Note: Descriptions are shown in the official language in which they were submitted.
- 1 132~7~
:
. .
,
~, MULTIPLE STAGE GAS ABSORBER
The present invention relates generally to
apparatus and method for absorbing a gas into a liquid,
and specifically to apparatus and method for
reintroducing gas effluent into a fresh liquid, and more
specifically to apparatus and method for sequentially
passing a volume of oxygen through a series of packed
bsds submerged in water wherein the oxygen is
substantially absorbed into fresh water and waste yases
1 10 such as nitrogen, argon and car~on dioxide are stripped
from the water.
Gas absorption means are well known in the
art. Examples are shown in U. S. Patent Nos. 3,771,492;
3,9~6,893; 3,948,608; 3,116,712; 2,259,034; 3,255,731;
; 15 and 4,116,164.
While the varieties of gas absorption means in
i the art are apparently well suited for their intended
purposes, unti.l the present invention there has been no
apparatus and method for use in sequentially
reintroducing a gas into a series of enclosed contact -
.f 20 chambers receiving un-gassified liquids. As the gas is
I absorbed by the liquid, other gases are stripped from
j the liquid into the gas phase resulting in the need to
purge contaminated gas continuously from the system.
The contaminated gas is repeatedly reintroduced into
, 25 fresh liquid to allow for absorption of substantially
~1 ~
, ~
: ' ,: . ''
132~7~8
-- 2
all available gas by a liquid. The use of relatively
small contact chambers operating in parallel with
regards to the liquid being treated, and in series in
the gas phase, reduces the head required to achieve an
acceptable degree of inlet gas absorption. It is for
this reason that the present gas absorber was invented.
The invention is capable of exploitation in
the chemical industry and is particularly useful in an
application where gas is moved into a liquid such as
aquaculture, waste water treatment, and chemical
processing.
` The present invention promotes absorption of
substantially all inlet oxygen by water, for uses such
as fish farming and waste treatment. It may also be
used to enhance absorption of other gases by a liquid
for other uses. The present invention is a multiple
stage yas absorber having a plurality of containers,
partially submerged in water, wherein oxygen is forced
into the bottom of the first container, some of which is
absorbed by the water while waste gases such as
; nitrogen, argon and carbon dioxide are stripped from the
water, and ar~ extracted as a waste gas effluent. The
waste gas effluent is sequentially reintroduced to each
container of fresh water until substantially all
available oxygen is absorbed.
The invention, will be understood from the
, following description taken in connection with the
accompanied drawings in which like parts are given like
identification numerals and wherein:
Fig. 1 is a schematic view of a first
embodiment of the present invention with portions
thereof removed for clarity;
Fig. 2 is a schematic view the self cleaning
mechanism of the present invention; and
Fig. 3 is a schematic view of a second
embodiment of the present invention.
For purposes of this specification, the
~ ' .
~L3~7~8
- 3 -
following definitions will apply.
- B = Bunsen's coefficient at a given
temperature and salinity,
- C = existing concentration of a gas in
solution
C* = saturation concentration of a gas in
solution
dc/dt = change in concentration of a gas in
solution with respect to time
KLa = overall gas transfer coefficient
d = diameter of a gas molecule (A)
K = ratio of molecular weight to molecular
' volume for a gas
X = mole fraction of the gas in the gas phase
Pv = vapor pressure of water
Pt = total pressure
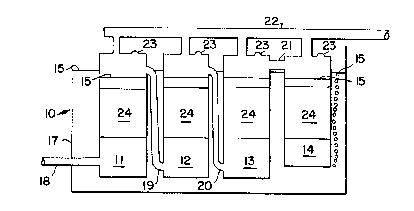
Referring now to Fig. 1, a first preferred
embodiment 10, uses a series of packed columns 11, 12,
13, 14. As used herein, packed beds are defined as
sealed columns packed with particles of high specific
surface area. The packing used is available in several
different sizes, shapes and materials. Liquid entering
the column is distributed uniEormly over the packing
` surface via a nozzle or perforated plate. The design of
packed columns for degassing applications is described
' in an article entitled Design of Packed Columns for
Degassing by John Colt and Gerald Bouck which appear at
Aquacultural Engineering 3 (1984) pages 251-273,
published by Elsevier Applied Science Publishers.
Absorber 10 comprises a large liquid container
, 17, a series of packed columns 11, 12, 13, 14, a gas
introduction pipe 18, a series of gas effluent
reintroduction pipes 19, 20, 21 sequentially connecting
each column 11, 12, 13, 14, and a liquid supply manifold
j 35 22 for continuously supplying fresh liquid to all
~, columns 11, 12, 13, 14. Container 17 is substantiallyfilled with water 15. Each column 11, 12, 13, 14 is
.
1 3~7~8
- 4 -
constructed in a non-permeable manner which forms an
~nclosed top, and sides, with a pressure relief valve 23
at the top of each column 11, 12, 13, 14. The columns
11, 12, 13, 14 are submerged in water 15 and a water
S seal is formed at the bottom of each column. Fresh
:~ water is continuously introduced (by pumping, by natural
water flow or by other means wherein the head pressure
is greater than the pressure drop across the system 10)
into all columns 11, 12, 13, 14 through manifold 22, and
lO substantially pure oxygen is continuously introduced
into the first column 11 through pipe 18. The packed
bed 24 within each column 11, 12, 13, 14 is designed to
cause the water to have minimum film thickness and to
promote maximum surface area for the absorption of
15 oxygen. In this example, approximately 30% of the
oxygen is absorbed by the water, in first column 11, and
waste gases such as argon, nitrogen, and carbon dioxide
are stripped from the water lS, replacing the oxygen and
resulting in a contaminated effluent gas which is forced
20 by incoming oxygen out of column 11, through
reintroduction pipe 19 into second column 12. In second
~! column 12, the effluent gas (@ 70% oxygen) is combined
with fresh water from manifold 22. Since the fresh
water has a higher capacity to accept oxygen exiting the
j 25 first column 11, about 20% of the total oxygen is
~ stripped from the effluent gas in second column 12.
`J Under pressure from the oxygen entering the first column
11, the 50% 2 effluent gas exits column 12 and enters
column 13, through reintroduction pipe 20, where about
30 10% of the total oxygen is absorbed in the manner as
`~ previously discussed, resulting in a 40% effluent gas
which similarly proceeds through reintroduction pipe 21
to the final column 14. Another 10% of the total oxygen
is absorbed by the fresh water in column 14. The
35 reintroduction pipe 21 reintroduces the gas to the top
of the final column 14 which is substantially shorter
than the previous columns 11, 12, 13, and the final
,j .
.
132~75~
effluent gas simply bubbles out of the bottom of column
14. The multi-stage packed column absorber 10 offers
the advantage of a high oxygen absorption efficiency
with minimal head (energy) requirement. The
configuration of this system is such that the gradient
between the saturation level of dissolved oxygen (D0~ in
:1
the water being treated and the ambient D0 concentration
is maintained so as to maximize oxygen transfer.
In a pure oxygen contact system, oxygen is
transferred from the gas phase to the liquid phase.
Concurrently, dissolved nitrogen, argon and carbon
dioxide will be stripped from the liquid phase into the
gas phase. The primary resistance to the movement of
these gases is usually provided by the stagnant liquid
film present at the interphase between the gas and the
liquid (Lewis and Whitman, 1924). The rate at which
transfer occurs is then proportional to the difference
between the existing and saturation concentration of the
gas in solution. In differential form, the relationship
1 20 is expressed as:
dc/dt = KLa ~C* - C); [Equation 1].
The overall transfer coefficient (KLa) will
1~ reflect the conditions present in a specific gas-liquid
-~ contact system. Conditions of importance include
turbulence, waste characteristics of the liquid, the
extent of the gas-liquid interphase and temperature.
Although each gas species in a contact system will have
a unique value of KLa, it has been established by E. C.
Tsivoglou, et al. in 1965 in an article entitled Tracer
~easurements Of Atmospheric Reareation - 1. Laboratory
~ Studies published at pages 1343-1362 of volume 37 of the
j Journal Of The Water Pollution Control Federation that
relative values for a specific gas pair are inversely
proportional to their molecular diameter, i.e.:
(KLa)1/(KLa)2 = (d)2/(d)1; [Equation 2].
The relationship above, based on Einstein's law of
~, diffusion, provides a convenient means of establishing
.:
~' ' ' . ,
~ 3~7~8
- 6 -
relative rates of gas transfer in a multicomponent
system ~eg. N2, 2 and CO2). Nitrogen, the major
dissolved gas component of national surface waters has a
` KLa representing 94% of that of oxygen. The saturation
concentration of gas in solution ~C*) will influence the
direction as well as the rate of gas transf~r [equation
2]. The C* of a gas is a function of its partial
pressure in the gas phase, liquid temperature, and
liquid composition as related by Henry's law. In
equation form:
C* = BKl(X(PT ~ Ph2O)/76O); [Equation 3]-
The C* of oxygen in the multi-stage absorber
; described here is increased by increasing the mole
fraction of 2 in the gas-phase [equation 3]. C* is
increased further by hydrostatic pressure [equation 3].
This pressurization is achieved by partially submerging
each column 11, 12, 13, 14 (packed-bed) below water
level in the vessel 17 receiving the absorber's
effluent, as shown in Fig. 2. Gas pressure within the
column 11, 12, 13, 14 is sufficient to keep water 15
from entering and flooding the packing 16. The increase
in C* achieved serves to (1) accelerate the rate of gas
transfer [equation 1], and (2) provide the capability of
achieving an e~fluent dissolved gas level in excess of
i 25 the air saturation concentration at local barometric
pressure.
The gradient between C* and C necessary for
gas transfer to occur ~equation 1] is maintained by
dividing the influent water flow into several portions,
each of which is directed through an individual packed-
bed 11, 12, 13, 14. All o~ the oxygen gas being metered
into the system is initially directed through a single
first column subsection 11. Some 2 is absorbed by
water passing through this segment 11. Concurrently,
and in agreement with equation 3, nitrogen and other
gases are stripped from solution into the gas phase.
This stripping action results in a reduction in the
~32~7~
- 7 -
oxygen purity level of the gas phase (X02 equation 4)
which, in turn, reduces C* and the rate of 02
~ absorption. An equilibrium X02 will eventually be
-~ established. The gas continuously exiting this column
11 is directed into a second packed column 12 allowing
more oxygen to be utilized as the gas is being exposed
to fresh watPr that has not as yet been oxygenated. In
this column 12 the mole fraction f 2 drops further due
again to gas stripping from solution. Since the gas
will still have a X02 greater than air (21%), the gas
from the second column 12 is directed through a third 13
and then a fourth 14. The number of column segments 11,
12, 13, 14 required to achieve an acceptable degree of
2 absorption will be related to packing height,
pressure, inlet gas flow, inlet dissolved gas
concentrations etc. Because the gas is reused, the
h~ight of the packed bed can be kept low, thus
minimizing the head (energy) required to operate the
system.
~j 20 In conventional packed bed 2 absorbers, as
I described by R. E. Speece in 1981 in a paper entitled
;~l Management Of Dissolved Oxygen And Nitrogen In Fish
Hatchery Waters found in the Proceedings Of The Bio-
engineering Symposium For Fish Culture at pages 53-62,
published by L. J. Allen and E. C. Kinney, Fish Culture
Section of the American Fisheries Society and the
Northeast Society of Conservation Engineers, Bethesda,
Maryland, the gas enters a single column at the bottom
~J, of the packing and exits at the top. Water in turn
enters the top of the column and exits the bottom.
Operating in the conventional manner requires a head
pressure or column height far in excess of that required
by the low profile multi-stage configuration 10 for the
same degree of 2 utilization.
A third improvement offered by the multi-stage
packed bed 10 is that provisions have been made so that
the packing can be cleaned within the column 11, 12, 13,
.
'~ ' . . '
, . .
: '. ,
.:
132~7~
-- 8
;,
14 to minimize labor requirements. Fixed beds 24, as
shown by Fig. 2, with plastic packing 25 will act as
mechanical filters and will, at periodic intervals,
require cleaning. Conventionally, this is achieved by
removing the packing 25 from the bed 24 by hand. With
the present system 10, removal of the packing 25 is not
required. Each of the individual columns has been
outfitted with a series of water nozzles 26 which direct
a high velocity water jet tangentially and at a slight
downward angle at the packing. During cleaning, the
beds 11, 12, 13, 14 are taken off-line. Gas within the
column is the bled out of valves 23 at the top of the
packed--bed unit 11, 12, 13, 14. This serves to flood
the beds 24 with water in which the columns 11, 12, 13,
14 have been partially submerged. The packing 25 is
nearly buoyant when flooded. Thus, when the water
nozzles 26 are activated, they cause the packing 25 to
`, spin around the central axis as well as to move
vertically up and down the column 11. The hydrostatic
sheariny forces imposed on the packing 25 by the action
of the water jets, combined with mixing and particle
contact, serve to dislodge entrapped particulate matter.
This matter is purged from the bed 24 during the
¦ cleaning operation and upon the removal of water from
the packing once the cleaning nozzles 26 have been
I turned off. Water is removed from the bed by
~! introducing gas under pressure into the packed column
~-l subsections 11, 12, 13, 14. At this time, the water
flow to the columns 11, 12, 13, 14 can be reestablished.
In situations where water quality is poor,
~` having a high amount particulate matter, the present
invention can be practiced without packing 25. The
packing 25 can be removed, and water is sprayed from
nozzles 26 into the unpacked column 24 creating a high
surface area water droplet spray and creating turbulence
in the existing water, thereby promoting absorption of
the available oxygen.
;'
, , .
..
~3~7~8
_ 9 _
As Fig. 3 of the drawings illustrates, the
second preferred embodiment of the present multiple
stage gas absorber 30, comprises a large liquid
container 31, a series of enclosure hoods 32, 33, 34,
35, and agitation means 36 within each hood 32, 33, 34,
35, a gas introduction pipe 37, a series of gas effluent
reintroduction pipes 38 sequentially connecting each
hood 32, 33, 34, 35, and a liquid supply manifold 39 for
continuously supplying fresh liquid to all hoods 32, 33,
34, 35. Fresh liquid may also be supplied without the
use of manifold 39 in a passive water introduction
:l environment such as a river or stream. The manifold 39
: and the container 31 may also be eliminated in
situations where this embodiment 30 is used in a pond or
lake (the container 31 becomes a pond or lake), and the
agitation means 36 such as a paddle wheel or an impeller
induces flow of water into the hoods 32, 33, 34, 35.
While other gases and liquids may be used, the primary
use is for introducing oxygen into water. Container 31
20 is substantially filled with water ~0. Hoods 32, 33,
34, and 35 are each constructed in a non-permeable
manner which forms an enclosed top and sides, leaving
:~ only the bottom unenclosed, Since each hood 32, 33, 34,
35 is partially submerged in water 40, a water seal is
25 formed at the bottom of each hood 32, 33, 34, 35. Fresh
.j water is continuously introduced into all hoods 32, 33,
- 34, 35 through manifold 39, and substantially pure
' oxygen is continuously introduced into the first hood 32
~' through pipe 37. Rotary agitation means 36 such as a
:` 30 turbine surface areator or a paddle wheel surface
areator are incorporated in each hood 32, 33, 34, 35 to
cause the agitated water to have minimum liquid film
thickness to promote maximum surface area for the
absorption of oxygen. Approximately 30% of the oxygen
is absorbed by the water in the first hood 32, and waste
gases such as argon, nitrogen, and carbon dioxide are
stripped from the water, replacing the oxygen, and
13~7~
-- 10 --
resulting in a contaminated effluent gas which is forced
by the incoming oxygen out of hood 32, through
reintroduction pipe 38 into second hood 33. In second
hood 33, the effluent gas (@ 70% oxygen) is agitated
with fresh water, which has a higher capacity to accept
oxygen from the contaminated gas than the water of the
previous hood 32, and about 20% o~ the total oxygen is
stripped from the effluent gas in the second hood 33.
Under pressure from the oxygen entering the first hood
10 32, the 50% effluPnt gas exits hood 33 and enters hood
34 where about 10% of the total oxygen is absorbed in
~, the manner as praviously discussed, resulting in a 40%
effluent gas which similarly proceeds to the last hood
35. As Fig. 3 illustrates, the previous hoods 32, 33,
- 15 34 extend deeper into the water 40 than does last hood
35, and last hood 35 has no exit pipe. The last hood 35
is shorter to allow gas to exit at the bottom of hood 35
before escaping the system at any other point, thereby
eliminating the need for compressors or other means to
20 force the gas from one hood to the next. There is also
no mechanical pressurizing means. As another 10% of the
total oxygen is absorbed by the fresh water of the last
~-l hood 35, substantially all available oxygen has been
l` extracted, and the final effluent simply bubbles out the
f 25 bottom o~ hood 35.
, .
'
:
,: . .