Note: Descriptions are shown in the official language in which they were submitted.
-` 133~420
Method of Consistently Producing a Copper
Deposit on a Substrate by Electroless Deposition
Which Deposit is Essentially Free of Fissures
BACKGROUND OF THE INVENTION
Electroless copper deposition solutions
comprise copper ions and a reducing agent for the copper
ions. The reducing agent oxidizes on a catalytic
surface, and provides electrons to the surface.
The general equations written for a system with
copper ions and alkaline formaldehyde are:
2HCHO + 40H- = 2HCOO- + 2H2O + H2 + 2e-, and
CuLn+2 + 2e- = cu + Ln
where e- designates an electron, L designates the ligand
necessary to prevent precipitation of basic copper
compounds in alkaline solution and n refers to the
valence of the ligand ion.
Copper deposits on substrates produced by
electroless deposition or electroless deposition
reinforced by electroplating are an important part of
many processes used for the manufacture of printed
circuits. Additive or fully additive printed wiring
boards are made with a process which uses 100%
electrolessly formed copper.
A specification, Mil Spec. P-55110-D, describes
tests which measure the performance of printed circuits
when subjected to conditions and environments the
printed circuits will be exposed to during manufacture
and use. In order to provide reliable printed circuits,
the criteria for printed circuits in military and some
commercial applications are based on the ability to meet
the requirements of this specification.
Heretofore, electroless copper deposits on FR-~
epoxy glass material using the fully-additive method of
making printed circuits have not been able to pass the `
35 Mil. Spec. P~55110-D thermal stress test. When exposed - ~
ycc/in ~-
2,~
to this test, plated-through holes would fracture during
a 10-second exposure to molten solder, the fracture
usually occurring at the intersection of the hole wall
with the surface, the corners of the holes.
The build-up of by-products and trace
contaminants in electroless copper deposition solutions
has had major detrimental effects on the quality of
copper deposits. In addition to the normal by-products
formed during operation, chemical contamination can
enter the plating solution through chemical additions,
water supplies, air or from the work placed in the
electroless copper bath. Many of the inorganic
contaminants, such as iron, cuprous ions, silver, gold,
antimony, arsenic and many other metals and their
compounds, as well as many organic contaminants, can
cause deleterious results for both bath operation and
the quality of the copper deposits, even when only
present in parts per million concentration. ~-
SUMMARY OF THE INVE~TION
Definitions:
By the term anodic reaction rate is meant the
rate of oxidation of the reducing agents on a metal
surface in an electroless metal deposition solution.
By cathodic reaction rate is meant the rate of
25 reduction of metallic ions to metal on a metallic ~ ;
surface in an electroless deposition solution.
By the intrinsic anodic reaction rate, r~', is
meant the anodic reaction rate as measured on a metallic
surface in an electroless plating solution by imposing a
potential slightly more positive than the mixed
potential on the metallic surface.
By the intrinsic cathodic reaction rate, rc',
is meant the cathodic rate as measured on metallic
surface in an electroless plating solution by imposing a
ycc/in
3 1 33~42~
potential slightly more negative than the mixed
potential on the metallic surface.
By the mixed potential, Emp~ is meant the
potential difference between a reference electrode and a
metallic surface on which both the anodic and the
cathodic reactions are proceeding, and metal is being
electrolessly deposited. Unless otherwise stated, the
reference electrode is a saturated calomel electrode,
SCE.
By the term thermal stress test is meant a test
of printed circuit specimens containing plated through -
holes wherein the specimens are conditioned at 120C to
150C for a period of 2 hours minimum to remove
moisture; after conditioning, the specimens are placed
in a dessicator on a ceramic plate to cool; the
specimens are then fluxed (type RMA of MIL F-14256) and
floated in a solder bath (Sn 63 + 5%), and maintained at
288 + 5C for a period of 10 seconds; after stressing,
the specimens are placed on a piece of insulator to
.:, :
cool; then the specimens are microsectioned in a
vertical plane at the center of the hole and examined
for cracks at 50 to 100 magnifications. A minimum of
one microsection containing at least three holes is made
for each sample tested. Any cracks forming in the
copper deposit on the specimens will indicate thermal
stress failure.
By an electroless plating reaction being under
cathodic control is meant that the cathodic reaction
: ,
controls the overall plating rate, i.e., the plating ;-~
rate depends on the concentration of the cathodic
reactants, the concentration of the metal ions, or the
concentration of depolarizers for the half reaction
involving the metal ions.
By an electroless plating reaction being under
anodic control is meant that the anodic reaction
controls the overall plating rate, i.e., the plating
ycc/in ;~
4 ~33:~420
rate depends on the concentration of the anodic
reactants, the concentration of the reducing agents for
the metal ions, or depolarizers for the half reaction
involving the reducing agents.
By the term high quality copper is meant copper
that has small crystals with a grain size less than 10
micrometers and low frequency of crystal dislocations,
defects and twinning. High quality copper on printed
circuit boards will pass the thermal stress test.
When referring to electrolessly deposited
copper, by the term satisfactory copper quality is meant ~ -
also high quality copper. ~ -
By fissure free copper deposits is meant
electroless copper deposits free from internal cracks or
fissures or internal defects capable of causing cracks
or fissures when the copper deposit is th~rmally ;
stressed. Fissure resistant copper means copper
deposits that will not form fissures or cracks when ~-
exposed to thermal stress, thermal cycling or in use.
20 Objects of the Invention: -
It is an object of this invention to provide -
copper metal deposits with good physical properties from
electroless plating solutions. -
It is also an object of this invention to
provide electrolessly deposited copper for printed
circuit boards which is resistant to crack formation
under thermal stress testing at 288C.
It is an object of this invention to provide
highly reliable printed wiring boards.
It is a further object of this invention to
provide a method of operating and maintaining an ;~
electroless copper plating solution which ensures the
deposition of copper having good physical properties and
being free of fissures.
ycc/in
. ~, ~ ,. .
33~20
4a
It is an object of this invention to provide a
method of formulating electroless copper plating
solutions that are capable ~:
~., .,:
~ .-. ::-.
' ;~''
, .
'..,~:: , :'
` 1 '~"'';~ ~,
.. ....
::
,: ~ ,. ..
.~
, ~", ".
ycc/in
':~ .' '-
.: ~. : ;;; `,. ,~ ,.
'' '~'~"',
; 133~2~
5of depositing copper free of fissures and resistant to
cracking under thermal stress.
It is an object of the present invention to
provide a method of formulating and operating an
electroless plating solution for forming copper deposits
being substantially free of fissures, the solution
comprising copper ions, a complexing ligand for copper
ions, a pH adjustor, a reducing agent, and at least one of
a stabilizer and ductility promoter and having a desired
10 initial ratio of the intrinsic anodic reaction rate to the :-
intrinsic cathodic reaction rate, characterized in that the
influence of build-up of by-products being formed during
bath operation is compensated for by raising the copper ion
concentration and the pH value or decreasing the
concentration of the reducing agent or by raising the
copper ion concentration and the pH value and decreasing
the concentration of the reducing agent, and thereby
maintaining the ratio of intrinsic anodic reaction rate to
intrinsic cathodic reaction rate at less than about 1:1 and
the original deposition rate as well as depositing
substantially fissure free copper.
Brief Description of the Invention:
This invention is based upon the discovery that, ~
in order to produce satisfactory copper the constituents .
comprising the electroless copper deposition solution are ~ -~
present in the solution in concentrations and under
operating conditions such that, at the operating
ycc/jj
!~jJ ~ , .
5a 133142~) :
temperature of the solution, the intrinsic anodic reaction
rate is not greater than the intrinsic cathodic reaction
rate.
In one aspect, this invention comprises a method
of monitoring and controlling electroless plating solutions
to obtain electrolessly formed me1:al deposits of high
quality, characterized in that the ratio of the intrinsic
reaction rates is maintained less than 1.1 during copper
deposition. In another embodiment, the invention comprises
a method of monitoring the ratio of the intrinsic anodic
and cathodic reaction rates of the electroless deposition
solution, and adjusting the solution composition and/or
operating conditions to maintain the intrinsic anodic
reaction rate less than 110% of the intrinsic cathodic
reaction rate.
In yet another embodiment, the invention ~
comprises methods of selecting an electroless copper ~- `
plat;ng baths which will operate under anodic control. -~ ;
Alkaline electroless copper plating baths comprise copper
20 ions, ligands to solubilize the copper ions, a reducing ~ -
agent capable of reducing the copper ions to metal, a pH
adjusting compound, and additives such as stabilizers,
accelerators, ductility promoters and surfactants.~`; f
. ,~; . i
Solutions under anodic control can be achieved by
25 maintaining the ratio of the mole concentration of the ~'~
reducing agent t:o the mole concentration of copper ions ;
,,.-:: ., ;
ycc/~c : .. ~
",~
,:, ~",
i~ ".,''~,'', ,':
5b 13 3 ~ 4 2 0
less than about 1.2. These embodiments include methods of
maintaining constant plating rates, and methods for
increasing plating rates.
Broadly, the present invention provides a method
of formulating and operating an electroless plating
solution for forming copper deposits which are
substantially free of fissures. This solution comprises
copper ions, a complexing ligand for copper ions, a pH
adjustor, a reducing agent, and at least one of a
stabilizer and ductility promoter and has a desired initial
ratio of the intrinsic anodic reaction rate to intrinsic
cathodic reaction rate. The method is characterized in
that the influence of build-up of by-products that are
formed during bath operation is compensated for by raising
the cooper ion concentration and the pH value or decreasing
the concentration of the reducing agent, or by raising the
copper ion concentration and the pH value and decreasing ~
the concentration of the reducing agent, so as to maintain -
the ratio of intrinsic anodic reaction rate to intrinsic `
cathodic reaction rate and the original deposition rate as
well as to deposit substantially fissure free copper.
The present invention also provides an ~-
electroless copper plating solution which comprises copper
ions, a complexing ligand for copper, a pH adjustor, a
reducing agent, a stabilizer or ductility promoter and an
accelerator which contains a delocalized pi bond. The
: .
yc¢/jo
` 5c ~.33~20 ~ ~
accelerator is selected from (i) heterocyclic aromatic
nitrogen and sulfur compounds, (ii) nonaromatic nitrogen ~:
compounds having at least one delocalized pi bond, and
(iii) aromatic amines, and mixtures of the foregoing. The
plating solution is characterized in that the mole
concentration of the reducing agent is no greater than the
mole concentration of the copper ions.
~, ,
... ...
',' ;; ~' " '~
,,,.-'.'.',.,' ' ';
,: .~ ~", ,,
'( ~;.,
.~ :
.:, . :
.,'' ,-, ''
'-. :,
ycctjc
~';~':
: ' ',: ' -
6 1331~20
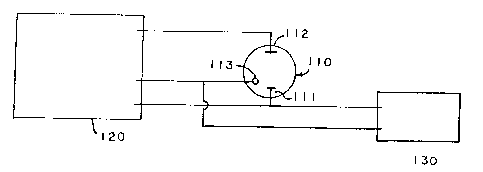
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a schematic diagram of apparatus
suitable for carrying out electrochemical measurements
according to this invention.
Fig. 2 is the plot of the potential applied in
making the measurements vs. time as described in Example
1.
Fig. 3 is the plot of the current produced vs.
the potential applied as described in Example 1.
DETAILED DESCRIPTION OF THE INVENTION
While the invention will be described in thecontext of alkaline electroless plating bath solutions,
its scope is not limited to such solutions.
There are many ele~troless copper plating
solution formulations which will initially deposit high
quality copper. However, experience has shown that as
the plating baths age, the quality of the deposit ~ -
deteriorates, and the bath must be replaced in whole or
in part. The age of an electroless plating solution is
determined by build-up of plating reaction by-products
and by build-up of contaminants. The build-up of by-
products can be measured by the change in solution ~ ;~
density or specific gravity. Under fixed operating
conditions, the contaminants also will build-up in -
proportion to the change in solution density~ The
teaching of this invention allows the extension of the
useful life of such solutions by controlling the mole
ratio of formaldehyde to copper, and increasing the
copper concentrations and the pH of such solutions to
30 obtain adequate plating rates at the desired mole ratio. ~ ; -
Aqueous electroless copper plating solutions
for use in the processes of this invention contain
copper compounds which serve as the source of copper
ions to form the copper metal deposits; reducing agents
which are themselves oxidized and provide the electrons
ycc/in
.- : .,.
`~~` 7 1331420
necessary to reduce the copper ions to copper metal
deposits; pH adjusting compounds which provide a pH
suitable for reduction of the copper ions by the
reducing agents; complexing agents to solubilize the
copper ions at the pH of the solutions; and additives to
stabilize the solution, brighten the deposits, reduce
surface tension, inhibit hydrogen inclusion in and
improve ductility of the copper metal deposits. -
Among copper compounds that are suitable as
sources of copper ions are copper sulfates, copper
nitrates, copper halides, copper acetates, copper -
phosphates, copper oxides, copper hydroxides, basic
copper sulfates, halides and carbonates and soluble
copper complexes. Copper(II) compounds are preferred, -
15 and copper(II) sulfate and copper(II) chloride are~; ~
commonly used. Another source of copper ions is -
metallic copper which may be electrochemically dissolved ~
into the electroless plating solution, or -
electrochemically dissolved into an electrolyte and
20 diffused through a membrane into the electroless plating ;~;~
solution.
The lower limit for the concentration of the
copper compound in the electroless plating solution
should be high enough to maintain the intrinsic cathodic
~5 reaction rate greater than 90~ of the intrinsic anodic
reaction rate. The upper limit is the concentration
where copper metal precipitates homogeneously throughout
the solution instead of only forming copper deposits on
preselected catalytic surfaces. The upper limit also
depends on the stabilizer additive used to control
homogeneous precipitation. For most electroless copper
plating bath formulations, the concentration will be
above 0.01 molar and below 0.2 molar, and increases as
the bath ages by build-up of plating by-products and/or
contamination.
ycc/in ;
~ ~ .
1331~20
In one embodiment of the invention, the copper
concentration and the pH of the electroless plating
solution are increased as the by-products and
contaminants build-up in the solution. In this
embodiment, in order to obtain fissure free copper
deposits when contaminants and/or by-products build-up
in the solution the copper concentration is increased 20
to 200% preferably 40 to 100% while the pH also is
increased.
Among the reducing agents that are suitable for
the reduction of copper ions are formaldehyde reducing
agents. Formaldehyde reducing agents include compounds
such as formaldehyde, formaldehyde bisulfite,
paraformaldehyde, dimethylhydantoin, and trioxane.
Other suitable reducing agents are boron hydrides such
as boranes and borohydrides such as alkali metal
borohydrideR.
The upper limit for the reducing agent in the
electroless plating bath is the concentration at which
the intrinsic anodic reaction rate is 110% the intrinsic
cathodic reaction rate. The lower limit is the
concentration at which copper plating on a clean copper
surface doesn't occur, i.e., the plating solution is
passive. Preferably, the lower limit is the
concentration at which the intrinsic anodic reaction
rate is 75% to 85% of the intrinsic cathodic reaction
rate. For formaldehyde reducing agents, the limits
depend on additives, pH and very strongly on the
temperature. In solutions where the intrinsic anodic
~0 and cathodic reaction rates have not been determined,
the concentration of formaldehyde will preferably be set
above 0.01 molar and below 1.2 times the molar
concentration of copper ions and more preferably
maintained at or below the molar concentration of the
copper ions.
ycc/in
~ .
. ;:
9 ~33~420
Suitable pH adjusting compounds include the
alkali metal hydroxides and copper oxide. In the
operation of an alkaline, electroless copper plating
solution, the pH usually drops during plating, and
hydroxides are added to raise or maintain pH. If the pH
needs to be lowered, an acidic compound would be used as
a pH adjusting ion. When the reclucing agent is a
formaldehyde reducing agent, the activity of the
reducing agent depends on the pH as well as the
concentration of the reducing agent. Therefore to
increase the activity of the reducing agent and thus
increase the intrinsic anodic reaction rate, as
described herein below, either the concentration of the
formaldehyde reducing agent or the concentration of the
hydroxide compound ~i.e., pH) may be increased. In
operating an electroless copper solution when the
intrinsic anodic reaction is to be increased, preferably ~;-
pH is increased and formaldehyde concentration is held
substantially constant or even decreased.
In one embodiment of this invention as the
solution ages, the intrinsic cathodic reaction rate is -~
increased by raising the copper concentration by 40 to
100% and the anodic reaction rate is increased less than
the cathodic reaction rate by raising the pH 0.1 to 1 pH ~ ~
25 unit, more preferably by 0.2 to 0.6 pH unit. ~ ;
For formulations with formaldehyde type ;
reducing agents, the pH (measured at room temperature) ;~ ~;
is usually set between 9.5 and 14. When the ratio of
the mole concentration of the reducing agent to the mole ~ ~
30 concentration of the metal ion is less than about 1.2 ~! ;,'~., ;~`
the pH is preferably greater than 11.9, more preferably
greater than 12.2.
Suitable complexing agents for electroless `
copper plating solutions are well known to those skilled
in the art. Among the complexing agents useful for
electroless copper plating solutions are ~
ycc/ln - -- ::
~::,
., ~
- ~33~2~
ethylenedinitrilotetra-acetic acid (EDTA), hydroxyethyl-
ethylenediaminetriacetic acid (HEDTA),
diethylenetrinitrilo-pentaacetic acid (DTPA),
nitrilotriacetic acid (NTA), triethanol-amine,
tetrakis(2-hydroxypropyl)ethylenediamine (THPED), penta-
hydroxypropyldiethylenetriamine, and tartaric acid and
its salts (Rochelle salts). Copper deposits without
fissures, and plated through hole printed circuits
capable of withstanding a thermal stress of 288OC for 10
seconds may be plated from solutions comprising these
complexing agents or mixtures thereof by the methods and
procedures of this invention.
Many additives have been proposed for use in
electroless copper plating solutions. The additives
which have been proposed may be classified by function
into different groups. Most additives have more than a
single effect on the electroless copper plating
solutions, so classification of additives into groups
may be somewhat arbitrary. There is some overlap
between the additive groups, and almost all the
additives affect the rate of the oxidation of the
reducing agent (the anodic reaction) or the reduction of
the copper ion to metal (the cathodic reaction).
One group of additives are surfactants or
wetting agents to control surface tension. Anionic,
nonionic, amphoteric or cationic surfactants may be
used. The choice of surfactants may vary depending on `~
the operating temperature and the ionic strength of the
electroless plating solution employed. Preferably, the
surfactant is used at solution temperatures and ionic
strengths below its cloud point. Surfactants containing
polyethyoxy groups or fluorinated surfactants are
preferred. Among the preferred surfactants are
alkylphenoxypolyethoxy phosphates, polyethoxypolypropoxy
block copolymers, anionic perfluoroalkyl sulfonates and
ycc/in ~ ~
.:
~ " .. .
~331~20
11
carboxylates, nonionic fluorinated alkyl alkoxylates and
cationic fluorinated quatenary ammonium compounds.
A second group of additives are stabilizers
which prevent the spontaneous decomposition of the
plating solution and/or the formation of undesired
copper deposits outside of, or extraneous to, the
desired deposit, so called "extraneous copper". Among
the additives that have found use as stabilizers and to
inhibit extraneous copper are oxygen (e.g., oxygen added
to the plating solution by stirring or air agitation of
the solution), divalent sulfur compounds (e.g., thiols,
mercaptans, and thioethers), selenium compounds (e.g.,
selenocyanates), covalent mercury compounds (e.g., `~
mercuric chloride and phenylmercury), and copper (I)
15 complexing agents (e.g., cyanides, 2,2'-dipyridyl and -
1,10-phenanthrolines). ,
A third group of additives may be classified as
ductility promoters and/or additives to retard hydrogen
inclusion in the deposit. This group would include
polyalkylene ethers, cyanides, nitriles, compounds of
vanadium, arsenic, antimony and bismuth, nickel salts,
2,2'-dipyridyl, 1,10-phenanthrolines and some organic ;
silicones.
The ductility promoters also act as stabilizers
and are used alone or in combination with other
stabilizers. Suitable concentrations for various
stabilizers and ductility promoters have been described
by Zeblisky et al., U.S. Patent 3,095,309; Schneble et ~ -
al., U.S. Patent Nos. 3,257,213, 3,310,430 and -i
3,361,580; Zeblisky et al., U.S. Patent No. 3,485,643;
Schneble, U.S. Patent No. 3,607,317; Underkofler et al., ~
U.S. Patent No. 3,844,799; Heymann et al., U.S. Patent -
No. 3,454,416; Clauss, U.S. Patent No. 3,492,135; Gulla
et al., U.S. Patent No. 3,663,242; Shipley et al., U.S. -
Patent No. 3,615,732; Jonker et al., U.S. Patent No.
3,804,638; Molenaar et al., U.S. Patent No. 3,843,373;
ycclin
12 1331~2~ ~
Morishita et al., U.S. Patent No. 4,099,974; Nakaso et
al., U.S. Patent No. 4,548,644; and Nakaso et al., U.S.
Patent No. 4,557,762.
The amount of stabilizPr and/or ductility
promoter in the electroless copper plating solution
depends on the stabilizers or ductility promoters
selected and on the concentration of copper ions,
reducing agent and pH. The concentrations range between
0.01 and 100 mg/l. Jackson, U.S. Patent No. 3,436,233
describes some stabilizers that are used in even larger
quantities up to 2 g/l. In general, stabilizers and/or
ductility promoters should be present in the electroless
plating solution in an amount sufficient to prevent
extraneous plating, i.e., plating on masks or resists,
and substantially less than the amount that would cause
passivation of metal surfaces being plated or that would -
stop the plating reaction.
A fourth class of additives is the group of
plating rate accelerators (also known as depolarizers)
as taught by McCormack et al., U.S. Patent No.
4,301,196. These accelerating agents are compounds
containing delocalized pi bonds such as heterocyclic ;
aromatic nitrogen and sulfur compounds, aromatic amines
and non-aromatic nitrogen compounds having at least one
delocalized pi bond. Among such compounds are purines,
pyrimidines, pyridines, thiazines, triazines and thiol
derivatives.
Preferably, the depolarizing or accelerating ~-~
agent will be present in a small effective amount, i.e., ~-
30 generally at least about 0.0001 to about 2.5 grams per
liter, more specifically about 0.0005 to 1.5 grams per ~ `
liter and preferably from about 0.001 to about 0.5 grams
per liter. In general, the amount of depolarizing or
accelerating agent used will vary depending upon the
particular agent employed and the formulation of the
solution.
ycc/in
.'.. ~ .... :
-: ~331~20 :
13 ~
,
Electrolessly deposited copper in plated
through holes must be thick enough to pass thermal
stress and thermal cycling tests. The minimum thickness
is about 10 micrometers, and preferably is at least 15%
of the height of the plated through hole. Preferably,
the copper thickness is at least 17~ of the height of
the plated through hole and more preferably, at least
20%. ~
Although electrolessly deposited copper has ! .~',
been known for many years to be inferior to
electrolytically deposited copper in resistance to
thermal stress, ductility and other physical properties,
surprisingly it has been found that if electroless
copper deposition solutions are formulated and
controlled to have an intrinsic anodic reaction rate
less than 110% of the intrinsic cathodic reaction rate,
copper deposits with superior physical properties,
including resistance to thermal stress, may be obtained.
The intrinsic rate ratio can b~ determined by
measuring the reaction rates for the two half reactions
in the neighborhood of the mixed potential, e.g., at
+lOmV for the one and at -lOmV for the other half
reaction; or by sweeping the potential on the one and
the other side of the mixed potential and measuring the -~
current.
In one method, the intrinsic anodic reaction
rate at the mixed potential is estimated from the
current required to vary the potential on a working
electrode which is electrolessly depositing copper. The
potential between the working electrode and a reference
electrode is varied in a potential ramp between En1p and
+30 mV from Emp by passing current between the working
electrode and a counter electrode and simultaneously
measuring the potential and the anodic current as the
potential changes. Alternatively, if the counter
electrode is at Emp and very much larger than the working -
ycc/in
. ~ '': '
331~20
14
electrode, it can also serve as a reference electrode
since the current passed between it and the working
electrode would be too small to shift the counter
electrode potential. The intrinsic anodic reaction rate
at Emp may be determined from the slope of a current vs.
voltage plot as it approaches E~p.
Similarly, the intrinsic cathodic reaction rate
may be determined from the slope of the current vs.
voltage plot between -3OmV from h~mp and En1p.
When the intrinsic cathodic deposition rate is
maintained greater than the intrinsic anodic deposition
rate, or when the ratio of the intrinsic anodic
deposition rate to the intrinsic cathodic deposition
rate, r'~/r'c, is less than 1.1, preferably is less than
1.05, and more preferably is less than 1.0, it had been
found that copper with superior physical properties is
deposited. In order to maintain the desired ratio, it -
may be desirable to increase the rate of the intrinsic ~ ~
cathodic reaction, or increase the intrinsic cathodic ~-
rate more than an increase in the rate of the intrinsic
anodic reaction.
Among the methods for increasing the rate of
the intrinsic cathodic reaction are (1) raising the
concentration of the cathodic constituent, i.e., the
metal ion concentration; (2) addition of a catalyst or
depolari~er to accelerate the cathodic reaction; and (3)
increasing the surface area available for the cathodic
reaction (e.g., by reducing the contaminants or the i~ `~
stabilizer concentration and the surface area blocked by
contaminants or stabilizer; this may be accomplished by
diluting the solution with fresh solution or by carbon
treatment of the solution to remove contaminants
blocking the surface area available for the cathodic
reaction). When the metal ion concentration becomes too
35 high, extraneous metal deposition in the bulk of the ;
solution or outside the desired metal pattern may be
ycc/in
,_, ! ,
15 ~331~20 :
observed. For many electroless copper plating
solutions, this occurs at copper ion concentrations
above the range of 0.08-0.12 moles per liter.
As electroless plating solutions build-up by-
products and contamination, the Ratio usually will
increase. The Ratio, r~/rC~ may k,e maintained less than
1 while increasing both the anodic and cathodic reaction
rates, by increasing the rate of the intrinsic anodic
reaction less than an increase in the rate of the
lo cathodic reaction. The rate of the intrinsic anodic
reaction may be increased by (1) decreasing the
concentration of the reducing agent (i.e., lower
formaldehyde) while increasing the pH; or (2) increasing
the concentration of anodic depolarizers such as
heterocyclic aromatic nitrogen or sulfur compounds. If
the concentration of the formaldehyde is lowered too
much, the Emp of the solution may rise by 50-200 mV and
the solution becomes passive, i.e., there is no
electroless deposition. Frequently, the solution will
become active again at a higher temperature. It has
been found that to increase the concentration of the
anodic reactants, the product of the formaldehyde
concentration and the square root of the hydroxide ion
concentration, [CH20][0H-]~s, must be increased.
Although the formaldehyde concentration may be
decreased, held constant, or even increased, the product
[CH2O][OH-]s, is increased to maintain the intrinsic
anodic reaction rate less than the cathodic rate as the
cathodic rate is increased.
For plating solutions operating above room
temperature, the square root of the hydroxide ion
concentration [oH-]05 may be conveniently estimated using
the room temperature (25C)pH of the solutions.
In the event, that bath contaminants cause
35 reduction of deposition rate and inadequate copper -
quality because of temporary, localized passivation of
,; ~
ycc/in
: .
16 1 3 3 1~12 ~
the plating surface, the condition must be compensated
for by increasing the plating current produced by the
anodic half-reaction, i.e., by increasing pH. Since
this will increase intrinsic anodic reaction rate, the
copper concentration must be increased to bring the
Ratio of r9/rC to the original value before the solution
became contaminated, or a value below 1.1 and adequate
for the resulting plating rate.
Measurement of the intrinsic rate of the partial
lo reactions
We have determined the ratio of the intrinsic
rate of the partial anodic and cathodic reactions from
measurements of current-potential relationships in a
narrow potential range (e.g., from -30 to +30 mV from
the mixed potential, Emp)~ This relationship is used in
two ways. Both methods give similar conclusions ~
regarding conditions for producing copper of preferred ~ -
qualities.
In one method, the cathodic current, ic, at the
potential which is 10 mV negative with respect to the Emp
(i.e., the overpotential, Eta = -10 mV vs. El~p) is taken ~;~
as the rate of the cathodic partial reaction, (rC)l~lvl or
simplified rc; the anodic current i~ at the potential
which is 10 mV positive with respect to the mixed
potential, Empr (i.e., the overpotential, Eta = +lOmV vs.
Emp) is taken as the rate of the anodic partial reaction,
(r9~+l~Vl or simplified r9.
Alternatively, in a computerized method, the
intrinsic rates of the partial reactions is determined0 using the rate expression
n n
r' = ~ [ijEj]/ ~ [(Ej)2]
j=l j=l ,.,~ ', ~ ,~
ycc/in
' ' '",:'.''
- ~33142~
17
where r' is the partial rate, ij is the current density -
at an overpotential, ~j(Eta), referenced to the mixed
potential, Emp~ and Ej is calculated from the
overpotential vs. Emp~ ~j (Eta), according to the equation
Ej = lO(~j/ba~ - 10(~ c)
where b~ and bc are the Tafel slopes. For an
electrochemical reaction, a plot of the overpotential,
~, from the thermodynamic equilibrium potential vs.
logarithim of the current, log i, was found by Tafel to
be of the form
~ = a - b(log i).
For many electroless solutions, the anodic reaction,
CH20 + 20H- =HCoo- + H20 + ~H2 + e-
the constant b~ has the value 940 mV/decade, and for the
cathodic reaction,
CuLn+2 + 2e- = Cu + Ln
bc has the value 310 mV/decade.
The rate of the cathodic partial reaction, rc',
is obtained, in this invention, by applying the above
equation to a set of pairs of experimental values (ij~Ej)
from the cathodic potential range which is, e.g., from -
3 0 mV vs. Emp to E~p . The rate of the partial anodic
reaction, ra', is obtained by applying the above equation
to a set of pairs of experimental values obtained from
the anodic potential range which is, e.g., from El"p to
E=+3 0 mV vs. Emp~
The currents used to calculate intrinsic
reaction rates are measured at potentials near Emp~ e.g.,
10 ' ~ 50 mV from Emp~ which may introduce some errors in
3 0 the determination of the intrinsic reaction rates. The
equations strictly apply only close to the mixed
potential. If one examines both positive and negative
overpotentials and currents for a particular solution,
one will find near the mixed potential, the over-
35 potential departs from the Tafel (semilogarithmic)ycc/in
, ~
133~20
18
relationship. The current measurements for
determination of the intrinsic anodic and cathodic
reaction rates must be in the range where the semi-
logarithmic relationship is non-]inear. This range is
often within + 40 mV of the Emp/ hut can be larger or
smaller depending on the electroless plating solution
formulation. The admissable error depends on the set
point of the ratio of the intrinsic anodic and cathodic
reaction rates and thus on the formulation of the ~-
electroless plating solution.
Procedure
An experimental setup for carrying out
electrochemical measurements of r~, r~', rc and r'c,
according to this invention, is shown in Fig. 1. The
setup shown in Fig. 1 is composed of an electrochemical
cell (110), a potentiostat with function generator (120)
and a recorder (130).
In a typical test, an all-glass, single
compartment cell with three electrodes was used. The
test electrode was a platinum wire, 3.8mm2 in area
(length 2.0 mm, diameter 0.6mm), and the auxiliary
electrode a platinum cylinder (about lOmm2 in area), both
electroplated with copper. Plating was done in an acid
copper solution (CuS04.5H20 - 188 g/l, H2S0~ - 74g/l) at
25 10 mA/cm2 for 1-5 min. A saturated calomel electrode
(SCE) was used as a reference electrode.
The current-potential curves were obtained with
an IBM Instruments Inc. EC/225 Voltammetric Analyzer~
(120 in Fig. 1) and recorded on an IBM Instruments Inc. ~ -`
30 7424 X-Y-T Recorder~` (130).
The test electrode, (111) in Fig. 1, an
auxiliary electrode, (112), and a reference electrode
(113) are connected to the potentiostat, (120). The
potentiostat with function generator was used in a DC
operating mode, for linear sweep voltammetry (LSV). The
ycc/in
. ~
-` 19 ~331~20
sweep waveform as shown in Fig. 2 is a linear rampJ the
current is continuously sampled; when the potential
reached a final value it is left at this value for a
short period of time and then reset to the initial
value, or an automatic scan reversal to the initial
value can be used.
Example 1 :
An electroless copper plating solution was :
prepared with a high copper concentration and a
correspondingly high specific gravity. The ratio of the
mole concentration of the formaldehyde reducing agent to
the mole concentration of the copper was 0.67. The
formulation was as follows:
Copper sulfate 0.12 moles/1
15 Ethylenedinitrilotetraacetic acid 0.20 moles/1
Formaldehyde 0.08 moles/1
pH (25C) 11.9
[CH2o][oH-]5 0.007 (m/1)~5
Cyanide (Orion electrode) 110 mV vs. SCE ~
20 Vanadium pentoxide 5 mg/1 ~ ~;
Specific gravity 1.124
Operating Temperature 75~C
rA 0.14 mA/cm2 ~:.
rc 0.16 mA/cm2 ;.~
25 Ratio (r~/rC) 0.88 . : .
r~' 1.13 mA/cm2 ;:~ :
rc. 1.96 mA/cm2 . ~.
Ratio' (r~'/r0') 0.58
Additive printed circuit boards were plated in
30 this solution and after plating, tested by the thermal `.
stress test at 288C for 10 seconds. There were no
cracks formed in the copper by the thermal stress test
ycc/in
"
. . ~ .
~ ~33l~2a
which confirmed the results from the ratio of the
intrinsic anodic and cathodic reaction rates.
Example 2
A solution from a working, production,
electroless copper, plating bath was operated to the
formulation below as far as its formulated bath
constituents are concerned. The formulation was known
to be able to produce high quality copper. However the
ratio of formaldehyde to copper was greater than 1.2 so
10 the solution would not consistently deposit high quality ~
copper as the by-products and contaminents build-up and ,
the ratio changed. Electrochemical analysis of the
solution gave a Ratio of 1.1 and a Ratio' of 1.05,
indicating borderline performance. The deviation of the
electrochemical Ratio results from the good Ratio
results indicate the presence of an unknown contaminant.
Fully additive printed wiring boards were prepared on
adhesive coated, epoxy glass laminates in this
electroless copper plating bath. Thermal stress testing
20 showed cracks in 20% of the copper hole walls. `~
'' ' ` ,~
'`
;' ''''','.''.'
ycc/in ~ ~
~" ~ ~S
~33142~
21
The solution had the following formulation:
Copper Sulfate moles/1 0.028
EDTA mo:Les/1 0.076
Formaldehyde mo:Les/1 0.049
pH (at 25C) 11.6
[HC~o][oH-]05 (moles/1/)~5 0.0031
Sodium Cyanide mV vs. SCE -110
(Orion electrode)
Vanadium Pentoxide grams/l 0.0012
Specific Gravity grams/ml 1.094
(at 25C)
Temperature C 75
r~ mA/cm2 0.33
rc mA/cm2 0.30
Ratio 1.10
r~' mA/cm2 2.87 I
rc' mA/cm2 2.74 : :
Ratio' 1.05
Thermal Stress cracks 20%
:
ycc/in
..
, ~ :
.
22 ~331~2~
In order to deposit copper that would pass the
thermal stress test, a similar solution was prepared
with a pH of 11.9 and a ratio of formaldehyde to copper ::
of 0.84. The solution had the following formulation: :
Copper sulfate 0.056 moles/l ~ :~
EDTA 0.110 moles/1
Formaldehyde 0.047 moles/l :; ::~
pH ~at 25C) 11.9
[CH2o~[oH-]5 0.0042 (moles/l)~5 ~p
Sodium Cyanide -100 mV vs SCE
(by Orion electrode)
Vanadium Pentoxide 0.004 grams/l :
Specific Gravity 1.066 (at 25C)
Temperature 75C
ra 0.33 mA/cm2
rc 0.40 mA/cm~
Ratio 0.83
r~' 1.69 mA/cm2 ~ ~ :
rcl 1.98 mA/cm2
Ratio' 0.85
: Thermal Stress no cracks : ;~
. ,, ~.
Because the solution was under anodic control
and the anodic rate was only slightly increased, the
increase in the copper ion concentration to twice the ~ ;
concentration did not cause a corresponding increase in
the plating rate. The copper metal was deposited at ~
approximately the same rate, and it required 17 hours to .:~:
deposit copper 35 micrometers thick.
, ~,.
. . ~. .,
~: j ., ,
ycc/in -
. ' ''~'
, ,~'`':.' '
23 13~1420
In order to accelerate the plating rate, since
the concentration of the cathodic reactant had already
been doubled, the concentration of the anodic reactants
was increased by increasing the pH to 12.2. The changes
in the formulation are shown below:
pH (at 25C) 12.2
[CH20][0H-]s 0.006 (moles/l) 1'5
Sodium Cyanide -110 mV vs SCE
Specific Gravity 1.070 (at 25C)
rn 0.47 mA/cm2
rc 0.49 mA/cm2
Ratio 0.96 `~
r~' 5.02 mA/cm2 -~
rc~ 5.30 mA/cm2
Ratio' 0.95
Thermal Stress no cracks
This solution deposited copper 35 micrometers
thick in less than 8 hours. This example illustrates
how the principles of this invention may he used to
obtain copper with superior physical properties at fast
plating rates.
Example 3
In this example, a test solution was
deliberately contaminated to show how the teaching of
this invention may be used to adjust the formulation, or
reset the control parameters, to obtain fissure free
copper deposits from a solution in which contaminants ~
have build-up over a period of time as the solution i9 ~,
used.
An electroless copper test solution was
prepared with a stabilizer system using both vanadium
and cyanide additions agents. In the table below, this
solution is marked A. The electrochemical analysis of
the solution gave a ratio of the intrinsic anodic
ycc/in
. . ~ :
--- 133l ~2~
2~
reaction rate to the intrinsic cathodic reaction rate,
Ratio' = r'~/r'c, o~ less than 1.1 indicating the
solution would deposit fissure free copper.
As a deliberate contaminant, 1 mg/1 of 2-
mercaptobenzothiazole (2-MBT), was added to the test
solution. The addition of the contaminant turned the
solution passive, i.e., stopped the electroless plating
reaction, and the mixed potential of the copper
electrode in the test solution was shiEted outside the
electroless plating range.
The conventional practice in the prior art was
to increase the formaldehyde and the pH in order to
regain a mixed potential sufficient for electroless
copper plating. Following this conventional procedure,
formaldehyde was added to the solution to triple the
concentration and enough sodium hydroxide was added to
increase the pH by one pH unit. In addition, copper was
added to increase the cathodic reaction rate. The -
modified formulation is listed in the table as solution
20 B. While these adjustments overcame the passivation and -~
increased the rate of deposition, the ratio of
formaldehyde to copper was 2.4, which is greater than
1.2. As expected the electrochemical analysis of the -;-;
intrinsic anodic and cathodic reaction rates gave a
~5 Ratio' greater than 1.1 indicating the copper deposits ;;i
would be subject to fissures.
To lower the intrinsic anodic reaction rate
relative to the intrinsic cathodic reaction rate the `~
solution was reformulated with the original formaldehyde
30 concentration and a formaldehyde to copper ratio of 0.7; , -~
this is solution C. The Ratio' was reduced to less than ,
1.1, so the solution would deposit copper resistant to '
fissures.
To achieve a preferred Ratio' of the intrinsic
anodic reaction rate to the intrinsic cathodic reaction
rate, the concentration of the anodic reactant, -~
ycc/in
1331420
formaldehyde, was further reduced. The formulation is
listed as solution D. The Ratio' of the intrinsic
anodic reaction rate to the intrinsic cathodic reaction
for this solution is less than 1.0, and thus the
solution can provide a high quality, fissure free copper
deposits.
. ,'., ~.
~. '
ycc/in :
'' .:.
26 ~331~2~
~D ~ Ul ' ~':
,~, "., ,~, o o
~1 ou~ O oo
. . .. . o ~ o ~ I
O O O ~ O ~ N ~ `J O
.
~o r~ I` .
In U~ ~t' O O O O ~ U~ ,
~) O rl O In o ~ O O
. . . . O ~1 0 r~ Ul 0~ '
O O O ,~ O ~ ~ ~ I ~ ~ ~I d' '
:'~ ' ' ' ; '`
`. ': `
O ~ O ~ O U~ O ~ : ,--':
m o ~ O
. . . . o ~ o ~ ~ 0~
o o o ~ o ~ ~ r~ I ~ ~ ,1 ~ ::,~ :: : : :
~, .,., . ~
.:.:..
i', ,.`".',,
I ~ o ~ O o ~ ~, ~ .....
~ O rl O ~ O ~ ~r ~ ~ I`
~, . O ,~ ~ o ~ i 0 ,~
'. . `'~ .; '
~, ~, ~, O ,~ ~;
:. ,. .
U~ O a~
1~ o7 ~ O O
,~ U~ ~C ~ X
O ~ ~ ~ ~ U
u~ ~ ~ Z ~ 4 4
IS) o Ln
.
27 1331420
This example shows that with
mercaptobenzothiazole as an accelerator or depolarizing
agent, increased copper concentration and pH with the
same or decreased formaldehyde concentration leads to
faster plating rates and high quality copper deposits.
The plating rate of solution A without the accelerator
or depolarizing agent was 1.4 micrometers per hour, The
plating rates of solutions C and l) with the accelerator
or depolarizing agent were 4.0 and 3.3 micrometers per
hour, respectively.
Example 4
The procedure of Example 3 was repeated using a
plating tank for 70 liters of the solution. The plating
tank was equipped with an electroless copper plating
bath controller which continuously measured the solution
parameters such as the copper and formaldehyde
concentrations, the pH, the cyanide ion activity and the
temperature. The plating bath controller automatically
compared the measured parameters to the set points and
made additions to the solution to maintain the solution
within the preset operating limits.
The plating solution was operated to deposit
approximately 6 turnovers. (A turnover is replacing the
copper salt content of the solution once). This raised
the specific gravity of the solution due to the
formation of by-product sodium sulfate and sodium
formate. The intrinsic anodic and cathodic reaction
rates were measured by electrochemical analysis, and the -
Ratio' of the intrinsic anodic reaction rate to the
intrinsic cathodic reaction rate was less than 1.1 which
indicates the copper deposit is resistant to fissures.
The solution was used to make additive printed circuits ;
by the electroless deposition of copper to form surface
conductors and plated through holes. The printed ~ -~
circuits were thermally stressed by contact with molten
ycc/in
1 331~2~
2~
solder at 288C for 10 seconds. After thermal stress,
the plated through holes were microsectioned and i
examined for cracks in the deposited copper. There was
no evidence of cracks or fissures in the copper
5 conductors or plated through holes. The formulation `
tested is shown in the table below.
The operating solution, found to deposit
fissure free copper, was then treated with 0.5 mg of 2-
mercaptcbenzothiazole (2-MBT) as a deliberate
contaminant to simulate the effect of contamination of
the plating solution by organic compounds. Organic! '
contamination is a frequent problem in electroless
copper plating, especially in solutions operated for ~ -
five or more turnovers. Sources of contamination ; -
include leaching from plastic substrates being
electrolessly plated, from the plating resist or from
fortuitous contamination.
After the addition of the contamination, the
plating solution became substantially passive. The
plating rate was about 0.03 micrometers of copper per
hour and the solution would no longer deposit copper on
the hole walls of the insulating base material to make `~
plated through holes. The Ratio' of the intrinsic
anodic and cathodic reaction rates was greater than 1.1, -
25 so even if copper would have deposited on the hole ~-
walls, the formed deposit, and thus the plated through -~
holes, would fail the thermal stress test. This
solution is more fully described below. ~;
Following the procedures of Example 3 in a ~
30 sample of the solution, the pH was raised to provide a ; ;;
more active platiny solution, and the copper
concentration was increased to adjust the Ratio' of the
intrinsic anodic and cathodic reaction rates to less
than 1.1. The increase in the copper concentration
reduced the ratio of formaldehyde to copper from 1.7 to
0.85. When the Ratio' of less than 1.1 was achieved ~ ~
ycct in ,,: . -
' : ~` .
33~2~
29
with the sample solukion, the set points on the
electroless plating bath controller for copper
concentration and pH were reset. Additive printed
circuit boards were plated in the contaminated
electroless plating solution using the new set points.
The copper deposited on these printed circuit boards was
tested by thermal stress with molten solder at 288C for
ten seconds and was found free of cracks or fissures.
The formulation, set points and test data for
this solution are also given below.
Original Solution
Good with
Solution Reset
Controls
CuS04 mol/1 0.028 0.040
EDTA mol/1 0.087 0.100
CH2O mol/1 0.047 0.047
pH 25C 11.75 12.40
[CH2o][oH-]o5 (m/1)~5 0.0035 0.007
Gafac RE-610 mg/l 40 40
NaCN (Orion elec-
trode vs. SCE) mV -130 -130
v2os mg/1 1 1 ~ `~
Specific gravity g/cm3 1.066 1.066
25 Temperature C 75 75
Emp vs. SCE mV -764 -687
Plating Rate m/hr 1.7 2.9
r~a mA/cm2 1.44 2.57
mA/cm2 1.39 2.40
Ratio' 1.04 0.93
Thermal stress pass pass
In this example, a passive, contaminated
solution was restored to active plating, and then by
adjustment of the formulation, according to the
ycc/in
1331~2~
teachings of this invention, the intrinsic anodic and
intrinsic cathodic reaction rates of the conkaminated
solution were adjusted to deposit high quality copper.
The addition of 2-mercaptobenzothiazole, a heterocylic
nitrogen and sulfur compound, and increasing the copper
concentration and pH resulted in a 70% increase in the
plating rate.
Example 5
In this example, fissure resistant copper was
deposited from an electroless copper deposition solution
operating at low temperature. A first electroless
copper plating solution was formulated to operate at
300C. The formaldehyde concentration was higher than -
similar solutions at 75C as is the common practice in
15 electroless copper solutions operating near room ;
temperature. The ratio of the formaldehyde ~ -
concentration to the copper concentration was 2.4. The
solution plated slowly, depositing 25 micrometers of
copper in three days. This first solution composition
is given in the table below. As reported in the table,
the ratio of the intrinsic anodic reaction rate to the
intrinsic cathodic reaction rate is greater than 1.1, -~ ~-
and the additive printed circuit boards prepared in the
solution failed the thermal stress test. ~
.; ~ '
,:'' ~,' '
ycc/in
, ;,''' ;~'~
. . ~ "::
-- 1 3 ~ 2 0
31
Following the teachings of this invention, the
concentration of the formaldehyde reducing agent was
reduced to lower the anodic reaction rate relative to
the cathodic reaction rate. The ratio of the
formaldehyde concentration to the copper concentration
was reduced to 0~5. The resulting solution is the 2nd
solution in the table below.
Solution -
1st 2nd
0 CUSO4 mol/1 0.02B 0.028
EDTA mol/1 0.087 0.087
Formaldehyde mol/1 0~067 0.013
pH 25C 12.5 12.5
CcH2o]~oH-]~s (m/1)~5 0.012 0.002
15 NaCN mg/l 20 20
v2os mg/l 3 3
Temperature C 30 30
Emp vs. SCE mV -783 -750
ra' mA/cm2 0.341 0.323
rc~ mA/cm2 0.280 0.304
Ratio' 1.22 1.06 ~ ~-
The second solution is used to plate additive
printed circuit boards with copper 25 micrometers thick.
It is difficult to initiate electroless plating on
catalytic adhesive and catalytic base materials at low
temperatures and low formaldehyde concentration.
Therefore, before plating the additive circuit boards,
the conductive~pattern including the plated through
holes is covered with a thin layer of copper about 0.2
micrometers thick in an electroless strike solution
which has a formaldehyde concentration of 0.13 -~
moles/liters.
ycc/in ~ ;~
,". ~.
~` 1331~2~
32
These additive printed circuit boards from the
second solution pass the thermal stress test,
demonstrating that maintaining a :Eormaldehyde to copper
ratio in an electroless plating solution less than 1.2 : .:
can provide fissure free copper dleposits.
- .
ycc/in : ~
... .