of the test compound is poured onto
i, barley plants about 8 cm in height. Care is taken that the sp~ay mixture
~ does not come into contact with the growing parts of the plants. The
I treated plants are infected 48 hours later with a conidia suspension of
thc fungus. The infected barley ~lants are then stood in a greenhouse at
about 22C and evsluation of infestation is made after 10 days.
The plants were free from infestatlon in both tests, whereas the control
pl~nt~ ttrt co~pltttly iDfested.
.,
r
: .
- 24 1 32q 1 60
,
Example 3.8: Action against Rhizoctonia solani (soil fungus on rice
plants)
Protective local soil application
A spray mixture ~0.002 % a.i.~ prepared from a formulation of the test
compound i9 poured onto 12-day-old rice plants without contaminating the
growing parts of the plants. To infect the treated plants, a suspension
of mycelium and sclerotia of R. solani is applied to the surface of the
soil. After incubation for 6 days at 27C (by day~ and 23C (by night)
and 100 % relative humidity (humidity box~ in a controlled environment
chamber, fungus attack on the leaf sheath, leaves and stem is evaluated.
.
No infestation occurred after treatment with the test compound.
,'
,,
~ t
,
'~!
... .
,'~
d
., .
.
3~
,,
~1
~,
;' '
.J
. , .
,: . .
^, :
1 '
" .