Note: Descriptions are shown in the official language in which they were submitted.
t 3 3 ~
¦ HE~BICIDAL AMIDES OF 4-TRIFLUOROMETHYL-3'-
J CARBOXY-4'-NITRO DIPHENYL ETHERS ~.
The Disclosure
This invention relates to novel compounds which show ~ -
5 activity as herbicides, to novel herbicidal compositions
which contain these compounds, and to new methods of
controlling weeds with these herbicidal compositions. ~ .
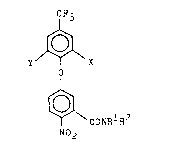
In accordance with the present invention, there is
provided a new class of novel diphenyl ethers having the
10 formula : ;
. CF3 :~.
.
,~
~COIRlR~
~33a~
-- 2 --
wherein X is a hydrogen atom, a halogen atom, preferably
a fluorine atom or a chlorine atom, a
trihalomethyl group, preferably a
trifluoromethyl group, an alkyl group,
pref~rably having l to 4 carbon atoms, most
preferably a methyl group, or a cyano group,
i Y is a hydrogen atom or a halogen atom,
i preferably a fluorine atom or a chlorine atom,
l is a hydrogen atom, a hydroxy group, a
(Cl-C43alkoxy group, an alkyl group,
preferably having 1 to 4 carbon atoms,
optionally substituted with one or more
~ halogen atoms, hydroxy groups, tCl-C4)
j alkoxy groups, cyano groups, thiol groups,
~1 15 (Cl-C4) alkylthio groups, carboxy groups,
or agronomically-acceptable salts thereof, or
carb(Cl-C4)alkoxy groups, or a cycloalkyl
:~ group, preferably having one carbocyclic ring
and 5 to 7 carbon atoms, and
R2 i5 a hydrogen atom, an alkyl group,
. preferably havlng l to 4 carbon atoms,
optionally substituted with one or more
halogen atoms, hydroxy groups, (Cl-C4)
alkoxy groups, cyano groups, thiol groups,
(Cl-C4) alkylthio groups, carboxy groups,
:~ or agronomically-acceptable salts thereof, or :
carb(Cl-C4)alkoxy groups, or a cycloalkyl
. group, prefera~ly havina one carbocyclic ring
~:~ and 5 to 7 carbon atoms, or
; 30 R1 and R2 can be taken together with the
attached nitrogen atom to form a saturated
heterocyclic ring having 4 to 6 carbon atoms
and up to one additional hetero nitrogen,
oxygen, or sulfur atom.
When the X, Rl, or R2 substituent is an alkyl group,
it can have either a straight- or branched-chain
;~
~ ~ ~ ' `~ ` ~ '` ` ` ` " ~ ' ' ' ` ! `" ~ ` i ! . ; ~ ~; ` . ` : . ~ ~
.~!'., ~.~ .. ' ' ' . ,'. ~ ` ' , ' .
~ ~ 3,~",;,"`-.,""` '~
~33~A~L~
configuration. When Rl or R2 is a substituted alkyl
group, it will preferably have a single substituent.
These novel compounds have been found to show
unexpected activity as weed control agents. In a
preferred embodiment of the invention, X i5 a halogen
atom, most preferably a chlorine atom, Y is a hydrogen
atom and Rl and R2 are individually a hydrogen atom
or a substituted or unsubstituted (Cl-C4)alkyl group,
more preferably a hydrogen atom or an unsubstituted ~ -
(Cl-C4) alkyl group. Most preferab~y either Rl or
R2 is a hydrogen atom, and the other an unsubstituted
(Cl-C4) alkyl group.
Examples of the compounds of the invention embraced
by Formula I include:
2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy)-N-
methylbenzamide
2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy)-N,N-
diethylbenzamide
2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy)-N-
t-butylbenzamide
2-Nitro-5-(2-bromo-4-trifluoromethylphenoxy)-N-
(2-hydroxyethyl)benzamide
` 2-Nitro-5-(2-fluoro-4-trifluoromethylphenoxy)-N,N-
4-oxapentamethylenebenzamide
2-Nitro-5-~2,6-dichloro-4-trifluoromethylphenoxy)-N-
methyl-N-(2-hydroxypropyl)benzamide
2-Nitro-5-(2 chloro-4-trifluoromethylphenoxy)-N-
~; cyclohexylbenzamide ~ ~
~ 2-Nitro-5-(2-cyano-4-trifluoromethylphenoxy)-N-
! i ~ ;~ 30 carboxymethylbenzamide
~-Ni~ro-5-(2,4-bistrifluoromethylphenoxy~-N-ethyl-
N-(methylthioethyl)benzamide
2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy)-N-
(2-ethoxyethyl)benzamide
; 35 2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy)-N-
ethylbenzamide
r ~; ~
~33~5
-- 4 --
2-Nitro-5-(2-fluoro-4-trifluoromethylphenoxy)-N-
methyl-N-(l-carbethoxy)ethylbenzamide
2-Nitro-5-(2-chloro-6-fluoro-4-trifluoromethylphenoxy)
-N-methylbenzamide
5 2-Nitro-5-(4-trifluoromethylphenoxy)-N,N-
, pentamethylenebenzamide
7 2-Nitro-5-(2-chloro-4-trifluoromethylphenoxy) -N-
¦ carboxymethyl-N-(2-hydroxyethyl)benzamide
2-Nitro-5-(2-cyano-4-trifluoromethylphenoxy)benzamide
! lo 2-Nitro-5-(4-trifluoromethylphenoxy)-N-
(2-cyanoethyl)benzamide
~, 2-Nitro-5-(2-chloro-4~trifluoromethylphenoxy)-N, Nl-bis-
A,j (2-hydroxyethyl)benzamide
2-Nitro-5-(2-fluoro-4-trifluoromethylphenoxy)-N-hydroxy-
benzamide2-Nitro-5-(2,6-dichloro-4-trifluoromethylphenoxy)-N-
methoxybenzamide
The novel diphenyl ethers of the invention are useful
both as preemergence and postemergence herbicides.
Preemergence herbicides are ordinarily used to treat the
soil in which the desired crop is to be planted by
application either before seeding, during seeding, or, as
in most applications, after seeding and before the crop
~- emerges. Postemergence herbicides are those which are
applied after the plants have emerged and during their
growth period. Compounds of the invention are
~ particularly active against broadleaf weeds.
: Among the crops on which the diphenyl ethers of the
invention can be advantageously employed are, for
example, soybeans, peanuts, cotton, corn, wheat, barley,
;~ rice and other cereal crops, and the like.
;~ When used in transplanted rice crops, the ethers can
be applied either preemergence or postemergence to the
; weeds--that is, they can be applied to the growth medium
of the transplanted plants either before the weed plants
~ have emerged or while they are in their early stages of
`, ';~
` ''
~, i,~". ~ ' ; .
133~
growth. The ethers can be applied to the growth medium
either before or after the rice has been transplanted to
that medium.
The diphenyl ethers of the invention can be applied
in any amount which will give the desired degree of weed
control. A preferred rate of application of the
herbicides of the invention is from about 0.1 to 12, and
most preferably about 0.25 to 4, pounds of the diphenyl
ether per acreO
~ 10 Under some conditions, the diphenyl ethers of the
¦ invention may be advantageously incorporated into the
soil or other growth medium prior to planting a crop.
This incorporation can be carried out by any convenient
means, including by simple mixing with the soil, by
applying the diphenyl ether to the surface of the soil
and then disking or dragging into the soil to the desired
depth, or by employing a liquid carrier to accomplish the
necessary penetration and impregnation.
A diphenyl ether of the invention can be applied to
the growth medium or to plants to be treated either by
itself or, as is generally done, as a component in a
herbicidal composition or formulation which also
comprises an agronomically acceptable carrier. By
agronomically-acceptable carrier is meant any substance
2S which can be used to dissolve, disperse, or diffuse a
herbicidal com?ound in the composition without impairing
the effectiveness of the herbicidal compound and which by
~ itsel has no detrimental effect on the soil, equipment,
r~: crops, or agronomic environment. Mixtures of the
diphenyl ethers of the invention may be used in any of
these herbicidal formulations. The herbicidal
compositions of the invention can be either solid or
- liquid formulations or solutions. For example, the
diphenyl ethers can be formulated as wettable powders,
emulsifiable concentrates, dusts, granular formulations,
~ `~ - aerosols, or flowable emulsion concentrates. In such
:~ , :~
~,
, 6 7, ~ , , ", . ~
1 3 3 ~
formulations, the compounds are extended with a liquid or
solid carrier and, when desired, suitable surfactants are
incorporated.
It is usually desirable, particularly in
postemergence applications, to include adjuvants, such as
wetting agents, spreading agents, dispersing agents,
stickers, adhesives, and the like, in accordance with
agricultural practices. Examples of adjuvants which are
commonly used in the art can be found in the John W.
McCutcheon, Inc. publication "Detergents and Emulsifiers
Annual. n
The diphenyl ether compounds of this invention can
be formulated in any appropriate solvent. Examples of
solvents which are useful in the practice of this
invention include water, alcohols, ketones, aromatic
hydrocarbons, halogenated hydrocarbons,
dimethylformamide, dioxane, dimethyl sulfoxide, and the
like. Mixtures of these solvents can also be used. The
concentration of the solution can vary from about 2% to
about 98% with a preferred range being about 25% to about
75%.
For the preparation of emuls~fiable concentrates,
the diphenyl ether can be dissolved in organic solvents,
such as benzene, toluene, xylene, methylated naphthalene,
;~ 25 corn oil, pine oil, o-dichlorobenzene, isophorone,
cyclohexanone, methyl oleate, and the like, or in
mixtures of these solvents, together with an emulsifying
agent which permits dispersion in water. Suitable
~ emulsifiers include, for example, the ethylene oxide
`~ 30 derivatives of alkylphenols or long-chain alcohols,
mercaptans, carboxylic acids, and reactive amines and
partially esterified polyhydric alcohols.
Solvent-soluble sulfates or sulfonates, such as the
alkaline earth salts or amine salts of
~; 35 alkylbenzenesulfonates and the fatty alcohol sodium
sulfates, having surface-active properties can be used as
:~
" ~
133~
emulsifiers either alone or in conjunction with an
ethylene oxide reaction product. Flowable emulsion
concentrates are formulated similarly to the emulsifiable
concentrates and include, in addition to the above
components, water and a stabilizing agent such as a
water-soluble cellulose derivative or a water-soluble
salt of a polyacrylic acid. The concentration of the
active ingredient in emulsifiable concentrates is
~ usually about 10% to 60% and in flowable emulsion
j 10 concentrates, this can be as high as about 75%.
Wettable powders suitable for spraying, can be
prepared by admixing the compound with a finely divided
solid, such as clays, inorganic silicates and carbonates,
and silicas and incorporating wetting agents, sticking
agents, and/or dispersing agents in such mixtures. The
concentratation of active ingredients in such
formulations is usually in the range of about 20% to 98%,
preferably about 40% to 75%. A dispersing agent can
constitute about 0.5% to about 3% of the composition, and
a wetting agent can constitute from about 0.1% to about
5% of the composition.
Dusts can be prepared by mixing the compounds of the
invention with finely divided inert solids which may be
organic or inorganic in nature. Materials useful for
this purpose include, for example, botanical flours,
silicas, carbonates and clays. One convenient method of
preparing a dust is to dilute a wettable powder with a
finely divided carrier. Dust concentrates containing
about 20% to 80% of the active ingredient are commonly
made and are subsequently diluted to about ]% to 10% use
concentration.
Granular formulations can be prepared by
impregnating a solid such as a granular fuller's earth,
vermiculite, ground corn cobs, seed hulls, including bran
; 35 or other grainhulls, or similar material. A solution of
<~`
~ 3 ~
one or more of the diphenyl ethers in a volatile organic
solvent is then removed by evaporation. The granular
- material can have any suitable size, with a preferable
size range of 16 to 60 mesh. The diphenyl ether will
usually comprise about 2 to 15% of the granular
formulation.
The diphenyl ethers of the invention can also be
mixed with fertilizers or fertilizing materials before
their application. In one type of solid fertilizing
composition in which the diphenyl ethers can be used,
particles of a fertilizer or fertilizing ingredients,
such as ammonium sulfate, ammonium nitrate, or ammonium
phosphate, can be coated with one or more of the ethers.
The solid diphenyl ethers and solid fertilizing material
can also be admixed in mixing or blending equipment, or
they can be incorporated with fertilizers in granular
formulations. Any relative proportion of diphenyl ether
and fertilizer can be used which is suitable for the
crops and weeds to be treated. The diphenyl ether will
commonly be from about 5% to about 25% of the fertilizing
composition. These compositions provide fertilizing
materials which promote the rapid growth of desired
plants, and at the same time control the growth of
` undesired plants. -
~ 25 The diphenyl ethers of the invention can be applied
;~ as herbicidal sprays by methods commonly employed, such
as conventional high-gallonage hydraulic sprays, low
gallonage sprays, airblast spray, aerial sprays and
dusts. For low volume applications a solution of the
~; 30 compound is usually used. The dilution and rate of
application will usually depend upon such factors as the
type of equipment employed, the methods of application,
the area to be treated and the type and stage of
development of the weeds.
~; 35 For some applications, i~ may be desirable to add
;:~
,: .,
;' ~- ~ .
~" ~33~
g
, one or more other herbicides along with diphenyl ethers of
i the invention. Examples of other herbicides which can be
incorporated to provide additional advantages and
effectivenss include:
Carboxylic Acids and Derivatives
2,3,6-trichlorobenzoic acid and its salts
2,3,5,6-tetrachlorobenzoic acid and its salts
2-methoxy-3,5,6-trichlorobenzoic acid and its salts
2-methoxy-3,6-dichlorobenzoic acid and its salts
~ 10 2,3-dichloro-6-methylbenzoic acid and its salts
2,4-dichlorophenoxyacetic acid and its salts and
esters
2,4,5-trichlorophenoxyacetic acid and its salts and
: esters
2-methyl-4-chlorophenoxyacetic acid and its salts and
: esters ;~
2-(2,4,5-trichlorophenoxy)propionic acid and its
: salts and esters
4-(2,4-dichlorophenoxy)butyric acid and its salts and
esters
4-(2-methyl-4-chlorophenoxy)butyric acid and its
salts and esters
~ 2,3,6-trichlorophenylacetic acid and its salts
; 3,6-endoxohexahydrophthalic acid
25 dimethyl 2,~5,6-tetrachloroterephthalate
trichloroacetic acid and its salts
2,2-dichloropropionic acid and its salts
k 2,3-dichloroisobutyric acid and its salts
Carbamic Acid Derivatives
30 ethyl N,N-di(n-propyl)thiolcarbamate
propyl N,N-di(n-propyl)thiolcarbamate
~ :~ ethyl N-ethyl-N-(n-butyl)thiolcarbamate
.~ propyl N-ethyl-N-(n-butyl~thiolcarbamate
~t, '`. 2-chloroallyl N,N-diethyldithiocarbamate
;; ~ 35 N-methyldithiocarbamic acid salts
!~`
,~, :~
;,
~, :: ~
,37~`'
~33~
-- 10 --
ethyl l-hexamethyleneiminecarbothiolate
isopropyl N-phenylcarbamate
isopropyl N-(m-chlorophenyl)carbamate
4-chloro-2-butynyl N-(m-chlorophenyl)carbamate
methyl N-(3,4-dichlorophenyl)carbamate
Phenols
dinitro-o-(sec-butyl)phenol and its salts
pentachlorophenol and its salts
Substituted Ureas
~ 10 3-(3,4-dichlorophenyl)~ dimethylurea
i 3-phenyl-l,l~dimethylurea
. 3-(3,4-dichlorophenyl)-3-methoxy-1,1-dimethylurea
3-(4-chlorophenyl)-3-methoxy-1,1-dimethylurea
3-(3,4-dichlorophenyl)-1-n-butyl-1-methylurea
3-(3,4-dichlorophenyl)-1-methoxy-1-methylurea
: 3-(4-chlorophenyl)-1-methoxy-1-methylurea
; 3-(3,4-dichlorophenyl)-1,1,3-trimethylurea
3-(3,4-dichlorophenyl)-1,1-diethylurea
~ dichloral urea
¦ 20 Substituted Triazines
t~j 2-chloro-4,6-bis(ethylamino)-s-triazine
2-chloro-4-ethylamino-6-isopropylamino)-s-triazine
2-chloro-4,6-bis(methoxypropylamino)-s-triazine
2-methoxy-4,6-bis(isopropylamino)-s-triazine
2-chloro-4-ethylamino-6-(3-methoxypropylamino)-s-
.~ : triazine
2-methylmercapto-4,6-bis(isopropylamino)-s-triazine
2-methylmercapto-4,6-bis(ethylamino)-s-triazine
: 2-methylmercapto-4-ethylamino-6-isopropylamino-s-
: 30 triazine
.~
2-chloro-4,6-bis(isopropylamino)-s-triazine
2-methoxy-4,6-bis(ethylamino)-s-triazine
. ~ 2-methoxy-4-ethylamino-6-isopropylamino-s-triazine
2-methylmercapto-4-(2-methoxyethylamino)-6-
isopropyoamino-s-triazine
~ `: :
'~
~ 3 3 ~
Diphenyl Ether Derivatives
2,4-dichloro-4'-nitrodiphenyl ether
2,4,6-trichloro-4'-nitrodiphenyl ether
2,4-dichloro-6-fluoro-4'-nitrodiphenyl ether :
3-methyl-4'-nitrodiphenyl ether
3,5-dimethyl-4~-nitrodiphenyl ether
2,4'-dinitro-4-trifluoromethyldiphenyl ether
2,4-dichloro-3'-methoxy-4'-nitrodiphenyl ether
2-chloro-4-trifluoromethyl-3'-ethoxy-4'-nitrodiphenyl
ether
2-chloro-4-trifluoromethyl-4'-nitrodiphenyl ether
1 2-chloro-4-trifluoromethyl-3'-carbethoxy-4'-
I nitrodiphenyl ether
2,4-dichloro-3'-carbomethoxy-4'-nitrodiphenyl ether
Anilides
N-(3,4-dichlorophenyl)propionamide
N-(3,4-dichlorophenyl)methacrylamide ~:
N-(3-chloro-4-methylphenyl)-2-methylpentanamide
N-(3,4-dichlorophenyl)trimethylacetamide
N-(3,4-dichlorophenyl)-a,a-dimethylvaleramide
N-isopropyl-N-phenylchloroacetamide
N-n-butoxymethyl-N-(2,6-diethylphenyl~chloroacetamide
N-n-methoxymethyl-N-(2,6-diethylphenyl)chloroacetamide
.
Uracils
25 5-bromo-3-s-butyl-6-methyluracil :
S-bromo-3-cyclohexyl-1,6-dimethyluracil
3-cyclohexyl-5,6-trimethyleneuracil
5-bromo-3-isopropyl~6-methyluracil
3-tert-butyl-5-chloro-6-methyluracil
~. :; _
Nitriles
2,6-dichlorobenzonitrile
diphenylacetonitrile
~ -
3,5-dibromo-4-hydroxybenzonitrile
3,5-diiodo-4-hydroxybenzonitrile
., '~ .
~ ~: :
~ , :
:::
a6 ,Ij~
~1 3 3 ~
- 12 -
I Other Organic Herbicides
j 2-chloro-N,N-diallylacetamide
N-(l,l-dimethyl-2-propynyl)-3,5-dichlorobenzamide
maleic hydrazide
3-amino-1,2,4-triazole
monosodium methanearsonate
disodium methanearsonate
N,N-dimethyl-~,-diphenylacetamide
N,N-di(n-propyl)-2,6-dinitro-4-trifluoromethylaniline
N,N-di(n-propyl)-2,6-dinitro-4-methylaniline
N,N-di(n-propyl)-2,6-dinitro-4-methylsulfonylaniline
0-(2,4-dichlorophenyl)-O-methyl-isopropylphosphor-
amidothioate
4-amino-3,5,6-trichlorop.icolinic acid
2,3-dichloro-1,4-naphthoquinone
e di(methoxythiocarbonyl)disulfide
3-isopropyl-lH-2,1,3-benzothiazidin~-(4)3H-one-2,2-
dioxide
6,7-dihydrodipyridoil,2-a:2',1'-c~pyrazidinium salts
20 1,1'-dimethyl-4,4'-bipyridinium salts
3,4,5,6-tetrahydro-3,5-dimethyl-2-thio-2~-1,3,5-
thiadiazine.
4-Am~no-6-~1,1-dimethylethyl)~3-methylthio-1,2,4-
triazin-5-~4H)-one
25 3-Amino-2,5-dichlorobenzoic acid
N3, N3-Diethyl-2,4-dinitro-6-trifluoromethyl-1,3-
phenylene-diamine
N-(2-chloroethyl?-2,6-dinitro-N-propyl-4-trifluoro- :
methylaniline
N-Cyclopropylmethyl- ~ ~, ~trifluoro-2,6-dinitro-N-
propyl-~-toluidine
N-(l-Ethylpropyl)-3,4-dimethyl-2,6-dinitrobenzeneamine
: S-(O,O-diisopropyl)phosphorodithioate ester of N-~2-
, ~ mercaptoethyl)benzenesulfonamide
2-~-Naphthoxy)-N,N-diethylpropionamide
,
` ~ ,~
~r~
133~
2-Chloro-N-(2-ethyl-6-methylphenyl)-N-t2-methoxy-1-
methylethyl)acetamide
Methylsulfanilylcarbamate
3-(~ -Chlorophenoxy)phenyl)-l,l-dimethylurea
Phosphonomethylglycine.
Methyl 2-(4-(2,4-dichlorophenoxy)phenoxy)propionate
Sodium 2- 4-[13,5-dichloro)-2-pyridyloxylphenoxy
propionate
Methyl 2-(4-(4-trifluoromethylphenoxy)phenoxy)-
propionate
When mixtures of herbicides are employed, the relative
proportions which are used will depend upon the crop to
; be treated and the degree of selectivity in weed control
which is desired.
15The ethers of the invention can be prepared by
reacting an acid halide, preferably an acid chloride of a
diphenyl ether of the formula .,.
~ y~X
rII)
I COOH
. N02
wherein X and Y are as defined above, with an excess of
~ 20 an amine of the formula
,~ HNRlR2 (III) .
/ .',,:
~:
`~: _
. ~
.~ ~ I " ~
. ~ ~
13 3 0 4 ~ 3
- 14 -
wherein Rl and R2 are as defined above. This
reaction is generally carried out in a solvent in which
the acid chloride is soluble, such as diethyl ether, a
chlorinated hydrocarbon, an aromatic hydrocarbon, or the
5 like, at a temperature of about 0 to about 100C,
preferably at about 0 to about 40C. The amination
reaction can also be carried out under Schotten-Bauman
conditions, where one equivalent of an amine of Formula
III is first added to a mixture of an organic solvent,
10 such as diethyl ether or the like, and an aqueous
solution of a strong inorganic base, such as sodium
hydroxide, or the like, followed by the slow addition of
an organic solution of the acid chloride, for example in
toluene, to the agitated two-phase system. This reaction
15 is generally carried out at a temperature of about 0 to
about 100C, preferably about 10 to about 40C.
, Compounds of the invention can also be prepared by
3 reacting an ester of a diphenyl ether of Formula II with
an amine of Formula III, using an acid catalyst, such as
20 ~-toluenesulfonic acid, hydrochloric acid, sulfuric acid
or the like in a suitable inert solvent, such as
tetrahydrofuran, glyme, toluene, xylene, or the like, at
a temperaturs of about 20 to the reflux temperature of
the solvent. In another route to the compounds of the
25 invention, an ammonium salt of a diphenyl ether of
Formula II, in which the cation has the formula
1 2
-NH2R R (IIIa)
~;~ wherein Rl and R2 are as defined above, is heated at
a temperature of about 100 to about 200 in a
~; 30 high-boiling inert solvent, such as toluene, xylene,
diglyme, or the like, to produce the amide directly.
;~ The diphenyl ether precursors can be prepared by -
reacting a suitably substituted phenol, or the potassium
:~
!. ~;'.'.``.. , ,'.j ., ,. i ' , ;' I . .
~ ~ '' "'' '' ~ ; ' ~ ` '~ ' ~ ~ ~'~ ;'''~' ''
1330.~A~
or sodium salt of the phenol, with a suitably substituted
halobenzene, such as a chloro- or fluorobenzene in the
presence of an alkaline agent. Such precursors and their
preparation are described in U.S. Patent 3,928,416, of Bayer et
al., granted December 23, 1975, and U.s.Patent 4,031,131, of
Johnson, granted June 21, 1977,
Another route to the compounds of the invention
involves the nitration of a diphenyl ether of the formula
CF3
Y~\X
o ~IV)
,~ ~''
;~
CONR R
wherein X, Y, Rl and R2 are as defined above, using
typical niteating agents such as potassium nitrate in
~ sulfuric acid, acetyl nitrate, mixed sulfuric acid/nitric
;~ aci~d, nitrosonium tetrafluoroborate, and the like. The
nitration reaction is generally caeried out at about -20
to about; 100C, ~rererably about 0 to about 5C, ~
optionally in the presence of an inert organic solvent,
such as methylene chloride or other chlorinated
hydrocarbon.
Amides of the invention can also be prepared by
condensing a phenol of the formula ~-
,
~,
, ~ ?
~ ~.s~i'
~ ~ ~.~,~,.,. ~ , ;'; ` '.,' ' ~` , - . ...
-
1330~
- 16 -
CH3
~/
Y I X
0~ .
wherein X and Y are as defined above with a substituted
halobenzene of the formula
xl
~ (Vl) ;
1 2
CONR R
NO 2
wherein X is a halogen atom, preferably a fluorine
atom, and Rl and R2 are as defined above. This
reaction is generally carried out at a temperature of
about 0 to about 250C, preferably about 75 to about
200C, optionally in the presence of an appropriate
;~ 10 solvent, such as sulfolane, dimethylsulfoxide,
dimethylformamide, hexamethylphosphoroustriamlde, or other
inert polar organic solvent.
The following examples will further illustrate this
invention but are not intended to limit it in any way.
In Table I, typical diphenyl ethers of the invention are
listed with their elemental analyses. A specific
illustrative preparation of the co~pound of Example 2 is
set forth before Table I. All temperatures are in
degrees Celsius and parts and percentages are by weight
unless otherwise indicated.
.~
,.
133~
Example 2
Experimental:
Preparation of 2-nitro-5-(2-chloro-4-
trifluoromethylphenoxy-N-methylbenzamide
A solution of 2-nitro-5-(2-chloro-4-trifluoro-
methylphenoxy)benzoyl chloride (10 g., 0.026 mole) and an
excess of methylamine was stirred at room temperature in
toluene for five hours. Amine hydrochloride was filtered
off and the filtrate stripped to yield 2-(nitro-S-
(2-chloro-4-trifluoromethylphenoxy)benzoyl chloride (8
g.) m.p. 118-120C.
TABLE I . :
Diphenyl Ethers - Phy cal Data
~Cl
f ~coNRlR2
NO2
~'
,
l :i~
;:
~ '
1 3 ~
-- 18 --
u~ O ~
~ ~ D O
tP
,1
Z c~ r o ~ c~ ~o 1~
dP
w r~
C~ I CD 00 G '1
t~ ~9 ~ o~r ~ _I ~ ~ c~ _I 1`
: ~
C ~n C ~nc ~ c ~n c ~q
~ o ~ o ~ o ~ o ~ o ~ o a~ ,
:: ~ o ~
:~ ~r ~ ~ o _I
~, _
c~ o u~
o
: ~ ~
~ :: . e E E El O13 E ::
. i,
.' ,'i
.~ V :
"
X
,~
.- a
. ~ ~ ~ E z ,~
'.~ X
-:~
: ~ ~
,~` ," o
.~
~ ~ .
`~
:~
~3~Q~
-- 19 --
The following examples show the herbicidal
properties of diphenyl ethers of the invention.
Example 6
This example shows the herbicidal activity of
diphenyl ethers of the invention towards a number of
common weeds. using the procedure described below,
diphenyl ethers are evaluated for control of various
plant species.
The following test procedure is employed. Seeds of
selected crops and weeds are planted in soil in trays.
~or preemergence tests, the trays are treated with the
test compound immediately after the planting. For
postemergence tests, the seeds are allowed to germinate,
and after two weeks the trays are treated with the test
compound immediately after the planting. The compound to
be evaluated is dissolved in acetone, diluted with water,
and sprayed over the trays using a carrier volume
equivalent to 50 gallons per acre at the rate of
application of two or four pounds per acre. About two
weeks after the application of the test compound, the
statè of growth of the plants is observed and the
phytotoxic effect of the compound is evaluated. The
results of typical tests are summarized in Table II,
which gives the aver~ge percent control achieved by the
test ~ompounds with 0 representing no weed control and
100 complete kill of the plant.
The following species were tested:
Monocots;
barnyardgrass Echinochloa crusgalli
30 downybrome Bromus tectorum
green ,oxtail Setaria vividis
johnsongrass Sorghum halepense
yellow nutsedge Cyperus esculentus
wild oats Avena fatua
^ ~
,~
~ 3 3 ~
- 20 -
Dicots
.
cocklebur Xanthium pensylvanicum
marigold Tagetes patula
tomato ~ycopersicum esculentum
5 morningglory Ipomoea sp
nightshade Solanum nigrum
velvetleaf Abutilon theophrasti
safflower ~ t IAC~Or;U~
'~ ~
''' : ':
,~
.,
:
, .
::
,
,'~
,.
.
,~
,
, ~
@``~
~ "i,;,"", ~ " ,, . - -.;, -` - ,,, ,,, , - " ~,
f ~
~330~
-- 21 --
~ahlo~
I o o a~ ~ o ~ o o o o
~al~,aAIa~ ~ O ~ a~ ~o ~ ~ o~
J ap~s~ O O t I t
o
.Iol~uFu.Io~ ~~ c4 ~o r ~ a ~ D
I o o u~ o o O O O
O~,~U10,~ 1 1 0 ~0 a~ ~r o o ~ ~
o o a~ o o o ~ u~
Pl~o~ ~o ~ I~ o o a~ a~
v oo oo oo oo oo
_l .mqal~o~ ~ co r~
O I o o u~ u~ O O a~ ~ o o
v s~o Pl~
c~o oo o o o ~ oa~
a9pas~nu Molla~ a. ~.~ o ~ o~ ut a~
3 v( S~u~lpaas )~ O O a~ o o o ~ o~
~ gSS~ UOSU~O~ 0~ ~ ~ O 1` ~ ~ ~ er D
cO, ~ .
~xo,~ uaal~ o r o o O ~ O oO a` o
O
C~ aulo~lq~6uMo~,o~ GO ~ ~ ~ ~ ~r
SSB.I~p~IB~UI~ cr~ a~ cra~ o ~ 0
. ~ n I
1~ . V V V ~ V
~ Z p~ O p~ ~ O O ~
.~ ~ E
3 ~
, ~ .
O u~
, ;~ .
~: ~ .$1~,
,~,,,, ,~,.
:~
13~0~
-- 22 --
V 13MO~ 2S ¦ I I I
a~Bal~,a~ o o o
~pB~S~ I I I I (~
I 00 00 00
~.IO~ U~U-IO~
00 00
o~UI,I, I_O, ,o~
a plo~F.Iz~l O O ~ o~ I I
00 00 00
_ o .mqal~l~o~ cr~ ~ er :
C O S~BO Pll~ ~ o o
Hl~ o a~pas~nu Molla~¦ ~n ~o
li3 aJ o ( S5~UFIp3a S ) I In ~ ~ ~
~: ~ o ~SSBl~uOsu~Orl ~ ~
~: ~IF~xo~ uaa~ O ~ 0~ O O
~ aUlO<Iq.~UMOa¦ 1~~ O ~0 0 o ~
: u~a~ oo co v ,
~ S9B.I~p-lBl~U-IBa a~ ~ v
r ~ :
,o
v v
æ ~ ~ ~ ~ ~ 4 v
~"; o s~
.~ 6 19 .,~
"~ C) Id ~ u7
~ O
:'~
,. ,
13304~
Example 7
This example shows the selectivity of the diphenyl
ethers of the invention towards several common crops,
including corn, cotton, rice, soybeans and wheat. Using
a procedure similar to Example 6, the following crops
were planted and treated--corn, cotton, rape, rice,
sugarbeet, soybeans and wheat. The results of typical
tests are summarized in Table III, which gives the
average percent injury caused by the test compound with O
representing no in~ury and 100 complete kill of the plant.
~ : ~
: ~:
.'
;v~
' '~ ' ' ' ' ' ' ' " . '
,'~ '
i(i~
, ~
1 3 ~ t~
-- 24 --
In O In u~ o ut Ul In ~ o u~ u~
LH~
o o o o o o o o ~ o U o
~os ~
I ooo oou~ ~oo oo~n
~S I ~ ~ ~ ~ U~
C)
o o o n ~ o o u~ In o
I~I ~ ,~
E ooo ooo ooo ooo
c ~~ ~ ~ co co
~q
O I 000 OoO u~ ooo
~~1 ~D~O ~er ~ ~ ~.
U'~ O O U~ O O U~ ~ O
N~
U~OO ooo ooo ~U~O
LHM ¦ ~
ooo ooo oo o ooo
H ~i O S t~
H ~ :
. ~ OoO oou~ lnoo o~o
: ~ a ~S o o o a~ o a~ cr ~ 1-- o a~ cs
U ~
OOU~ 000 000 000 ~ '
~ I~;I ~ ~ ~
::' aJ o . .
a~ O O O O O O O O U--l O U~ O
~ Y~ 000 0_10 ooa~ o~a
0~ ~ ~
LO~l ooo ooo ooo ooo
'~:'
~, OU~O 000 ~00 000
- N;:) co ~ H -I
~:
~ ~ O 1~
. . c a~ z Z x
Z Z
I
,~
,~ o _,
.~
~ ~`
~ `
'i~
;g;: ~ :
~330~5
It is to be understood that changes and variations
can be made without departing from the spirit and scope
of this invention as defined by the appended claims.
,:;:
:
:
:' . ''
, '
~:
'',
ii ~ ~
~ : .
~,,,~ .
~ `'~
~,, :