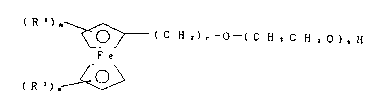Note: Descriptions are shown in the official language in which they were submitted.
13~9622
FERROCENE DERIVATIVES AND PROCESS FOR PRODUCING
ORGANIC THIN FILMS
TECHNICAL FIELD
The present invention relates to ferrocene
derivatives and a process for producing organic thin films,
and more particularly to novel ferrocene derivatives,
surfactants containing them, and a process for producing
organic thin films using various ferrocene derivatives
including said novel ferrocene derivatives.
BACKGROUND ART
In general, coloring matters such as
phthalocyanine or its derivatives and the like are insoluble
in water, and although they are soluble in organic solvents
such as dimethylformamide (DMF), tetrahydrofuran (THF) and
the like, their soluble amounts are small and the solubility
is only several milligrams (mg).
Surfactants to make the phthalocyanine and the
like soluble in water have heretofore been investigated,
but no satisfactory surfactant has been developed. It is
reported that functional group-substituted phthalocyanine
derivatives can be dissolved in water to some extent by
the use of sulfone-based surfactants. However, the
solubility is not always sufficiently high and further
unsubstituted phthalocyanine cannot be dissolved at all.
In connection with polymer insoluble in water,
surfactants to make them soluble in water have been
1329S22
investigated in the same manner as described above. In
fact, however, no satisfactory results have been obtained.
The present inventors have made extensive
investigations to develop surfactants to make coloring
matters such as phthalocyanine or its derivatives and the
like, or water-insoluble polymers and the like, soluble
in water.
In the course of the study, it has been found
that ferrocene derivatives are promising as surfactants
having the aforementioned performanc~. As a result of
further investigations based on the above findings, the
present inventors have discovered that new ferrocene
derivatives derived by introducing a polyoxyethylene chain
or a specified substituent containing pyridinium ion, in
ferrocene or its derivatives can achieve the object. At
the same time, they have discovered that a water-insoluble
(hydrophobic) organic thin film can be efficiently produced
from various ferrocene derivatives including the new
ferrocene derivatives by electrochemical techniques.
An object of the present invention is to provide
novel ferrocene derivatives. Another object of the present
invention is to provide surfactants having superior
performance, containing the novel ferrocene derivatives.
Another object of the present invention is to provide a
process for efficiently producing thin films of hydrophobic
organic substances.
DISCLOSURE OF INVENTION
1~29S22
That is, the present invention provides ferrocene
derivatives represented by the general formula:
R ' ~ C n H 2 n_ ~ N~-- R
F e ... ( I
R 2~
(wherein R1 and R2 are each a hydrogen, a methyl group,
an ethyl group, a methoxy group or a carbomethoxy group,
R3 is hydrogen, a methyl group, an ethyl group, a methoxy
group, a carbomethoxy group, a hydroxyl group, a carboxyl
group or a sulfonic acid group, and X is a halogen, and
CnH2n is a straight chain or branched chain alkylene group
having 4 to 16 carbon atoms),
the general formula:
R '
~~-( C H z) .--0--( C H 2 C H z O ) ~ H
F e
R Z ~ ~ lI A )
25 (wherein r is an integer of 11 to 18, s is a real number
of 2.0 to 50.0, and Rl and R are the same as described
above). or the general formula:
132~622
R '
~~ ( C H 2) w--C --O--( ~ H ~ C H 2 0 ) S H
F e O .. t ~ B )
R 2
(wherein w is an integer of 2 to 20, and s, Rl and R2 are
the same as described above).
The invention provide5 a ferrocene derivative
represented by the general formula-:
R ~--C, H ' ~--X N~ R '
R~ (I)
(wherein Rl and R are each independently a hydrogen, a
methyl group, an ethyl group, a methoxy group or a
carbomethoxy group, R3 is a hydrogen, a ~ethyl group, an
ethyl group, a methoxy group, a carbomethoxy group, a
hydroxyl group, carboxyl group or a sulfonic acid group, X
is a halogen, and CnH2n is a straight or branched
hydrocarbon group having 4 to 16 carbon atoms); or the
general formula:
25(R '~( C H ~),~0~(C H t C H ~ O), H
(R 2~
~B 4
_,
...4A
1329622
1 (wherein ~ is an integer of 2 to 4, n is an integer of 2 to
5, r is an integer of 11 to 18, s is a real number of 2.0 to
70.0, and Rl and R2 are the same as described above), or the
general formula: `
5 ~R ~ ( C H ~ C --O~( C H t C H ~ O ), ~{
F~ O
(R')~ ! (8)
(wherein w is an integer of 2 to 18, and m, n, 9, R and R2
are the same as described above)
The present invention further provides surfactants
containing the ferrocene derivatives represented by the
above general formula (I), (IIA) , (IIB), (A) or (~)~
The present invention further provides a process
for producing organic thin films which comprises making
hydrophobic organic substances soluble in an aqueous medium
by the use of surfactants (micelle forming agents) comprising
ferrocene derivatives, and electrolyzing the micelle solution
thus obtained to form a thin film of the above hydrophobic
organic substance.
The novel ferrocene derivatives of the present
invention are the novel compounds represented by the general
formula (I), (IIA) or (IIB). In accordance with the process
2~ of the present invention, using these novel ferrocen~
- 4A -
...4B
'~.
132~622
1 derivatives or other ferrocene derivatives as surfactants,
thin films of hydrophobic organic substances can be formed
efficiently and further in the desired thickness.
BRIEF DESCRIPTION OF DRAWINGS
- 4B -
1329622
Fig. 1 is a view schematically illustrating the
principle of the process of the present invention, wherein
1 indicates a ferrocene derivative; 2, a hydrophobic organic
substance 3, micelle; 4, an oxidized ferrocene derivative;
5, an anode; 6, a cathode; Fc, ferrocene; and e , an
electron.
Fig. 2 is a proton nuclear magnetic resonance
(lHNMR) spectrum of the ferrocene derivative obtained in
Example 1.
Fig. 3 is an infrared (IR) absorption spectrum of the
ferrocene~derivative.
Fig. 4 is an ultraviolet-visible (UV-VIS)
absorption spectrum of the ferrocene derivative.
Fig. 5 indicates W-VIS absorption spectra of
the supernatants obtained in Example 2 and Comparative
Example 1.
Fig. 6 is an electron micrograph showing the
surface structure of the thin film formed in Example 3.
Fig. 7 indicates ultraviolet tUV) absorption
spectra of the ethanol solutions of the thin films formed
in Examples 3 and 4.
Fig. 8 is an electron micrograph showing the
surface structure of the thin film formed in Example 4.
Fig. 9 is lH-NMR of the ferrocene derivative
obtained in Example 5.
Fig. 10 is lH-MMR of the ferrocene derivative
obtained in Example Ç.
1329622
Fig. 11 is H-NHR of the ferrocene derivative
obtained in Example 7.
Fig. 12 indicates visible (VIS) absorption spectra
of the supernatants obtained in Examples 8 to 12.
Fig. 13 indicates visible absorption spectra
of the coloring matter thin films on IT~ as obtained in
Examples 13 and 14.
Fig. 14 is an electron micrograph showing the
surface structure of the thin film formed in Example 13.
Fig. 15 indicates UV absorption spectra of the
ethanol solutions of the thin films formed in Examples 4,
15 and 16.
Fig. 16 is an electron micrograph showing the
surface structure of the thin film formed in Example 17.
Fig. 17 is a UV absorption spectrum of the
methanol solution of the thin film formed in Example 18.
Fig. 18 is an electron micrograph showing the
surface structure of the thin film formed in Example 19.
Fig. l9(a) is an electron micrograph showing
the surface structure of the thin film before post-treatment
as formed in Example 20.
Fig. l9(b) is an electron micrograph showing
the surface structure of the thin film after post-treatment
as formed in Example 20.
Fig. 20 is a Fourier transformation infrared
absorption spectrum of the thin film formed in Example 20.
Fig. 21 is a IR absorption spectrum using a KBr
-- 6 --
1329S22
l pellet of the polymer used in Example 20.
Fig. 22 is a graph showing a relation between
the film thickness of the thin film formed in Examples 20
and 21, and the amount of electricity having passed per
unit area of ITO.
Fig. 23 is a UV absorption spectrum of the thin
film formed in Example 21.
Fig. 24 is an electron micrograph showing the
surface structure of the thin film formed in Example 21.
BEST MODE FOR CARRYING OUT THE INVENTION
The novel ferrocene derivatives of the present
invention are represented by the general formula (I), (IIA)
or (IIB). In the general formula (I), R and R are each
a hydrogen, a methyl group, an ethyl group, a methoxy group
or a carbomethoxy group, R3 is a hydrogen, a methyl group,
an ethyl group, a methoxy group, a carbomethoxy group, a
hydroxyl group, a carboxyl group or a sulfonic acid group,
and X is a halogen, that is, chlorine, bromine, iodine,
fluorine and the like. CnH2n indicates a straight or
branched alkylene group having 4 to 16 carbon atoms (that
is, n is an integer of 4 to 16 ). Specific examples are
straight alkylene groups exemplified by polymethylene groups:
(CH2)n, such as a tetramethylene group, a pentamethylene
group,an octamethylene group, an undecamethylene group,
a dodecamethylene group, a hexadecamethylene group and the
like~ or branched alkylene groups such as a
2-methylundecamethylene group, a 4-ethylundecamethylene
1329622
1 group and the like.
The ferrocene derivatives represented by the
general formula (I) can be produced by various methods.
For example, they can be produced by adding pyridine-based
compounds represented by the general formula:
R ~
~O) ( I -- b )
(wherein R3 is the same as described above) to
halogen-containing ferrocene derivatives represented by
the general formula:
D I
~~-- C n H Z n X
F ~ ( I - a )
R 2 ~
(wherein Rl, R2, X and CnH2n are the same as described above)
and reacting them for about 1 to 5 hours in an atmosphere
25 of inert gas such as nitrogen gas and the like at a
temperature of 20 to 70C while sufficiently stirring.
Thereafter, the product is washed with diethyl ether and
1329622
1 the like and dried, and then dissolved in a polar solvent
such as acetone, methanol, ethanol, tetrahydrofuran and
the like. The resulting solution is poured in diethyl ether
and the like to precipitate. This operation is repeated
several times, and upon filtration, the ferrocene derivatives
of the general formula (I) can be obtained in a high purity.
On the other hand, in the ferrocene derivatives
represented by the general formula (IIA), r is an integer
of 11 to 18, and s is a real number of 2.0 to 50Ø Since
r is an integer of 11 to 18 as described above, an alkylene
group having 11 to 18 carbon atoms, such as an undecyl group,
a dodecyl group and the like, is present between a
ring-forming carbon atom and an oxygen atom (oxygen atom
nearest the ferrocene structure). s means not only an integer
between 2.0 to 50.0 but also a real number including them,
and indicates an average repeating number of the oxyethylene
group (-CH2CH2O-) constituting the ferrocene derivative.
The ferrocene derivatives of the general formula
(IIA) can be produced by various methods. For example,
they are produced as follows:
For example, the ferrocene derivatives represented
by the general formula (IIA) are obtained by adding an alkali
metal (metallic sodium, metallic potassium and the like)
to polyethylene glycol represented by the general formula:
HO~CH2CH2O~sH ................... (II-a)
(wherein s is the same as described above), stirring the
resulting mixture for several minutes to several days at
1329622
1 a temperature of ordinary temperature to 200C, adding a
halogencontaining ferrocene compound represented by the
general formula:
R '
~~( C H 2)~ X I
F e -- ( 11 -- b )
R 2
(wherein R1, R2 and r are the same as described abovel and
xl is a halogen atom), reacting them with stirring, and
then extracting and purifying.
On the other hand, the ferrocene derivatives
5 represented by the general formula (IIB) can be obtained
by adding concentrated sulfuric acid to polyethylene glycol
represented by the above general formula (I~-a), stirring
the resulting mixture for several minutes to several days
at a temperature of ordinary temperature to 200C, adding
carboxyl group-containing ferrocene compounds represented
by the general formula:
~~ ( C H z) w C O O H
F e ( ~ -- c )
R 2~
-- 10 --
1329622
1 (wherein R1, R2 and w are the same as described above),
reacting with stirring, and then extracting and purifying.
That is, in accordance with this method, the ferrocene
derivatives represented by the general formula (IIB) are
S obtained.
In producing the ferrocene derivatives represented
by the general formulas (IIA) and (IIB), similar polyethers
can be used in place of the polyethyene glycol of the general
formula (II-a). It suffices that the extraction treatment
after the reaction is carried out using alcohol, THF and
the like, and the purification is carried out by
chromatographic purification and the like.
The ferrocene derivatives of the present invention
as represented by the general formula (I), (IIA) or (IIB)
which are obtained by the methods as described above are
effective as surfactants and can be used particularly as
surfactants (micelle forming agents) to make hydrophobic
organic substances soluble in water or an aqueous medium.
In this case, ferrocene derivatives of the general formula
(IIA) wherein r is 11 to 15 and specifically ll to 13 are
suitably used as surfactants. In the general formula (IIB~,
ferrocene derivatives in which w is 7 to 15 are particularly
suitably used as surfactants.
The surfactants of the present invention contain
the ferrocene derivatives of the general formula (I), (IIA)
or (IIB) as the major component, and other various additives
can be added thereto, if necessary. When the surfactants
1329622
1 of the present invention are used, various hydrophobic
organic substances can be made soluble in water or in an
aqueous medium.
A process for production of organic thin films
of the present invention will hereinafter be explained.
In the process of the present invention, the ferrocene
derivatives are used as surfactants (micelle forming agents).
As the ferrocene derivatives, not only the ferrocene
derivatives of the above general formula (I), (IIA) or (IIB),
but also various ferrocene derivatives can be used.
Examples of such ferrocene derivatives include,
as well as those represented by the general formula (I),
(IIA) or (IIB), ferrocene derivatives of the general formula
(IIA) wherein r is 2 to 10, and ferrocene derivatives in
which a ferrocene compound (ferrocene or ferrocene having
a suitable substitutent (an alkyl group, an acetyl group
and the like)) is bonded to a cationic surfactant of the
ammonium type (preferably the quaternary ammonium type)
having a main chain having 4 to 16 carbon atoms (preferably
2~ 8 to 14). If the number of carbon atoms in the main chain
is too small, no micelle is formed, and if it is too large,
the resulting ferrocene derivatives are not soluble in water.
The ferrocene compound is bonded to the surfactant in various
embodiments. Main embodiments are an embodiment in which
the ferrocene compound is bonded to the terminal of the
main chain of the surfactant, an embodiment in which the
ferrocene compound is bonded to an intermediate point of
- 12 -
1329622
1 the main chain, directly or through an alkyl group, and
an embodiment in which the ferrocene compound is incorporated
in the main chain. Ferrocene derivatives of this type are
represented by the general formula:
R ' R 5
M
~ ( C H 2 ) u
( C H z) ~ H F e
o ~T
~wherein R4 and R5 are each a hydrogen or an alkyl group
having 1 to 4 carbon atoms (but not exceeding t as described
hereinafter), M and T are each a hydrogen or a substituent,
X is a halogen, and t and u are integers satisfying the
requirements: t ~ O, u ~ O, and 4 _ t + u s 16), the general
formula:
R R S
N ~ X ~
~ t C H 2 ) j C H ( C H 2 ) 11--H
( C H 2) h H
( C H 2) D
F e
~ T
-- 13 --
13~9622
1 (wherein R4, R5, X, M and T are the same as described above
(provided that the number of carbon atoms of R4 and R5 does
not exceed h as described hereinafter), and h, j and k are
integers satisfying the requirements: h > O, i 2 O, k , 1
and 3 < h + j + k < 15), the general formula:
N X ~ ) ~ ~ C H 2 ) ~ H
( C H z)x H F e
lo ~T
(wherein R4, R5, X, M and T are the same as described above
(provided that the number of carbon atoms of R4 and R5 does
not exceed x as described hereinafter), and x, y and z are
integers satisfying the requirements: x > O, Y ~ , Z 2 1,
and 4 s x + y + z < 16), or the general formula:
R 4 R '
~ M
N X~ ~
( C H 2~ ~C H ~ ( C H 2) :~ H
(wherein R4, R5, M, T, x, y and z are the same as described
above).
In the process of the present invention, as
- 14 -
13~9622
1 ferrocene derivatives to be used as the micelle forming
agent, those derived by replacing a part of the alkyl chain
of the general surfactant (surface active agent) with
ferrocene can be used.
Representative examples of ferrocene compounds
as the micelle forming agent (surfactant) are shown below.
C H
C H ~ 7 N ~-- C,, H
lo C H 3 F e
C H
\ B r~
C H 7 N ' C H z
C 1 2 H Z5 F e
C H 3
\ B r~
C I b H ~ F e
-- i5 --
1329622
1 In the process of the present invention, a
surfactant (micelle forming agent) comprising the
aforementioned ferrocene derivative, a supporting salt and
a hydrophobic organic substance are introduced in an aqueous
medium and thoroughly dispersed by the use of supersonic
waves, a homogenizer, or a stirrer and the like to form
a micelle and then, if necessary, an excess of the
hydrophobic organic substance is removed and the micelle
solution thus obtained is subjected to electrolytic treatment
using the aforementioned electrode while allowing it to
stand or somewhat stirring it. During the electric
treatment, the hydrophobic organic substance may be
supplementarily added to the micelle solution, or there
may be provided a recycle circuit in which the micelle
solution in the vicinity of the anode is withdrawn out of
the system, the hydrophobic organic substance is added to
the withdrawn micelle solution and thoroughly stirred, and
then the resulting solution is returned to the vicinity
of the cathode. Electrolytic conditions are determined
appropriately depending on various circumstances. Usually
the liquid temperature is 0 to 70C and preferably 20 to
30C, the voltage is 0.03 to l.S V and preferably 0.1 to
0.5 V, and the current density is not more than 10 mA/cm2
and preferably 50 to 300 ~A/cm2.
On performing this electrolytic treatment, the
reaction as illustrated in Fig. 1 proceeds. Explaining
in connection with the behavior of Fe ion of the ferrocene
- 16 -
1329622
1 derivative, Fe2 is converted into Fe3 on the anode, leading
to break-down of the micelle, and particles (about 600 to
900 A) of the hydrophobic organic substance are deposited
on the anode. On the other hand, on the cathode, Fe3
S oxidized on the anode is reduced to Fe2 , recovering the
original micelle and, therefore, a film forming operation
can be carried out repeatedly using the same solution.
By the electrolytic treatment as described above,
a thin film made from particles about 600 to 900 A in size
of the desired hydrophobic organic substance is formed on
the anode.
The supporting salt (supporting electrolyte)
to be used in the process of the present invention is added,
if necessary, in order to control the electrical conductance
of the aqueous medium. The amount of the supporting salt
added is usual~y about 10 to 300 times and preferably about
50 to 200 times that of the above surfactant (micelle forming
agent~. The type of the supporting salt is not critical
as long as it is able to control the electric conductance
of the aqueous medium without inhibiting the formation of
the micelle and the deposition of the above hydrophobic
organic substance.
More specifically, sulfuric acid salts (salts
of lithium, potassium, sodium, rubidium, aluminum and the
like) and acetic acid salts (salts of lithium, potassium,
sodium, rubidium, magnesium, calcium, strontium, barium,
aluminum ahd the like) are suitable.
~32~22
1 The electrode to be used in the process of the
present invention may be a metal more noble than the
oxidation potential (against +0.15 V saturated calomel
electrode) of ferrocene, or an electrically conductive
substance. More specifically, IT0 (mixed oxide of indium
oxide and tin oxide), platinum, gold, silver, glassy carbon,
an electrically conductive metal oxide, an electrically
conductive organic polymer and the like can be used.
Various hydrophobic organic substances can be
used in the production of organic thin films according to
the process of the present invention. As well as coloring
matters for optical memory and organic coloring matters,
such as phthalocyanine, metal complexes thereof, and
derivatives thereof, naphthalocyanine, metal complexes
thereof and derivatives thereof, porphyrin and its metal
complexes, and the like, electrochromic materials such as
1,1,-diheptyl-4,4'-bipyridinium dibromide,
1,l'didodecyl-4,4'-bipyridinium dibromide and the like,
lightsensitive materials (photochromic materials) and light
sensor materials, such as
6-nitro-1,3,3-trimethylspiro-(2'H-l'benzopyran-2,2'-indoline)
(commonly called spiropyran) and the like, liquid crystal
display coloring matters such as p-azoxyanisole and the
like, electrically conductive organic materials and gas
sensor materials, such as the 1:1 complex of
7,7,8,8-tetracyanoquinonedimethane (TCNQ) and
tetrathiafulvalene ( TTF ), light curing paints such as
1329622
1 pentaerythritol diacrylate and the like, insulating materials
such as stearic acid and the like, diazo-type light-sensitive
materials and paints such as 1-phenylazo-2-naphthol and
the like, and the like can be used. In addition,
water-insoluble polymers, for example, general purpose
polymers such as polycarbonate, polystyrene, polyethylene,
polypropylene, polyamide, polyphenylene sulfide (PPS),
polyphenylene oxide (PP0), polyacrylonitrile (PAN) and the
like, polyphenylene, polypyrrole, polyaniline, polythiophene,
acetyl cellulose, polyvinyl acetate, polyvinyl butyral,
and various polymers (polyvinyl pyridine and the like) and
copolymers (a copolymer of methyl methacrylate and
methacrylic acid) can be used.
The present invention will hereinafter be explained
in more detail with reference to Examples and Comparative
Examples.
EXAMPLE 1
0.5 g of 1-ferrocenyl-12-bromoundecane and 0.1
ml of pyridine were mixed and reacted for 120 hours in a
nitrogen atmosphere while heating at 60C on a water bath.
In 4 hours from the start of the reaction, at least 95%
of the reaction was completed. This reaction mixture
solidified with an advance of the reaction and finally
solidified. This solid powder was well washed by adding
2S 10 ml of dimethyl ether. After washing, the powder was
separated by filtration. After the powder was fully dried,
10 ml of acetone was added thereto to dissolve it therein.
- 19 -
1~29622
1 Upon addition of 10 ml of dimethyl ether to the solution,
a precipitate was obtained. This operation was repeated
three times, and after drying, 0.22 g of a purified product
was obtained (yield, 38%).
The elemental analytical values of the substance
were as shown below. The results of measurement of proton
nuclear magnetic resonance ( H-NMR) spectrum (CDC13, TMS
standard) are as shown in Fig. 2, the results of measurement
of infrared (IR) absorption spectrum (KBr tablet method,
25C) are as shown in Fig. 3, and the results of measurement
of ultraviolet-visbiel (UV-VIS) absorption spectrum are
as shown in Fig. 4.
Elemental Analytical Values (%)
Carbon Hydrogen Nitrogen
Calculated 62.67 7.28 2.81
Found 62.08 7.65 2.73
The above results confirmed that the above
substance was a ferrocene derivative represented by the
formula:
,~ ( C H 2 ) I I B r -
F e
EXAMPLE 2
To 100 ml of water, 99.6 mg of the ferrocene
-- O
13~22
1 derivative obtained in Example ] as a surfactant (micelle
forming agent) and 2.56 y of lithium sulfate as a supporting
salt were added, and 10 mg of 1-phenylazo-2-naphthol was
added and dispersed and dissolved by application of
supersonic waves for 10 minutes. The resulting mixture
was further stirred for two days and nights with a stirrer,
and then the micelle solution thus obtained was subjected
to centrifugal separation at 2,000 rpm for one hour. A
UV-VIS absorption spectrum of the supernatant is shown in
Fig. 5 (indicated by (1)). This confirmed that
1-phenylazo-2-naphthol was made soluble in the micelle
solution. The solubility was 59 M/2mM micelle forming
agent solution. For comparison, a solution of only the
surfactant without addition of lphenylazo-2-naphthcl was
prepared, and its UV-VIS absorption spectrum is shown in
Fig. 5 (indicated by (3)).
COMPARATIVE EXAMPLE 1
To 100 ml of water, 95.6 mg of a ferrocene
derivative having the formula:
C H 3
C H 3 \/ N ~-- C,, H 2 2
C H 3 F e
~
as a surfactant (micelle forming agent) and 2.56 g of lithium
- 21 -
1329622
l sulfate as a supporting salt were added, and lO mg of 1-
phenylazo-2-naphthol was added and dispersed and dissolved
application of supersonic waves for 10 minutes. The
resulting mixture was further stirred for two days and nights
by the use of a stirrer, and the micelle solution thus
obtained was subjected to centrifugal separation at 2,000
rpm for one hour. A UV-VIS absorption spectrum of the
supernatnat is shown in Fig. 5 (indicated by (2)). The
solùbility of the l-phenylazo-2~naphthol was 38 ~M/2mM
micelle forming agent solution.
From the above results, it can seen that when
the micelle forming agent of Example 1 is used, l-phenylazo
-2-naphthol is dissolved in an amount of about 1.5 times
that when the micelle forming agent of Comparative Example
l is used.
EXAMPLE 3
In lO0 ml of water, 0.02 mol of lithium sulfate
as a supporting salt was dissolved, and as a micelle forming
agent, 0.2 m mol of the ferrocene derivative obtained in
Example 1 was added and dispersed by application of
supersonic waves to form a micelle. Then, 0.2 m mol of
a coloring matter (1-phenylazo-2-naphthol, which was a
hydrophobic organic substance, was added and incorporated
in the micelle by application of supersonic waves. After
the mixture was stirred for two days and nights, an excess
of the coloring matter was removed by cnetrifugal separation
to obtain a micelle solution. Using the micelle solution
- 22 -
1329622
1 as an electrolyte, IT0 as the anode, platinum as the cathode,
and a saturated calomel electrode as a reference electrode,
electrolytic treatment was performed under the conditions
of temperature 25C, applied voltage 0.3 V, current density
36 ~A/cm2. After 60 minutes, coloring matter thin film
having primary particles having an average particle size
of 700 to 1,000 A was obtained on the IT0.
A scanning type electron microscope (SEM)
photograph (magnification, 35,000 using JSM-T220 produced
by Nippon Denshi Co., Ltd.) of the coloring matter thin
film formed is shown in Fig. 6. A W absorption spectrum
of the thin film dissolved in ethanol is shown in Fig. 7
~Curve (3)). A UV absorption spectrum of the above coloring
matter in eth~nol is shown in Fig. 7 (Curve (1)). Since
the absorption peaks of Curves (3) and (1) are in agreement
with each other, it can be seen that the thin film on the
IT0 is made of the above coloring matter.
The deposited amount of the thin film was 18 nano
mol/cm2 .
EXAMPLE 4
In 100 ml of water was dissolved 0.02 mol of
lithium sulfate as a supporting salt, and as a micelle
forming agent, 0~2 m mol of the same ferrocene derivative
as used in Comparative Example 1 was added and dispersed
by application of supersonic waves to form a micelle. Then,
0.2 m mol of a coloring matter (1-phenylazo-2-naphthol)
which was a hydrophobic organic substance was added to the
- 23 -
1329622
1 micelle solution and then incorporated in the micelle by
application of supersonic waves. After the resulting mixture
was stirred for two days and nights, an excess of the
coloring matter was removed by centrifugal separation to
obtain a micelle solution. Using this micelle solution
as an electrolyte, ITO as the anode, platinum as the cathode,
and a saturated calomel electrode as a reference electrode,
electrolytic treatment was performed under the conditions
of temperature 25C, applied voltage 0.3 V, current density
35 ~A/cm2. After 60 minutes, a coloring matter thin film
having primary particles having an average particle size
of 700 A was obtained on the ITO.
A scanning type electron microscope (SEM)
photograph (magnification, 35,000, using JSM-T220 produced
by Nippon Denshi Co., Ltd.) of the coloring matter thin
film formed is shown in Fig. 8. A UV absorption spectrum
of the thin film dissolved in ethanol is shown in Fig. 7
(Curve (2)). A UV absorption spectrum of the above coloring
matter dissolved in ethanol is shown in Fig. 7 (Curve (1)).
Since the absorption peaks of Curves (2) and (1) are in
agreement with each other, it can be seen that the thin
film on the ITO is made of the above coloring matter.
The deposited amount of the thin film was 12 nano
mol/cm .
EXAMPLE 5
0.064 g of metallic sodium was added to 6.5 g
of polyethylene glycol (average molecular weight, 600),
1329$22
1 and stirred at 70C for one day and night. Then, 1.1 g
of 1-ferrocenyl-12-bromoundecane was added and reacted at
110C for 10 hours. This reaction solution was extracted
with a 1:1 mixture of water and n-butanol. The extract
was washed with water and then was subjected to
chromatographic purification by developing on a silica gel
column with a mixture of benzene and ethanol (benzene :
ethanol = 5:1) as a solvent. After drying, a purified
product was obtained, and the yield was 41% and the amount
was 0.96 g. The elemental analytical values of the purified
product were carbon 60.21%, hydrogen 9.46%, nitrogen 0.00%.
The results of measurement of the proton nuclear magnetic
spectrum (lHNMR) are as shown in Fig. 9.
From the above results, it can be seen that the
above purified product is a ferrocene derivative having
the following structure:
~ ( C H 2 ) I , 0 ( C H ~ C H z 0 ) I 2 . S H
F e
EXAMPLE 6
The procedure of Example 5 was repeated with the
exception that as the polyethylene glycol, polyethylene
glycol having an average molecular weight of 1,000 was used.
- 25 -
1329622
l For the purified product obtained, the yield was 31~ and
the amount was 2.15 g. The results of measurement of H-
NMR oE the purified product are as shown in Fig. 10.
From the above results, it can be seen that the
above purified product is a ferrocene derivative having
the following structure:
( C H z) , I O ( C H 2 C H ~ O ) , 7 H
F e
10 ~
EXAMPLE 7
The procedure of Example 5 was repeated with the
exception that 6 g of polyethylene glycol (average molecular
weight, 600) and 0.1 cc of concentrated sulfuric acid were
added to 0.29 g of ferrocenyldodecanic acid and reacted
at 80 C for 6 hours. For the purified product obtained,
the yield was 62% and the amount was 0.44 g. The results
of measurement of H-NMR of the purified product are as
shown in Fig. 11.
From the above results, it can be seen that the
above purified product is a ferrocene derivative having
the following structure:
- 26 -
132~622
.~ ( C H z ), I C -- O -- ( C H z C H z O ), z . 5 H
F e O
EXAMPLE 8
To 31.5 ml of water, 1.13 mg of the ferrocene
derivative obtained in Example 5 as a surfactant (micelle
forming agent) was added, and lO mg of phthalocyanine was
added and dispersed and dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred
for two days and nights by the use of a stirrer, and then
the micelle solution thus obtained was subjected to
centrifugal separation at 2,000 rpm for one hour. A visible
absorption spectrum of the supernatant is shown in Fig.
12 (indicated by A). This confirmed that the phthalocyanine
was made soluble in the micelle solution. The solution
was 4.4 mM/4 mM micelle forming agent solution.
EXAMPLE 9
The procedure of Example 8 was repeated with the
exception that the phthalocyanine was replaced by a
phthalocyanine-iron complex. A visible absorption spectrum
of the supernatant is shown in Fig. 12 (indicated by B).
This confirmed that the phthalocyanine was made soluble
in the micelle solution. The solubility was 0.72 mM~4 mM
micelle forming agent solution.
1~29~2
1 EXAMPLE 10
The procedure of Example 8 was repeated with the
exception that the phthalocyanine was replaced by a
phthalocyaninecobalt complex. A visible absorption spectrum
of the supernatant is shown in Fig. 12 (indicated by C).
This confirmed that the phthalocyanine was made soluble
in the micelle solution. The solubility was 0.22 mM/4 mM
micelle forming agent solution.
EXAMPLE 11
The procedure of Example 8 was repeated with
the exception that the phthalocyanine was replaced by a
phthalocyanine-copper complex. A visible absorption spectrum
of the supernatant is shown in Fig. 12 (indicated by D).
This confirmed that the phthalocyanine was made soluble
in the micelle solution. The solubility was 0.11 mM/4 mM
micelle forming agent solution.
EXAMPLE 12
The procedure of Example 8 was repeated with the
exception that the phthalocyanine was replaced by a
phthalocyanine-zinc complex. A visible absorption spectrum
of the supernatant is shown in Fig. 12 (indicated by E).
This confirmed that the phthalocyanine was made soluble
in the micelle solution. The solubility was 0.41 mM/4 mM
micelle forming agent solution.
EXAMPLE 13
To 10 ml of the micelle solution prepared in
Example 8 was added 0.22 g of lithium sulfate (Li2S04) to
- 28 -
1329622
l obtain a 0.44 mM phthalocyanine/2 mM micelle forming agent/
0.2 M lithium sulfate solution. Using this solution as
an electrolyte, ITO as the anode, platinum as the cathode
and a saturated calomel electrode as a reference electrode,
constant voltage electrolysis of applied voltage 0.5 V and
current 7 ~A was performed at 25C for 2 hours. As a result,
a coloring matter thin film having primary particles having
an average particle size of 1,000 A was formed on the ITO.
A SEM photograph (magnification, 20,000, using JSM-T220
produced by Nippon Denshi Co., Ltd.) of the coloring matter
thin film is shown in Fig. 14.
A visible absorption spectrum of the coloring
matter thin film on the ITO is shown in Fig. 13 (indicated
by A). Since the visible absorption spectra shown in Fig. 13
(indicated by A) and Fig. 12 (indicated by A) were in
agreement with each other, it was confirmed that the coloring
matter thin film on the ITO was made of the phthalocyanine.
EXAMPLE 14
The procedure of Example 13 was repeated with
the exception that the electrolytic time was changed to
40 minutes.
A visible absorption spectrum of the coloring
matter thin film thus formed is shown in Fig. 13 (indicated
by A). By comparison of A of Fig. 13 with B of Fig. 13,
it can be seen that the thin film formed has a small
absorption spectrum as compared with Example 13, and the
film thickness can be controlled by the electrolytic time.
- 29 -
1329622
l EXAMPLE 15
The procedure of Example 4 was repeated with the
exception that platinum was used as the anode and the current
density was changed to 38 ~A/cm .
A UV absorption spectrum of the formed thin film
dissolved in ethanol is shown in Fig. 15 (Curve B). A UV
absorption spectrum of the coloring matter (l-phenylazo-
2-naphthol) dissolved in ethanol is shown in Fig. lS (Curve
C), and a UV absorption spectrum of the thin film formed
in Example 4, as dissolved in ethanol is shown in Fig. 15
(Curve D).
EXAMPLE 16
The procedure of Example 4 was repeated with the
exception that glassy carbon was used as the anode and the
current density was changed to 40 ~Atcm2.
An ultraviolet absorption spectrum of the formed
thin film dissolved in ethanol is shown in Fig. 15 (Curve A).
EXAMPLE 17
The procedure of Example 4 was repeated with the
exception that as the micelle forming agent, a compound
having the formula:
C H
C H 3 \/ N ~--C H 2
C 1 2 H zs F e
- 30 -
1329622
1 was used, and the current density was changed to 30 ~A/cm2.
A SEM photograph (magnification, 35,000, using JSM-T220
produced by Nippon Denshi Co., Ltd.) of the thin film formed
is shown in Fig. 16.
EXAMPLE 18
A thin film wzs formed on ITO in the same manner
as in Example 4 except that as the coloring matter, 1,1'-
didodecyl-4,4'-bipyridinium dibromide was used, and the
current density was changed to 58 ~A/cm2.
A UV absorption spectrum of the formed thin film
dissolved in methanol is shown in Fig. 17 (Curve B). A UV
absorption spectrum of the above coloring matter dissolved
in methanol (concentration, 0.042 m mol/l) is shown in Fig.
17 (Curve A). Since the absorption peaks of Curves A and
B are in agr~ement with each other, it can be seen that
the thln film on the ITO is made of the above coloring
matter.
EXAMPLE 19
A thin film was formed in the same manner as in
Example 18 except that glassy carbon was used as the anode,
and the current density was changed to 60 ~A/cm2. A SEM
photograph (magnification, 1,000, using JSM-T220 produced
by Nippon Denshi Co., Ltd.) of the thin film is shown in
Fig. 18.
EXAMPLE 20
0.02 mol (concentration, 0.2 M) of lithium sulfate
as a supporting salt was dissolved in 100 cc of secondary
- 31 -
- 1329622
1 distilled water, and 0.3 m mol (concentration, 3 mM) of
the same surfactant (micelle forming agent) comprising a
ferrocene derivative, as used in Comparative Example 1 was
added thereto and dispersed by stirring to form a micelle.
0.82 nano mol (concentration, 8.2 nM) of a water
insoluble copolymer of methyl methacrylate and methacrylic
acid (molecular weight, 1 x 10 ) was added to the micelle
solution and incorporated in the micelle by application
of supersonic waves and stirring for one day and night.
Using ITO as the anode, platinum as the cathode,
and a saturated calomel electrode as a reference electrode,
electrolytic treatment was performed under the conditions
of temperature 25C applied voltage 0.3 V and current density
10 ~A/cm to obtain a polymer film on the ITO. This ITO
was washed with water and then, upon application of cyclic
voltammetry in an aqueous solution containing only a
supporting salt (lithium sulfate, concentration 0.2 M),
an oxidation reduction wave due to the micelle forming agent
incorporated in the film was observed. However, by sweeping
continuously 20 times 0 to + 0.5 V (against the saturated
calomel electrode) at a sweeping speed of 20 mV/sec in the
above aqueous solution, the height of the wave was decreased
to 10~ of the initial value. That is, 90% of the micelle
forming agent incorporated in the film could be removed
by this post-treatment.
A SEM photograph (magnification, 20,000, using
JSM-T220 produced by Nippon Denshi Co., Ltd.) of the polymer
132~522
1 film formed is shown in Fig. 19 (a), (b). Fig. l9(a) is
a photograph of the film before post-treatment, and Fig.
l9(b) is a photograph of the film after post-treatment (film
thickness- 1,800 A amount of electricity: 0.1 Coulomb/cm2;
film area: 0.91 cm2).
A Fourier transformation infrared (FT-IR)
absorption spectrum of the polymer film is shown in Fig.
20 (film thickness: 5,600 A; amount of electricity: 0.31
Coulomb /cm2; film area: 1.64 cm2), and an IR absorption
spectrum with a KBr pellet of the polymer used as the
material is shown in Fig. 21. Since the absorption peaks
of Figs. 20 and 21 are in agreement with each other, it
can be seen that the film on the ITO is made of the above
polymer.
A relation between the film thickness and the
amount of electricity having passed per unit area of the
ITO is shown in Fig. 22. Since, as can be seen from Fig.
22, there is a straight line relation (parallel relation)
between the film thickness and the amount of electricity
having passed, it can be seen that the film thickness can
also be controlled at will by controlling the amount of
electricity.
EXAMPLE 21
The procedure of Example 20 was repeated with
the exception that as the polymer, poly
(4-vinylpyridine)(molecular weight, 50,000, concentration,
7.9 ~M produced by Polyscience Inc.) was used, and the
- 33 -
1329622
1 concentration of the micelle forming agent was changed to
2.0 mM.
A UV absorption spectrum of the formed film (film
thickness: 400 A; amount of electricity: 0.019 Coulomb/cm2;
film area: 1.05 cm2) dissolved in 5 ml of ethanol is shown
in Fig. 23 (Curve a). A SEM photograph (magnification,
20,000, using JSM-T220 produced by Nippon Denshi Co., Ltd.)
of the formed thin film is shown in Fig. 24. A UV absorption
spectrum of the above polymer dissolved in ethanol (polymer
concentration, 0.25 ~M) is shown in Fig. 23 (Curve b).
Since the absorption peaks and wave forms of Curves a and
b are in agreement with each other, it can be seen that
the film on the ITO is made of the above polymer. Curve
c of Fig. 23 is a W absorption spectrum of a washing liquid
resulting from washing of ITO with 5 ml of ethanol, said
ITO having been obtained by electrolysis of a micelle
solution not containing a polymer.
A relation between the film thickness and the
amount of electricity having passed through per unit area
of the ITO is shown in Fig. 22. Since, as can be seen from
Fig. 22, there is a straight line relation (parallel
relation) between the film thickness and the amount of
electricity having passed, it can be seen that the film
thickness can also be controlled at will by controlling
2S the amount of electricity.
EXAMPLE 22
To 31.5 ml of water was added 1.13 mg of the
- 34 -
132~622
1 ferrocene derivative obtained in Example 7, as a surfactant
(micelle forming agent), and 10 mg of phthalocyanine was
added and dispersed and dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred
for two days and nights by the use of a stirrer, and micelle
solution thus obtained was subjected to centrifugal
separation at 2,000 rpm for one hour. A visible absorption
spectrum of the supernatant confirmed that the phthalocyanine
was made soluble in the micelle solution. The solubility
was 8.9 mM/4 mM micelle forming agent solution.
INDUSTRIAL APPLICABILITY
The f~rrocene derivatives of the present invention
are novel compounds and can be used in various applications,
for example, as surfactants, catalysts, auxiliary fuels,
depressors, dispersants and the like. The novel ferrocene
derivatives, when used as surfactants, form micelles in
an aqueous solution system and, therefore, coloring matters
such as phthalocyanine, having a wide variety of applications
and water-insoluble polymers can be made soluble.
If the process of the present invention is carried
out using the novel ferrocene derivatives or other ferrocene
derivatives as surfactants (micelle forming agents), an
organic thin film greatly small in thickness can be formed
by aqueous solution electrolysis and utilizing the gathering
or scattering of micelles. This process for production
of an organic thin film can be utilized, as well as coating
and coloring of various products, in production of electronic
- 35 -
132~22
1 materials such as photoconductor materials, solar batteries,
secondary batteries, electric power apparatus materials,
display device materials and the like, and further in
production of light-sensitive materials, insulating
materials, light memory materials, light sensor materials,
gas sensor materials and the like.
In addition to the subject matter described in the
principal disclosure, this invention includes the following
subject matter.
- 36 ~
132~22
DESCRIPTION
FERROCENE DERIVATIVES, SURFACTANTS CONTAINING THEM
AND PROCESS FOR PRODUCING ORGANIC THIN FILMS
Technical Field
The present invention relates to ferrocene derivatives,
surfactants containing them and a process for producing
organic thin films, and more particularly to novel ferrocene
derivatives containing a pol~oxyethylene chain, surfactants
containing said ferrocene derivatives and capable of making
coloring matter, e.g. phthalocyanine, soluble, and a process
for producing a thin film of a hydrophobic organic substance
using these surfactants.
Background Art
In general, coloring matter such as phthalocyanine or
its derivatives are insoluble in water, and although they are
soluble in organic solvents such as dimethylformamide (DMF~
or tetrahydrofuran (THF), their soluble amounts are small and
the solubility is only several milligrams (mg).
Surfactants to dissolve ohthalocyanine and the like in
water have heretofore been investigated, but no satisfactory
surfactant has been developed. It is reported that
functional group-substituted phthalocyanine derivatives can
be dissolved in water to some extent by using sulfone-based
surfactants. However, the solubility is not always
sufficiently high, and furthermore they cannot dissolve at
all unsubstituted phthalocyanines.
In connection with water-insoluble polymers, surfactants
3 ~
1~9622
to make them soluble in water have been investigated in the
same manner as above. In fact, however, no satisfactory
results have been obtained.
The present inventors have made extensive investigations
to develop surfactants to make coloring matters such as
phthalocyanine or its derivatives, or water-insoluble
polymers and the like soluble in water.
In the course of the study, it has been found that
ferrocene derivatives are promising as surfactants having the
aforementioned performance. As a result of further
investigations based on the above findings, the present
inventors have discovered that new ferrocene derivatives
obtained by introducing a specified substituent containing a
polyoxyethylene chain in ferrocene or its derivatives can
achieve the object. At the same time, they have discovered
that a water-insoluble (hydrophobic) organic thin film can be
efficiently produced by applying an electrochemical technique
using the above new ferrocene derivatives.
The present invention has been completed after the study
as described above.
Disclosure of Invention
That is, the present invention provides ferrocene
derivatives represented by the general formula (A):
t C H ~ O -t C H , ~ H ~ O ). H
F.e t ~ )
( R ' ) .--
-- 38 --
X
.. . . . . .
132~S22
(wherein Rl and R are independently hydrogen, a methyl
group, an ethyl group, a methoxy group or a carbomethoxy
group, m is an integer of 1 to 4, n is an integer of 1 to 5,
r is an integer of 11 to 18, and s is a real number of 2.0 to
70.0), or the general formula ( B!:
( C H 2 ~Y C - 0 -( C N 2 C H z O ), H
F e 0 ( B
( R ~) n~
(wherein w is an integer of 2 to 20, and Rl, R2, m, n and s
are the same as above), and also provides, surfactants
containing ferrocene derivatives represented by the general
formula (A) or (B ).
Moreover, the present invention provides a process for
production of an organic thin film which comprises making a
hydrophobic organic substance soluble in an aqueous medium
with surfactants containing ferrocene derivatives represented
by the general formula (A) or (B ), or ferrocene derivatives
represented by the general formula (A ):
~~ ( C H ~ ~ ~ Q--( C H 2 C H 2 0 ~s H
F e ~ ( A
( R 2)~
(wherein t is an integer of 2 to 10, and Rl, R2, m, n and s
- 39 -
.
132~622
are the same as above), and electrolyzing the resulting
micelle solution to form a thin film of the above hydrophobic
organic substance on an electrode.
Brief Description of Drawings
Fig. 1 is an explanation view schematically illustrating
the principle of the process of the present invention,
wherein 1 indicates a ferrocene derivative; 2, a hydrophobic
organic substance 3, a micelle; 4, an oxidized ferrocene
derivative; 5, an anode; 6, a cathode; Fc, ferrocene and e ,
an electron.
Fi. 9 is a lH-MMR spectrum of the ferrocene derivative
obtained in Example 5, Fig. lOis a H-MMR spectrum of the
ferrocene derivative obtained in Example 6, Fig.25 is a H-
MMR spectrum of the ferrocene derivative obtained in Example
7, Fig. æ is a lH-MMR spectrum of the ferrocene derivative
obtained in Example 24 Fig. D is a H-NMR spectrum of the
ferroc~ne derivative obtained in Example ~, Fig.~ is a lH-
NMR spectrum of the ferrocene derivative obtained in Example
25, and Fig. 23 is a lH-NMR spectrum of the ferrocene
derivative obtained in Example a~
Fig~ 12 is a visible absorption spectrum of the
supernatants obtained in Examples 8 to 12, and Fig.13 is a
visible absorption spectrum of coloring matter thin films on
I~O as obtained in Examples 13 and 14.
Fig. 30 indicates a visible absorption spectrum of the
supernatant obtained in Example 27 and a visible absorption
spectrum of the coloring matter thin film on ITO, Fig.~l
- 40 -
`X
--- 132~622
indicates a visible absorption spectrum of the supernatant
obtained in Example 28 and a visible absorption spectrum of
the coloring matter thin film on ITO, Fig. 32 indicates a
visible absorption spectrum of the supernatant obtained in
Example 25 and a visible absorption spectrum of the coloring
matter thin film on ITO, Fig. 33 indicates a visible
absorption spectrum of the supernatant obtained in Example 30
and a visible absorption spectrum of the coloring matter thin
film on ITO, Fig. 34 indicates a visible absorption spectrum
of the supernatant obtained in Example 31 and a visible
absorption spectrum of the coloring matter thin film on ITO,
Fig. 35 indicates a visible absorption spectrum of the
supernatant obtained in Example 32 and a visible absorption
spectrum of the coloring matter thin film on ITO, and Fig. 36
indicates a visible absorption spectrum of the supernatant
obtained in Example 33 and a visible absorption spectrum of
the coloring matter thin film on ITO.
Fig. 14 is an SEM photograph illustrating the surface
structure of the thin film formed in Example 25, Fig. 37 is
an SEM photograph illustrating the surface structure of the
thin film formed in Example 27, Fig. 38 is an SEM photograph
illustrating the surface structure of the thin film formed in
Example 28, and Fig. 39 is an SEM photograph illustrating the
surface structure of the thin film formed in Example 29.
X
1329622
l Figures A2 to A9 indicate visible absorption
spectrums obtained from Examples A; Figures Bl to B6
indicate visible absorption spectrums obtained from
Examples B;-and Figures Cl, C2 and C4 indicate visible
absorption spectrums obtained from Examples C.
Best Mode for Carrying Out The Invention
The ferrocene derivatives of the present invention
are represented by the general formula (A~ or (~) . In the
- 42 -
iX
~329622
general formula (A), Rl and R2 are independently hydrogen, a
methyl group, an ethyl group, a methoxy group or a
carbomethoxy group, m is an integer of 1 to 4, n is an
integer of 1 to 5, r is an integer of 11 to 18, and s is a
real number of 2.0 to 70Ø Since r is, as described above,
an integer of 11 to 18, an alkylene group (polymethylene
group) having 11 to 18 carbon atoms, e.g. an undecamethylene
group, a dodecamethylene group or a tridecamethylene group is
present between a ring-constituting carbon atom and an ether
oxygen atom nearest said carbon atom. s means not only an
integer between 2.0 and 70.0 but also a real number including
them, and indicates a mean value of a repeating number of an
oxyethylene group ~-CH2CH20-~ constituting a ferrocene
derivative.
On the other hand, since w of the general formula (B )
indicates an integer of 2 to 20, an alkylene group
(polymethylene group) having 2 to 20 carbon atoms, e.g. an
ethylene group or a propylene group is present between a
ring-constituting carbon atom and an oxycarbonyl group. Rl,
R2, m, n and s are the same as described abo~e.
These ferrocene derivatives represented by the general
formula (A) or ( ~) can be prepared by various methods. For
example, the ferrocene derivatives represented by the general
formula ~ ) are prepared as follows. That is, an alkali
metal (metallic sodium, metallic potassium, etc.) is added to
polyethylene glycol represented by the ~eneral formula:
HO--~CH2CH2 ~ ...~. (III)
- 43 -
-- 1329622
(wherein s is the same as described above), s.irred at
ordinary temperature to 200C for several min~tes to several
days, and then a halogen-containing ferrocene compound
represented by the general formula:
(R ')~ ~
( C H 2 ) r X
F e -- ( IV )
( R Z) n~
(wherein X is a halogen atom, and Rl, R2, m, ~ and r are the
same as described above) is added and reacted while stirring.
Thereafter, upon extraction and purification, a ferrocene
derivative represented by the general formula (A) is
obtained. A halogen-containing ferrocene com~ound of the
general formula (IV) can be prepared, for exa~ple, by
convertingW -halogenocarboxylic acid represen~ed by the
general formula: HOOC(CH2)r lX
(wherein r and X are the same as described ab~ve) into acid
halide (acylated product) represented by the general formula:
X OC(CH2)r lX (wherein Xl is a halogen atom resulting from a
halogenating atent, and r and X are the same as described
above) by the use of a suitable halogenating agent (thionyl
chloride, etc.), reacting the acid halide with ferrocene or
its derivative represented by the general for~ula:
( R ' ) .,~
F o - ~ V
( P~ ~ ) .~
- 44 -
- 132~622
(wherein Rl, R2, m and n are the same as described above) to
obtain a ferrocenylketone derivative represented by the
general formula:
( R ' ) ~
~ 0~ C ( C H z ), , X
,.~
( R Z)
(wherein Rl, R , m, n and r are the same as described above),
and further reducing the ferrocenylketone derivative.
On the other hand, a ferrocene derivative represented by
the general formula (B ) can be obtained by adding
concentrated sulfuric acid to polyethylene glycol represented
by the general formula (III), stirring at ordinary
temperature to 200C for several minutes, then adding a
carboxyl group-containing ferrocene compound represented by
the general formula:
( R ')~
~~( C H 2 )~ C O O H
F e ( Yll
( R ~) ,.~
(wherein Rl, R2, m, n and w are the same as described above)
and reacting while stirring, and then extracting and
purifying. A carboxyl group-containing ferrocene compound of
the general fo,rmula (VII) can be prepared, for ex~mple, as
- 45 -
132~S22
follows: that is, the carboxyl group-containing ferrocene
compound represented by the general formula (VII) can be
prepared by reacting alkoxycarbonylic acid halide represented
by the general formula: X OC(CH2)w 1COOR (wherein X is a
halogen atom, R is an alkyl group, and w is the same as
described above) with ferrocene or its derivative represented
by the general formula (V) to obtain ferrocenoylcarboxylic
acid ester represented by the general formula:
O
( R ' ) ~
~C ( C H 2)~-1 C O O R
F e
( R 2) ~
(wherein Rl, R2, m, n and w are the same as described above),
then hydrolyzing to obtain the corresponding carboxylic acid,
and then reducing or alternatively reducing and then
hydrolyzing.
The ferrocene derivatives of the present invention as
represented by the general formula (A) or ( B~ can be
produced as described above. In production of these
ferrocene derivatives, the polyethylene glycol of the general
formula (III) can be replaced by similar polyethers.
Extraction treatment after the reaction can be carried out
using alcohol, THF and the like, and purification can be
carried out by chromato purification and so forth.
~ he ferrocene derivatives represented by the general
- 46 -
-~ - 132~622
formula (A) or (B ) as obtained by the method as described
above are effective as surfactants and can be used
particularly as surfactants (micelle forming agents) to make
hydrophobic organic substances soluble in water or an aqueous
medium.
The surfactants of the present invention contain the
ferrocene derivatives of the general formula (A) or (8 ) as
the major component, and other various additives can be
added, if necesary. Use of the surfactants of the present
invention permits to make various hydrophobic organic
substances soluble in water or an aqueous medium. There are
various hydrophobic organic substances. Examples are
coloring matters and organic coloring matters for light
memory, e.g. phthalocyanine, phthalocyanine derivatives,
metal complexes of phthalocyanine, metal complexes of
phthalocyanine derivatives, naphthalocyanine,
naphthalocyanine derivatives, metal complexes of
naphthalocyanine, metal complexes of naphthalocyanine
derivatives, porphyrin, porphyrin derivatives
(tetraphenylporphyrin and the like), metal complexes of
porphyrin, and metal complexes of porphyrin derivatives;
electrochromic materials, e.g. l,l'-diheptyl-4,4'-
bipyridinium dibromide, and l,l'-didodecyl-4,4'-bipyridinium
dibromide; light-sensitive materials (photochromic materials)
and light sensor materials, e.g. 6-nitro-1,3,3-
trimethylspiro-(2'H-l'-benzopyran-2,2'-indoline) (commonly
called spiropyran) liquid crystal display coloring matters,
- 47 -
132~S22
e.g. p-azoxyanisole; organic electrically conductive
materials and gas sensor materials, e.g. a 1:1 complex of
7,7,8,8-tetracyanoquinonedimethane (TCNQ) and
tetrathiafulvalene (TTF); light-curable paints, e.g.
pentaerythritol diacrylate; insulating materials, e.g.
stearic acid, and diazo type light-sensitive materials and
paints, e.g. 1-pheny~azo-2-naphthol. Other examples include
water-insoluble polymers, for example, general purpose
polymers such as polycarbonate, polystyrene, polyethylene,
polypropylene, polyamide, polyphenylene sulfide (PPS),
polyphenylene oxide (PPO), and polyacrylonitrile (PAN), or
polyphenylene, polypyrrole, polyaniline, polythiophene,
acetyl cellulose, polyvinyl acetate, polyvinyl butyral and
other various polymers (polyvinylpyridine and the like) or
copolvmers (a copolymer of methyl methacrylate and
methacrylic acid, and the like).
In use of the ferrocene derivatives of the present
invention as surfactants, there are various embodimetns.
Particularly in production of the organic thin film of the
present invention, they are effectively used as micelle
forming agents. In the process of the present invention, a
surfactant (micelle forming agent) comprising a ferrocene
derivative represented by the general formula (~) or (B ), or
a ferrocene derivative represented by the general formula
(A ), a supporting salt, and a hydrophobic organic substance
are placed in an aqueous medium and thoroughly dispersed by
~ha use of supersonic waves, a homogenizer, or a stirrer, for
- 48 -
.~
132~622
example, to form a micelle. Thereafter, if necessary, an
excessive hydrophobic organic substance is removed, and the
micelle solution thus obtained is subjected to electrolytic
treatment using the aforementioned electrode while allowing
to stand or somewhat stirring. During the electrolytic
treatment, the hydrophobic organic substance may be
supplementarily added to the micelle solution, or there may
be provided a recycle circuit in which the micelle solution
in the vicinity of the anode is withdrawn out of the system,
the hydrophobic organic substance is added to the withdrawn
micelle solution and throughly stirred, and then the
resulting solution is returned to the vicinity of the
cathode. Electrolytic conditions are determined
appropriately depending on various circumstances. Usually,
the liguid temperature is O to 70C and preferably 20 to
30C, the voltage is 0.03 to 1.5 V and preferably 0.1 to 0.5
V, and the current density is not more than 10 mA/cm and
preferably 50 to 300~ A/cm .
On performing this electrolytic treatment, the reaction
proceeds as illustrated in Fig. 1. Explaining in connection
with the behavior of Fe ion in the ferrocene derivative, Fe2
is converted into Fe on an anode 5, leading to the break-
down of the micelle, and particles (about 600 to 900 ~) of a
hydrophobic organic substance are deposited on the anode. On
the other hand, on a cathode 6, Fe oxided on the anode 5 is
reduced to Fe2 , recovering the original micelle 3 and,
therefore, a film forming operation can be carried out
~9
/
132~622
repeatedly using the same solution.
Electrolytic treatment as described above forms a thin
film comprised of about 600 to 900 ~ particles of the desired
hydrophobic organic substance on the anode.
The supporting salt (supporting electrolyte) to be used
in the process of the present invention is added, if
necessary, in order to control the electrical conductance of
the aqueous medium. The amount of the supporting salt added
is usually about 10 to 300 times, preferably about 50 to 200
times that of the above surfactant (micelle forming agent).
The type of the supporting salt is not critical as long as
capable to control the electric conductance of the aqueous
medium without inhibiting the formation of the micelle and
the deposition of the above hydrophobic organic substance.
More specifically, sulfuric acid salts (salts such as
lithium, potassium, sodium, rubidium or aluminum) and acetic
acid salts (salts such as lithium, potassium sodium,
rubidium, beryllium, magnesium, potassium, strontium, barium
or aluminum), which are generally widely used as supporting
salts, are suitable.
The electrode to be used in the process of the present
invention is sufficient to be a metal more noble than the
oxidation potential lagainst +0.15 V saturated calomel
electrode) of ferrocene, or an electrically conductive
substance. ~ore specifically, ITO (mixed oxide of indium
oxide and tin oxide), platinum, gold, silver galassy carbon,
electrically conductive metal oxides, electrically conductive
1329622
organic polymers, and the like can be used.
Example
The present invention is described in greater detail
with reference to examples.
Preparation Example 1
(1) ll-undecanic acid chloride prepared from SO.O g of 11-
bromoundecanic acid and 90.0 g of thionyl chloride, 37.6 g of
anhydrous aluminum chloride, and 35.0 g of ferrocene were
reacted at 5C for 3 hours in a methylene chloride solvent.
After the completion of the reaction, the reaction mixture
was treated with diluted hydrochloric acid and then purified
with a silica gel column to obtain 56.9 g of 10-bromo
undecanyl ferrocenyl ketone represented by the following
formula:
11
--C ---( C H z), O B r
F e
(2) In the presence of amalgam prepared from 65.4 g of zinc
and 27.2 g of mercuric chloride, 56.9 g of 10-bromodecanyl
ferrocenyl ketone prepared in (1) above was refluxed for 6
hours in a mixed solvent of concentrated hydrochloric acid
and ethanol to perform a reduction reaction.
After the completion of the reaction, the reaction
mixture was extracted with ethyl acetate and purified on a
silica gel column to obtain 42.1 g of l-ferrocenyl-ll-
-- 1329~22
bromoundecane represented by the following formula:
-- ( C H 2) ', B r
F e
Example 5
0.064 g of metallic sodium was added to 6.5 g of
polyethylene glycol (average molecular weight, 600) and
stirred at 70C for one day and night. Then, 1.1 g of 1-
ferrocenyl-ll-bromoundecane (obtained in Preparation Example
1) was added thereto and reacted at 110C for 10 hours. This
reaction mixture was extracted with a mixture of equal
amounts of water and n-butanol. The extract was washed with
water and then was subjected to chromatographic purification
by developing on a silica gel column using a mixture of
~enzene and ethanol (benzene: ethanol=5:1) as a solvent. For
the purified product obtained after drying, the yield was 41%
and the amount was 0.96 g. The elemental analytical values
were: carbon, 60.21% hydrogen, 9.46%; nitrogen, 0.00%. The
results of measurement of proton nuclear magnetic resonance
spectrum (lH-MMR) were as shown in Fig. 9.
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure.
--( C H 2~ ~. O ~ C H 2 C H 20)12. ~ H
F e
- 52 -
13296~2
Example 6
The procedure of Example 5 was repeated with the
exception that polyethylene glycol having an average
molecular weight of 1,000 was used as the polyethylene
glycol. For the purified product obtained, the yield was 31%
and the amount was 2.15 g. The results of H-NMR measurement
were as shown in Fig. lO.
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure:
- ( C H z) , , O ( C H z C H z O ) 1 7 H
F e
C~
Preparation Example 2
(1) In the presence of 9.6 g of anhydrous aluminum chloride,
13.5 g of ferrocene and 19.9 g of ll-ethoxycarbonylundecanic
acid chloride (known as described in J. Amer. Chem. Soc., 69,
2350 (1947)) were reacted at room temperature for 2 hours in
a methylene chloride solvent.
After the completion of the reaction, the reaction
mixture was treated ~ith diluted hydrochlorice acid and then
purified with a silica gel column to obtain 13.7 g of ethyl
ferrocenoylundecanate represented by the following formulas
O O
~ e_( c H ~,~e O c ~ H 5
F e
- 53 -
1329622
(2) 12.4 g of ethyl ferrocenoylundecanate prepared in (1)
above and 2.9 g of potassium hydroxide were refluxed for 2
hours in an ethanol solvent and then was subjected to acid
treatment to obtain 11.3 g of ferrocenoylundecanic acid
represented by the following formula;
Ol O
--C --( C H z ~, O - C O H
F e
~ . .
(3) In the presence of zinc amalgam prepared from 6.5 g of
zinc and 2.7 g of mercuric chloride, 6.0 9 of
ferrocenoylundecanic acid prepared in (2) above was reacted
at 80C for 3 hours in a mixed solvent of.concentrated
hydrochloric acid and ethanol.
After the completion of the reaction, the reaction
mixture was extracted with ethyl acetate and purified with a
silica gel column to obtain 4.8 g of ferrocenyldodecanic acid
repre~sented by the following formula:
o
( C H ~ ) r~ C O H
F e
Example 7
The procedure of Example 5 was repeated with the
exception that 6 g of polyethylene glycol (average molecular
- 54 -
'
~` 1329622
weight, 600) and 0.1 cc of concentrated sulfuric acid were
added to 0.29 g of ferrocenyldodecanic acid (obtained in
Preparation Example 2) and reacted at 80C for 6 hours. For
the purified product obtained, the yield was 62% and the
amount was 0.44 g. The results of H-MMR measurement were as
shown in Fig. 25.
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure:
( C H z ), I C -- O -- ( C H 2 C H 2 0 ) I 2 . ~ H
Fe O
Preparation Example 3
(1) In the same manner as in Preparation Example 2 (1)
except that in place of ll-ethoxycarbonylundecanic acid
chloride shown in Preparation Example 2 (1), 35.0 g of 10-
ethoxycarbonyldecanic acid chloride was used, and 17.7 g of
anhydrous aluminum chloride was used and 24.7 g of ferrocene
was reactedS 23.0 g of ethyl ferrocenoyldecanate represented
by the formula shown below was obtained.
O O
li 11
C ( C H 2) ~ C O C 2 H,
F e
- 55 -
X
132~62~
(2) In the same manner as in Preparation Example 2 (2)
except that in place of ethyl ferrocenoylundecanate shown in
Preparation Example 2 (2~, 5.0 g of ethyl ferrocenoyldecanate
(obtained in (1) above) was used, and 1.2 g of potassium
hydroxide was used; 4.7 g of ferrocenoyldecanic acid
represented by the formula shown below was obtained.
O O
--C -( C H 2 ) 9 C O H
F e
(3) In the same manner as in Preparation Example 2 (3)
except that in place of ferrocenoylundecanic acid shown in
Preparation Example 2 (3), 4.7 g of ferrocenoyldecanic acid
(obtained in (2) above) was used, and 6.6 g of zinc and 2.7 g
of mercuric chloride were used; 3.4 g of ferrocenylundecanic
acid represented by the formula shown below was obtained.
( C H ~ ) r~C O H
F e
Example 23
The procedure of Example 5 was repeated with the
exception that 39~14 g of polyethylene glycol (average
~Z .
132~622
molecular weight, 600) and 0.1 cc of concentrated sulfuric
acid were added to 3.02 g of ferrocenylundecanic acid
obtained in Preparation Example 3, and reacted at 80C for 6
hours. For the purified product obtained, the yield was
51.5~ and the amount was 4.00 g. The results of lH-NMR
measurement were as shown in Fig. 26. Elemental analytical
values were as follows:
Carbon Hydrogen Nitrogen t%)
61.03 8.68 0.00
59.82 8.71 0.00 (Calculated)
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure.
( C H 2 ) I o C - O -- t C H 2 C H 2 0 ) I ~ . 2 H
Fe O
Preparation Example 4
(1~ In the same manner as in Preparation Example 2 (1)
except that in place of ll-ethoxycarbonylundecanic acid
chloride shown in Preparation Example 2 (1), 19.3 g of 9-
ethoxycarbsnylnonanic acid chloride was used, and 10.4 g of
anhydrous aluminum chloride was used and reacted with 14.0 of
ferrocene; 23.4 9 of ethyl ferrocenoylnonanate represented by
the formula shown below was obtained.
- 57 -
1329622
o o
--C--( C H 2)~C O C 2 H 5
F e
(2) In the same manner as in Preparation Example 2 (2)
except that in place of ethyl ferrocenoylundecanate shown in
Preparation E~ample 2 (2), 20.5 of ethyl ferrocenoylnonanate
(obtained in (1) above) was used, and 5.1 g of potassium
hydroxide was used 19.7 g of ferrocenoylnonanic acid
represented by the formula shown below was obtained.
O O
11 11
~ C--( C H 2 ) 8 C O H
(3) In the same manner as in Preparation Example 2 (3)
except that in place of ferrocenoylundecanic acid shown in
Preparation Example 2 (3), 11.1 g of ferronoylnonanic acid
(obtained in (2) above) was used, and 13.1 g of zinc and 5.5
g of mercuric chloride were used; 8.3 g of ferrocenyldecanic
acid represented by the farmula shown belo~ was obtained.
( C H 2 ) ~ --C O H
58
132~622
Example 24
The procedure of Example 5 was repeated with the
exception that 82.7 g of polyethylene glycol (average
molecular weight, 600) and 0.1 cc of concentrated sulfuric
acid were added to 8.19 g of ferrocenyldecanic acid obtained
in Preparation Example 4 and reacted at 80C for 6 hours.
For the purified product obtained, the yield was 49.2~ and
the amount was 10.60 g. The results of H-NMR measurement
were as shown in Fig. ~. Elemental analytical values were as
follows:
Carbon Hydrogen Nitrogen (~
60.02 8.63 0.00
59.43 8.63 0.00 (Calculated)
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure:
( C H 2 ~ ~ C -- O -- ( C H 2 C H 2 0 ~ . 2 H
Fe o
Preparation Example 5
(1) In the same manner as in Preparation Example 2 (1)
except that in place of ll-ethoxycarbonylundecanic acid
chloride shown in Preparation Example 2 (1), 29.0 g of 5-
ethoxycarbonyl~aleric acid chloride was used, and 32.4 g of
anhydrous aluminum chloride was used and reacted with 45.2
_ 59 _
1329622
of ferrocene; 44.1 g of ethyl ferrocenoylvalerate represented
by the formula shown below was obtained.
O O
Il 11
C--( C H z ) ~ C O C z H s
F e
(2) In the same manner as in Preparation Example 2;(2)
except that in place of ferrocenoylundecanic acid shown in
Preparation Example 2 (2), 44.1 g of ethyl
ferrocenoylvalerate (obtained in (1) above), and 13.3 g of
potassium hydroxide was used; 36.0 g of fe~rocenoylvaleric
acid represented by the formula shown below was obtained.
O
C ( C H z ) ~ - C O H
F e
(3) In the same manner as in Preparation Example 2 (3)
except that in place of ferrocenoylundecanic acid shown in
Preparation Example 2 (3), 9.4 g of ferrocenoylvaleric acid
(obtained in (2) above) was ~sed, and 13.1 g of zinc and 5.5
g of mercuric chloride were used; 6.9 g of ferrocenylhexanic
acid represented by the formula shown below was obtained.
( C H 2 ) 5 C O H
F e
6c>
1~29622
Example 25
The procedure of Example 5 was repeated with the
exception that 184.80 g of polyethylene glycol (average
molecular weight, 1,000) and 0.1 cc of concentrated sulfuric
acid were added to 6.90 g of ferrocenylhexanic acid obtained
in Preparation Example 5 and reacted at 80C for 6 hours.
For the purified product obtained, the yield was 39.5~ and
the amount was 11.68 g. The results of H-MMR measurement
were as shown in Fig. ~. Elemental analytical values were as
follows:
Carbon Hydrogen Nitrogen (~)
_
56.25 9.38 0.00
56.85 9.40 0.00 ~Calculated)
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure:
( C H z ) s C -- O -- ( C H z C H 2 0 ) 2 Z . ~ H
F e O
Preparation Example 6
Il) In the same manner as in Preparation Example 2 (lj
except that 16.0 g of octamethylferrocene (known as described
in Chem. Ztg., 19?6, 100 (3), 143 (Ger)~ was used in place of
ferrocene shown in Preparation Example 2 (1), 13.3 g of 9-
ethoxycarbonylnonanic acid chloride was used in place of 11-
- 61 -
- 1329622
ethoxycarbonylundecanic acid chloride, and further 7.2 g of
anhydrous aluminum chloride was used and reacted with 16.1 g
of ferrocene 6.4 g of ethyI octamethylferrocenoylnonanate
represented by the formula shown below was obtained.
O O
~ C ( C H 2 ) 8 C O C z H s
(2) In the same manner as in Preparation Example 2 (2)
except that in place of ethyl ferrocenoylundecanate shown in
Preparation Example 2 (2), 6.4 g of ethyl
octamethylferrocenoylnonanate (obtained in (1) above) was
used, and 1.1 g of potassium hydroxide was used 6.0 g of
octamethylferrocenoylnonanic acid represented by the formula
shown below was obtained.
O O
~ C --( C H z ) ~ C O H
(3) In the same manner as in Preparation Example 2 (3)
except that in place of ferrocenoylundecanic acid shown in
Preparation Example 2 (3), 6.0 g of
octamethylferrocenoylnonanic acid (obtained in (2) above) was
used, and 8.1 g of zinc and 3.3 g of mercuric chloride were
used; 2.1 g of octamethylferrocenyldecanic acid represented
6Z
1329622
by the formula shown below was obtained.
O
~> ( C H z ) q C O H
,~
Example 26
The procedure of Example 5 was repeated with the
exception that 86.64 g of polyethylene glycol (average
molecular weight, 2,000) and 0.1 cc of concentrated sulfuric
acid were added to 2.03 g of octamethylferroçenyldecanic acid
obtained in Preparation Example 6 and reacted at 80C for 6
hours. For the purified product obtained, the yield was
15.2~ and the amount was 1.61 g. The results of lH-NMR
measurement were as shown in Fig. 29- Elemental analytical
values were as follows:
Carbon Hydro~en Nitrogen (~)
58.51 9.23 0.00
57.84 9.15 0.00 (Calculated)
From the above results, it can be seen that the above
purified product was a ferrocene derivative having the
following structure.
C H , ) . ICI -- O --( C H , C H . O ) ~ . H
- 63 -
13~9622
Example 8
1.13 g of the ferrocene derivative obtained in Example5
was added to 31.5 ml of water as a surfactant (micelle
forming agent), and 10 mg of phthalocyanine was added and
dispersed or dissolved by stirring for 10 minutes by the use
of supersonic waves. The resulting mixture was stirred for
two days and nights with a stirrer, and the micelle solution
thus obtained was subjected to centrifugal separation at
2,000 rpm for one hour. A visible absorption spectrum of the
supernatant is shown in Fig. 12 (Curve A). This confirmed
that phthalocyanine was soluble in the micelle solution. The
solubility was 4.4 mM/4 mM micelle forming agent solution.
Example 9
The procedure of Example 8 was repeated with the
exception that phthalocyanine iron complex was used in place
of phthalocyanine. A visible absorption spectrum of the
supernatant is shown in Fig. 12(Curve B). This con~inmed
that the phthalocyanine was soluble in the micelle solution.
The solubility was 0.72 mM/4 mM micelle forming agent
solution.
Example 10
The procedure of Example 8 was repeated with the
exception that phthalocyanine cobalt complex was used in
place of phthalocyanine. A visible absorption spectrum of
the supernatant is shown in Fig. ~ (Curve C). This con~irmed
~hat the phthalocyanine was soluble in the micelle solution.
The solubility was 0.22 mMJ4 mM micelle forming agent
- 64 -
1~29622
solution.
Example 11
The procedure of Example 8 was repeated with the
exception that phthalocyanine copper complex was used in
place of phthalocyanine. A visible a~sorption spectrum of
the supernatant is shown in Fig. ~(Curve D). This confirmed
that the phthalocyanine was soluble in the micelle solution.
The solubility was 0.11 mM/4 mM micelle forming agent
solution.
Example 12
The procedure of Example 8 was repeated with the
exception that phthalocyanine zinc complex was used in place
of phthalocyanine. A visible absorption spectrum of the
supernatant is shown in Fig. ~ (Curve E). This confirmed
that the phthalocyanine was soluble in the micelle solution.
The solubility was 0.41 mM/4 mM micelle forming agent
solution.
Example 13
0.22 g of lithium sulfate (Li2SO4) was added to 10 ml of
the micelle solution prepared in Example 8 to prepare a 0.44
mM phthalocyanine/2 mM micelle forming agent/0.2 M lithium
sulfate solution. Using this solution as an electrolyte, and
ITO as an anode, platinum as a cathode and a saturated
calomel electrode as a reference electrode, constant-electric
potential electrolysis of applied voltage 0.5 V and current
7 ~ A was carried out at 25-C for 2 hours. As a result, a
coloring matter thin film having primary particles 1,000 ~ in
- 65 -
` 1329622
average particle diameter was formed on the ITO. A scanning
electron microscope (SEM) photograph (magnification: 20,000
using JSM-T220 produced by Nippon Denshi Co., Ltd.) of the
coloring matter thin film is shown in Fig. 14. A visible
absorption spectrum of the coloring matter thin film on ITO
is shown in Fig. 13 (Curve AJ. By agreement of Fig. 13
(Curve A) with Fig. ~ (Curve A) in visible absorption
spectrum, the coloring matter thin film on ITO was identified
to be composed of phthalocyanine.
Example 14
The procedure of Example 13 was repeated with the
exception that the term of electrolysis time was changed to
40 minutes.
A visible absorption spectrum of the formed coloring
matter thin film is shown in Fig. 13(Curve A). By
comparison of curve A with curve B in Fig. 13 it can be seen
that the formed thin film had a small absorption spectrum as
compared with Example 13 and the film thickness could be
controlled by the electrolysis time.
- 66 -
1329~22
Example 27
0.193 g of the ferrocene derivative obtained in Example
7 was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyanine was added
thereto and dispersed or dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solution (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig. 30 (Curve A). This confirmed that
phthalocyanine was soluble (dispersed) in the micelle
solution. The solubility was phthalocyanine 6.4 mM/2 mM
micelle forming agent solution. To this solution, LiBr as a
supporting salt was added in such a manner that the
concentration was 0.1 M and stirred for 10 minutes with a
stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturated calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density 45~ ~/cm2 was
carried out at 25-C for 30 minutes. The amount of
electricity passed in this case was 0.09 coulomb.
As a result, a thin film of phthalocyanine was obtained
on the ITO transparent glass electrode. A visible absorption
spectrum of pkthalocYanine on the ITO transparent glass
- 67 -
1329622
electrode is shown in Fig. 30 (Curve B). 8y agreement of
Fig. 30 (Curve A) with Fig. 30 (Curve B), it was confirmed
that the thin film on the ITO transparent glass electrode was
phthalocyanine. An ultraviolet (UV) absorption spectrum
showed that the thickness of the thin fiLm was 0.31~ m.
An SEM photograph (magnification: 30,000; using JSM-T220
produced by Nippon Denshi Co., Ltd.) of the thin film
obtained is shown in Fig. 37.
Example 28
0.190 g of the ferrocene derivative obtained in Example
was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyanine was added
thereto and dispersed or dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solution (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig. ~1 (Curve A). This confirmed that
phthalocyanine was soluble (dispersed1 in the micelle
solution. The solubility was phthalocyanine 7.8 mM/2 mM
micelle ~orming agent solution. To this solution, LiBr as a
supporting salt was added in such a manner that the
concentration was 0.1 M and stirred for 10 minutes with a
stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
- 68 -
1329~22
cathode and a saturated calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density q8~ A/cm2 was
carried out at 25~C for 30 minutes. The amount of
electricity passed in this case was 0.09 coulomb.
As a result, a thin film of phthalocyanine was obtained
on the ITO transparent glass electrode. A vi5ible absorption
spectrum of phthalocyanine on the ITO transparent glass
electrode is shown in Fig. 31 (Curve B). By a~reement of
Fig. 31 (Curve A) with Fig. 31 (Curve B), it was confirmed
that the thin film on the ITO transparent glass electrode was
phthalocyanine. An W absorption spectrum showed that the
thickness of the thin film was l.OS~ m.
An SEM photograph (magnification: 30,000; using JSM-T220
produced by Nippon Denshi Co., Ltd.) of the thin fiLm
obtained is shown in Fig. 38.
Example 29
0.187 g of the ferrocene derivative obtained in Example
~ was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyanine was added
thereto and dispersed or dissolved by stirring for 10 minutes
with sup~rsonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solution (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig.. 32 (Curve A). This confirmed that
- 69 -
- 1329622
phthalocyanine was soluble (dispersed) in the micelle
solution. The solubility was phthalocyanine 8.2 mM/2 mM
micelle forming agent solution. To this solution, LiBr as a
supporting salt was added in such a manner that the
concentration was 0.1 M and stirred for 10 minutes with a
stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturated calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density 72~ A/cm2 was
carried out at 25C for 30 minutes. The amount of
electricity passed in this case was 0.13 coulomb.
As a result, a thin film of phthalocyanine was obtained
on the ITO transparent glass electrode. A visible absorption
spectrum of phthalocyanine on the ITO transparent glass
electrode is shown in Fig. 32 (Curve B). By agreement of
Fig. 32 (Curve A) with Fig. 32 (Curve B), it was confirmed
that the thin film on the ITO transparent glass electrode was
phthalocyanine. An UV absorption spectrum showed tAat the
thickness of the thin film was 1.85~ m.
An SEM photograph ~magnification: 30,000; using JSM-T220
produced by Nippon Denshi Co., Ltd.) of the thin film
obtained is shown in Fig. 39.
Example 30
0.176 g of the ferrocene derivative obtained in Example
2~as added to 100 cc of water as a surfactant (micelle
- 70 -
--- 1329622
forming a~ent), and 100 mg of phthalocyanine was added
thereto and dispersed or dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solution (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig. 33 tcurve A). This confirmed that
phthalocyanine was soluble (dispersed) in the micelle
solution. The solubility was phthalocyanine 1.8 mM/2 mM
micelle forming agent solution. To this solution, LiBr as a
supporting salt was added in such a manner that the
concentration was 0.1 M and stirred for 10 minutes with a
stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturated calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and cùrrent density 17~ A/cm was
carried out at 25-C for 30 minutes. The amount of
electricity passed in this case was 0.04 coulomb.
As a result, a thin film of phthalocyanine was obtained
on the ITO transparent glass electrode. A visible absorption
spectrum of phthalocyanine on the ITO transparent glass
electrode is shown in Fig. 33 (Curve 8). By agreement of
Fig. 33 (Curve A) with Fig. 33 (Curve B), it was confirmed
that the thin film on the ITO transparent glass electrode was
- 71 -
- 132~622
phthalocyanine. An UV absorption spectrum showed that the
thickness of the thin film was 0.04 ~m.
Example 31
0.210 g of the ferrocene derivative obtained in Example
was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyanine was added
thereto and dispersed or dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solu~ion (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig. 34 (Curve A). This confirmed that
phthalocyanine was soluble (dispersed) in the micelle
solution. The solubility was phthalocyanine 4.0 mM/2 mM
micelle forming agent solution. To this solution, LiBr as a
supporting salt was added in such a manner that the
concentration was 0.1 M and stirred for 10 minutes with a
stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturat~d calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density 124 ~A/cm2 was
carried out at 25C for 30 minutes. The amount of
electricity passed in this case was 0.23 coulomb.
As a result, a thin film of phthalocyanine was obtained
- 72 -
1329622
on the ITO transparent glass electrode. A visible absorption
spectrum of phthalocyanine on the ITO transparent glass
electrode is shown in Fig. 34 (Curve B). By agreement of
Fig. 34 (Curve A) with Fig. 34 (Curve B), it was confirmed
that the thin film on the ITO transparent glass electrode was
phthalocyanine. An UV absorption spectrum showed that the
thickness of the thin film was 4.6~ m.
Example 32
0.188 g of the ferrocene derivative obtained in Example
5 was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyar.ine iron complex was
added and dispersed or dissolved by stirring for 10 minutes
with supersonic waves. The mixture was further stirred for
two days and nights with a stirrer, and then the micelle
solution (dispersed solution) thus obtained was subjected to
centrifugal separation at 2,000 rpm for 30 minutes. A
visible absorption spectrum of the supernatant obtained is
shown in Fig. 35 ~Curve A). This confirmed that the
phthalocyanine iron complex was soluble (dispersed) in the
micelle solution. The solubility was phthalocyanine iron
complex 4.1 mM/2 mM micelle forming a~ent solution. To this
solution, Li8r as a supporting salt was added in such a
manner that the concentration was 0.1 M and stirred for 10
minutes with a stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturated calomel electrode as a reference
- 73 ~
132~622
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density 14~ A/cm2 was
carried out at 25C for 30 minutes.
As a result, a thin film of phthalocyanine iron complex
was obtained on the ITO transparent glass electrode. A
visible absorption spectrum of the phthalocyanine iron
complex on the ITO transparent glass electrode is shown in
Fig. 35 icurve B). Because of agreement of Fig. 35(Curve A)
with Fig. 35 ~Curve ~), it was confirmed that the thin film
on the ITO transparent glass electrode was the phthalocyanine
iron complex. An UV absorption spectrum showed that the
thickness of the thin film was 0.16~ m.
Example.21
0.188 g of the ferrocene derivative obtained in Example
5 was added to 100 cc of water as a surfactant (micelle
forming agent), and 100 mg of phthalocyanine copper complex
was added thereto and dispersed or dissolved by stirring for
10 minutes with supersonic waves. The mixture was further
stirred for two days and nights with a stirrer, and then the
micelle solution ~dispersed solution) thus obtained was
subjected to centrifugal separation at 2,000 rpm for 30
minutes. A ~-isible absorption spectrum of the supernatant
obtained is shown in Fig. 36 ~urve A). This confirmed that
phthalocyanine copper complex was soluble (dispersed) in the
micelle solution. The solubility was phthalocyanine copper
complex 3.8 mM/2 mM micelle forming agent solution. To this
solution, LiBr as a supporting salt was added in such a
- 74 -
13296~2
manner that the concentration was 0.1 M and stirred for 10
minutes with a stirrer.
Using the obtained solution as an electrolyte, and an
ITO transparent glass electrode as an anode, platinum as a
cathode and a saturated calomel electrode as a reference
electrode, constant electric potantial electrolysis of
applied voltage 0.5 V and current density 43~ A/cm2 was
carried out at 25C for 30 minutes. The amount of
electricity passed in this case was 0.11 coulomb.
As a result, a thin film of phthalocyanine copper
complex was obtained on the ITO transparent glass electrode.
A visible absorption spectrum of the phthalocyanine copper
complex on the IT0 transparent glass electrode is shown in
Fig. 36 (Curve B). ~ecause of agreement of Fig. 36 (Curve A)
with Fig. 36 (Curve B), it was confirmed that the thin film
on the ITO transparent glass electrode was the phthalocyanine
copper complex. An UV absorption spectrum showed that the
thickness of the thin film was 0.08~ m.
Industrial A~e~ica ~
The ferrocene derivatives of the present invention are
novel compounds and can be used in various applications, for
example, as surfactants ~micelle forming agents), catalysts,
auxiliary fuels, depressors or dispersants. The novel
ferrocene dervativec, when used particularly as surfactants,
form micelles in an aqueous solution system and, therefore,
coloring matters such as phthalocyanine, having a wide
variety of applications and various hydrophobic polymers are
- 75 -
1~29~22
made soluble. In accordance with the process of the present
invention in which the above surfactant (micelle forming
agent) is used and at the same time~ gathering or scattering
of the micelles is utilized, an organic thin film having a
greatly small film thickness can be formed.
This application includes directly following this
page:
~a) Examples A disclosed on pages A1 to A6, following
and described with reference to Figures A2 to A9;
(b) Examples B disclosed on pages Bl to B8; following
and described with reference to Figures Bl to B6;
and
(c) Examples C disclosed on pages Cl to C3, following
and described with reference to Figures Cl, C2 and
C4.
here are no Figures Al or C3.
~ 32962'~
Example~ to~35
To a prescribed amount of water, micelle forming agent
was added to make a 2 mM solution, a hydrophobic organic
substance having a prescribed particle diameter was added
thereto, and the mixture was stirred with application of
supersonic waves fcr 10 minutes. The micelle solution thus
obtained was subjected to centrifugal separation at 2000 rpm
for one hour. A visible absorption spectrum of the
supernatant of the said solution is shown in Figs.~2 toA9
(indicated by A) (Examples~5,6,11,15,18,19,25 and 26).
The above results confirmed that these hydrophobic
organic substances are solubilized to the micelle solution.
To the said micelle solution, a prescribed supporting
electrolyte was added to prepare an electrolyte, and constant
potential electrolysis was performed under the conditions of
a temperature of 25C, an applied vol~age of 0.5 V so as to
reach the prescribed amount of electricity, by us~ of ITO or
GC (glassy carbon) as the anode, platinum as the cathode, and
a saturated calomel electrode as the reference electrode.
As the result, a thin film of the hydrophobic organic
substance used was formed on the anode. The visible
absorption spectrum on the anode is shown in Figs.A2 to~9
(indicated by B) (ExamplesA5,6,11,15,}8,19,25 and 26). Since
the absorption peaks of A and B are coincident, it was
confirmed that the thin film on the anode was made of the
1329622
hydrophobic organic substance used.
The thickness of the thin film was determined by UV
absorption spectrum.
The conditions and result of the operation mentioned
above are shown in Table~l.
?~
~ ~ 1329622 C C c c c
E E~ a) ~ o, a.
n ~ c
e ~o c ~ ,~
s O~ c c 3 3 ~ ~ 3 E~ ~ 3 E~ m
o o~ .
c c E ` ^' ~ ` '~ ~ ~ ~ ` ~ ~ ~ r7 ,~ ~ ~o
O c o ~ o o o o oo ~ _~ ' o
E~
~
o~ I ~ O ~D
c L- E o ~ O ~ ~` O ~ ~ O o _, ., ~
Eo o _i o o o, o o o o o 0 0 0 0 0 0 0
~ I
~ol ~
~C ~ 1 H 1~ H ~--1
~ O
~0 r~
~C-- OOOOOOOOOOOOOOOO
C C
~ ~ ~1 ~ ~ ' ,., L~ 5.~ 0~
~ Y m * * N ID * * m ~N m * u~ o ~ N
E'~ ~ ~ J ,~ V ~ _l Z
0 Cn r- 0
-~ e v _~ _
_1- c ~ ¢ ~S ~ 6 ¢ ¢ ¢ ¢ ¢ ¢
U Ll cn L~ 2
~ ~o ¢
,,~ ~ O 11~ O ~ ~ ~ X N I X X
~0v ~~ ~r _ o
n 0
~n_~
U ~ V E E o o _ _ o _ _ --I _ _ o o --I o o o
c~ ~ a_ o o o o o o o o o o o o o o o o
u ~
- ~ o ~ :1
¢ ~ o c ca m m
o C 5: ~ It C .C L O o _I
E ol _ ~ ~ ~ ," ~ ~~ o ~ ~ ~ _ ~
7q
l ~1 3 3 132962~ 3
IE O ~
_l E~ ~ ~ c o o
~ ~ ~ 0 ~ ID C
C O v v v v v v ~ ~D C t~, v ~ v V V v
.,~ O c l' c r c ~ c ~1 c .
~ ~J 3 3 3 3 3 3 ~ ~ 0 a m 3 ~ 3 3 3 3
. ~EI 0 ~ - 0 ~
~ _ --~ ~ O _~ N _~ _1
E-'
(~ v E
V O D ~ 1~ ~ N (''t ~` ~ 1` _ 0~ ~ U~ 1~ ~ ~ ~r N ~1
c 1~ E O O O 'O O _ ~1~ N O~
E U--I 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 o O o O
~U
al EO~
~:~ 1~ 1 ~ W ~ ~ H ~ ~ ~ 1-~
O
.
vV ~ N ~ N ~ N ~ N
__l~O~ O OO O O O O O O O O O O O O O
_~ .~ ~.1
,o ~ ~1 ~ ~ ~ ~ ' ~ ~J ~1o ~
_~ I N m o ~ N m Oc m" ~ m ~ O m m ,~ ~ O ~ N
cn ~ z v ~ z ~ ~ ~, Z Z ~ Z
D ~I C V
E~ lU E 11~ ¢ ~ ~ .
U ~I -- ~ E~ E~ E~ ~ E~ ~ ~ E'~ E'' ~ ~ ~ ~ D~ D. G E~
'~ ~ ~ ~ ~ ~ ~
~ .
V~
_~_ ~C ~ o
~:qJ 1~ ~ E~ o O N O O o o
3 o N o 1-~ _I N 'D N O O
N
~ ~U~D4
Ul~ V ~0 1` N ~ N O
E-- r~ ~o ~~ N N 0 '1 0
E~_ O O O O O' O O O O O o O O ~ 7 0 _ u-
O
U ,
,0,
o ~c ~ ^ Y ~ O ~ ~~
.1 v u~ ~E E -~
_ ,n D- ~O 1` --I ~ ~ Il~ C ,e _ _, N 1~- S m ~ c Ll c ' _ _ _
o ~ ~ ~ mm ~ ~ ~ ~ v ~ ~ u ~ a-
.C ~: ~ N ~ ~ O O O~ ~ C N ~ ~1 al ~ C O
U ~ ~ ~ ~ C
_ O 1~ ~^V ~ O-- N1'1~r1/7 'O 1-- ~ ~ O --~ N t~
~ Z _ _ _ N ~ N N ~ N ~ NN ~ 1~1 I't l''t rl 1'1 1`'1
W ~ C ~ ~
8~
1329~22
*l produced by Tokyo Kasei Kogyo Co., Ltd.
*2 produced by Kanto Chemical Co., Inc.
~3 distearyldimethylammonium chloride produced by Tokyo
Kasei Kogyo Co., Ltd.
*4 1,1'-didodecyl-4,4'-bipyridiniumdibromide produced by
Tokyo Kasei Kogyo Co., Ltd.
*5 1,3,3-trimethylindolino-6'-nitrobenzopyrylospiran
produced by Tokyo Kasei Kogyo Co., Ltd.
*6 poly(4-vinylpyridine), weight average molecular weight:
50000, produced by Polyscience Co.
*7 poly(2-vinylpyridine) produced by Aldrich Chemical Co.,
Inc.
*8 polyvinylbutylal, weight average molecular weight:
85000, 19% hydroxyl group/1% acetate group/80% butylal
group produced by Scientific Polymer Products Co.
*9 produced by Aldrich Chemical Co., Inc.
*10 4,4'-azoxyanisole produce by Aldrich Chemical Co., Inc.
*il dibenzo-18-crown-6 produced by Nisso Co., Ltd.
*12 poly(4-vinylpyridine), weight average molecular weight:
50000, produced by Polyscience Co.
~13 manganese tetraphenylporphyrin
*14 3.~ mM in monom~r unit
*15 1.0 mM in monomer unit
~16 1.2 mM in monomer unit
~1 .
1329622
*17 compound represented by the formula:
~ ?~, I H 2 Z ~0 C H z C H 2) ~ z . ~ O H
*18 compound represented by the formula:
C H3
C H 3 \/ N ~--C " H 2Z~
C H3 Fe
1329622
Example~l
To 20 ml of water, 198 mg of a compound (FPEG)
represented by the formula:
~C ~ I H 2Z tO C H 2 C H 2)12. zO H
F e
was added as a nonionic micelle forming agent, 115 mg of Pc-
Cu (alpha-type, produced by Dainichiseika Color & Chemicals
Mfg. Co., Ltd.) was added, and stirred with the application
of supersonic waves for 10 minutes. The micelle solution
thus obtained was subjected to centrifugal separation at 2000
rpm for one hour. A visible absorption spectrum of the
supernatant is shown in Fig.Bl (indicated by A). From the
above results, it was confirmed that Pc-Cu (alpha-type) is
solubilized to the micelle solution. The solubility was 5.3
mM/2 mM micelle forming agent solution.
To 20 ml of the said micelle solution, 0.210 g of LiBr
was added as a supporting electrolyte to form 5.3 mM Pc-Cu
(alpha-type)/2 mM micelle forming agent/0.1 M LiBr solution.
By use of the resulting solution as an electrolytic solution,
ITO as the anode, platinum as the cathode and a saturated
calomel electrcde as a reference electrode, constant
potential electrolysis was performed for 30 minutes under the
conditions of a temperature of 25-C, an applied voltage of
~3
1329622
0.500 V, and a current density of 8.5 ~A/cm . The amount of
electricity passed was 0.021 coulomb.
As the result, a thin film of Pc-Cu (alpha-type) was
formed on the anode. A visible absorption spectrum of the
said thin film on the anode is shown in Fig.81 (indicated by
B). Since the absorption peaks of A and B in Fig.Bl are
coincident, it was confirmed that the thin film of coloring
matter on the anode consists of Pc-Cu (alpha-type) used.
The thickness of the thin film was found to be 0.8 ~m
~rom the measurement of UV absorption spectrum.
Example B2
To 20 ml of water, 198 mg of FPEG used in Examplee~ was
added as nonionic micelle forming agent, and 115 mg of Pc-Cu
(beta-type) (produced by Dainichiseika Color & Chemicals Mfg.
Co., Ltd.) was added thereto, and then stirred with the
application of supersonic waved for 10 minutes. The
resulting micelle solution was subjected to centrifugal
separation at 2000 rpm for one hour. A visible absorption
spectrum of the supernatant of the solution is shown in Fig.B
2 (indicated by A). The above confirms that Pc-Cu (beta-
type) is solubilized into micelle solution. The solubility
was 5.1 mM/2 mM micelle forming solution.
Next, to 20 ml of the said micelle solution, 0.210 g of
LiBr was added as a supporting electrolyte to obtain a
solution of 5.1 mM Pc-Cu (beta-type)/2 mM micelle forming
agent/0.1 M LiBr. By the use of the above solution as an
e7ectrolytic solution, I~O zs the anode, platinum as the
1329622
cathode and a saturated calomel electrode as a reference
el~ctrode, constant potential electrolysis was performed for
30 minutes under the conditions of a temperature of 25C, an
applied voltage of 0.500 V, and a current density of 4.3
~A/cm2. The amount of the electricity passed was 0.012
coulomb.
As the result, a thin film of Pc-Cu (beta-type) was
formed on the anode. The visible absorption spectrum of the
thin film on the anode is shown in Fig.B2 (indicated by B).
Since the absorption peaks of A and B in Fig.~2 are
coincident, it was confirmed that the thin film of the
coloring matter on the anode consists of Pc-Cu (beta-type)
used.
The thickness of the said thin film was found to be
o . 3 ~m by the measurement of UV absorption spectrum.
Example B3
To 20 ml of water, lg8 mg of FPEG used in Exampleal was
added as nonionic micelle forming agent, and 229 mg of C116-
Pc-Cu (Phthalocyanine Green) (produced by Tokyo Kasei Kogyo
Co., Ltd.) was added thereto, and then stirred with the
application of supersonic waved for 10 minutes. The
resulting micelle solution was subjected to centrifugal
separation at 2000 rpm for one hour. A visible absorption
spectrum of the supernatant of the solution is shown in Fig.B
3 (indicated by A). The above confirms that C116-Pc-Cu is
solubilized into micelle solution. The solubility was
1.5 mM/2 mM micelle for~ing solution.
1329622
Next, to 20 ml of the said micelle solution, 0.210 g of
LiBr was added as a supporting electrolyte to obtain a
solution of 1.5 mM C116-Pc-Cu/2 mM micelle forming agent/0.1
M LiBr. By the use of the above solution as an electrolytic
solution, ITO as the anode, platinum as the cathode and a
saturated calomel electrode as a reference electrode,
constant potential electrolysis was performed for 30 minutes
under the conditions of a temperature of 25C, an applied
voltage of 0.500 V, and a current density of 12.6 ~A/cm2.
The amount of the electricity passed was 0.023 coulomb.
As the result, a thin film of C116-Pc-Cu was formed on
the anode. The visible absorption spectrum of the thin film
on the anode is shown in Fig.~3 (indicated by B). Since the
absorption peaks of A and B in Fig.a3 are coincident, it was
confirmed that the thin film of the coloring matter on the
anode consists of C116-Pc-Cu used.
The thickness of the said thin film was found to be
0.1 ~m by the measurement of UV absorption spectrum.
Example~4
To 20 ml of water, 198 mg of FPEG used in ExampleBl was
added as nonionic micelle forming agent, and 122 mg of Cl-Pc-
Cu (Phthalocyanine Blue) (produced by Tokyo Kasei Kogyo Co.,
Ltd.) was added thereto, and then stirred with the
application of supersonic waved for 10 minutes. The
resulting micelle solution was subjected to centrifugal
separation at 2000 rpm for ore hour. A visible absorption
spectrum of the supernatant of the solution is shown in Fig~ B
1329622
4 (indicated by A). The above confirms that Cl-Pc-Cu is
solubilized into micelle solution. The solubility was
4 . 2 mM/2 mM micelle forming solution.
Next, to 20 ml of the said micelle solution, 0.210 g of
LiBr was added as a supporting electrolyte to obtain a
solution of 4.2 mM Cl-Pc-Cu/2 mM micelle forming agent/0.1 M
LiBr. By the use of the above solution as an electrolytic
solution, IT0 as the anode, platinum as the cathode and a
saturated calomel electrode as a reference electrode,
constant potential electrolysis was performed for 30 minutes
under the conditions of a temperature of 25~C, an applied
voltage of 0.500 V, and a current density of 27 ~A/cm . The
amount of the electricity passed was 0.05 coulomb.
As the result, a thin film of Cl-Pc-Cu was formed on the
anode. The visible absorption spectrum of the thin film on
thç anode is shown in Fig. B4 ( indicated by B). Since the
absorption peaks of A and B in Fig. B4 are coincident, it was
confirmed that the thin film of the coloring matter on the
anode consists of Cl-Pc-Cu used.
The thickness of the said thin film was found to be
0.8 ~m by the measurement of ~V absorption spectrum.
Example~5
To 20 ml of water, 198 mg of FPEG used in Exampl~Bl was
added as nonionic micelle forming agent, and 282 mg of
CllOBr6-Pc-Cu (Heliogen Green) (K8730) (produced by BASF
Japan Co., Ltd.) was added thereto, and then stirred with the
application of supersonic waved for 10 minutes. The
~7
- 1329622
resulting micelle solution was subjected to centrifugal
separation at 2000 rpm for one hour. A visible absorption
spectrum of the supernatant of the solution is shown in Fig.
5 tindicated by A). The above confirms that Cl1OBr6-Pc-Cu is
solubilized into micelle solution. The solubility was
4.2 mM/2 mM micelle forming solution.
Next, to 20 ml of the said micelle solution, 0.210 g of
LiBr was added as a supporting electrolyte to obtain a
solution of 4.2 mM CllOBr6-Pc-Cu/2 mM micelle forming
agent/0.1 M LiBr. By the use of the above solution as an
electrolytic solution, ITO as the anode, platinum as the
cathode and a saturated calomel electrode as a reference
electrode, constant potential electrolysis was performed for
30 minutes under the conditions of a temperature of 25C, an
applied voltage of 0.500 V, and a current density of
8.2 ~A/cm2. The amount of the electricity passed was 0.015
coulomb.
As the result, a thin film of CllOBr6-Pc-Cu was formed
on the anode. The visible absorption spectrum of the thin
film on the anode is shown in Fig.B5 (indicated by B). Since
the absorption peaks of A and B in Fig.~5 are coincident, it
was confirmed that the thin film of the coloring matter on
the anode consists of Cl1OBr6-Pc-Cu used.
The thickness of the said thin film was found to be
0.9 ~m by the measurement of UV absorption spectrum.
ExampleB6
To 20 ml of water, 198 mg of FPEG used in Example~l was
~3q
1329622
added as nonionic micelle forming agent, and 300 mg of
C18Br8-Pc-Cu (Heliogen Green) (K9360) (produced by BASF Japan
Co., Ltd.) was added thereto, and then stirred with the
application of supersonic waved for 10 minutes. The
resulting micelle solution was subjected to centrifugal
separation at 2000 rpm for one hour. A visible absorption
spectrum of the supernatant of the solution is shown in Fig.
6 (indicated by A). The above confirms that C18Br8-Pc-Cu is
solubilized into micelle solution. The solubility was
3.8 mM/2 mM micelle forming solution.
Next, to 20 ml of the said micelle solution, 0.210 g of
Li8r was added as a supporting electrolyte to obtain a
solution of 3.8 mM C18Br8-Pc-Cu/2 mM micelle forming
agent/0.1 M LiBr. By the use of the above solution as an
electrolytic solution, ITO as the anode, platinum as the
cathode and a saturated calomel electrode as a reference
electrode, constant potential electrolysis was performed for
30 minutes under the conditions of a temperature of 25~C, an
applied voltage of 0.500 V, and a current density of
11.2 ~A/cm2. The amount of the electricity passed was 0~018
coulomb.
As the result, a thin film of C18Br8-Pc-Cu was formed on
the anode. The visible absorption spectrum of the thin film
on the anode is shown in Fig.B6 (indicated by B). Since the
absorption peaks of A and B in Fig.B6 are coincident, it was
confirmed that the thin film of the coloring matter on the
anode consists of C18Br8-Pc-Cu used.
~0~
1329622
The thickness of the said thin film was found to be
0.7 ~m by the measurement of UV absorption spectrum.
~a
1329622
Example~l
In 100 ml of water, 0.099 g of a compound (FPEG)
represented by the formula-
~C I, H 2 2 ~0 C H 2 C H 2~ 0 ~I
F e
was dissolved as nonionic micelle forming agent to make a 1mM solution. Further, 0.52 g of LiBr as a supporting salt
was added so as to have a concentration of 0.1 mM.
Then, an excess amount (1.0 g) of magnesium
phtharocianine (Pc-Mg) was added to the solution, which was
subjected to supersonic wave treatment for 10 minutes. After
that, the solution was stirred for one day and night, and
after the said operation was repeated, centrifugal separation
was performed at 2000 rpm for one hour to prepare a micelle
solution. A visible absorption spectrum of the supernatant
of the said solution is shown in Fig.~l (indicated by the
solid line). This confirmed that Pc-Mg is solubilized in
micelle solution. A visible absorption spectrum of the
ethanol solution of the said Pc-Mg is also shown in Fig.~l
(indicated by the broken line).
Subsequently, the above micelle solution ~saturated
solubilized aqueous solution of Pc-Mg) was electrolyzed for
two hours with a voltage of ~.5 V applied on the I~O
1329622
transparent glass electrode against the saturated calonel
electrode (SCE).
The visible absorption spectrum of the thin film
resulted on ITO glass transparent electrode according to the
above operation is shown in Fig.C2 (indicated by the solid
line). The visible absorption spectrum of the supernatant is
shown in Fig.C2 (indicated by the broken line) (which is the
same as the solid line in Fig.~l)
ExampleC2
In 100 ml of water, 0.198 g of the same FPEG as used in
Example~l was dissolved as nonionic micelle forming agent to
make a 2 mM solution. Further, 0.52 g of LiBr as a
supporting salt was added so as to have a concentration of
0.1 mM.
subsequently, an excess amount (1.0 g) of chlorine-
containing aluminum phtharocianine (Pc-AlCl) was added to the
solution, which was subjected to supersonic wave treatment
for 10 minutes. Then, the solution was stirred for one day
and night, and after the said operation was repeated,
centrifugal separation was performed at 2000 rpm for one hour
to prepare a micelle solution.
Subsequently, the above micelle solution (saturated
solubilized aqueous solution of Pc-AlCl) was electrolyzed for
two hours with a voltage of ,0.5 V applied on the ITO
transparent glass electrode against SCE.
The visible absorption spectrum of the thin film
resulted on ITO glass transparent electrode according to the
`- 1329622
above operation is shown in Fig.C4 (indlcated by the solid
line). The visible absorption spectrum of the supernatant of
the micelle solution is shown in Fig.C4 (indicated by the
broken line).
93