Note: Descriptions are shown in the official language in which they were submitted.
; 1 332286
METHbD OF REFINING OXIDE NICKEL ORE OR THE LIKE
Background of this invention
Field of the invention
This invention is related to method of producing a
ferro-nickel luppe containing iron and nickel by using a
rotary kiln with a drying and pre-heating means, such as a
grate, a shaft furnace or the like. This method comprises
steps of dividing the nickel oxide ore into two groups, dry
crushing the first group nickel oxide ore, while wet
crushing the second group nickel oxide ore, mixing and
kneading the dry crushed and the wet curshed
nickel oxide ores, together with a solid reducing agent,
such as cokes or the like, forming the resultant mixture
into briquettes, drying and preheating the briquettes using
the grate or shaft furnace of the rotary kiln, and sintering
the resultant preheated briquettes using the rotary kiln,
thereby and other metal compronents contained in the nickel
oxide ore and simulataneously causing growth of nickel and
other metal component as luppe or growth of nickel and other
metal component as luppe or particles and obtaining the
luppe containing iron and nickel. In detail, in the
briquette formatlon step, large amounts of the solid
reducing agent such as cokes and slag formation agent such
as limestone are preliminarily uniformly incorporated in the
~ ~ :
~: : :: ~
. .
:. ~
1 3~2~86
briquettes and also the water con-tent of the brique-ttes is
optimized to a modera-te quantity. As a result, the reducing
reaction of the nickel or other metal compornents is
promoted and the slag formation has a remarkable increase
during sintering step of the briquettes. Furthermore, a
large reduction of heat energy consumption and dust
scattering is carried out during drying and pre-heating
step using the grate or shaft furnace.
Description of the prior art
Nickel is an essential component of the austenitic
stainless steel. Usually, nickel silicate ores, such as
garnierite or the like, are improted to Japan as nickel ore,
which is to be used as a raw material for nickel. Nickel
silicate ores are refined, thereby obtaining nickel ~ -;
component as ferro-nickel. Nickel silicate ore to be used
as a raw material is usually powdery and has a large amount
of water therein. Consequently, nickel silicate ores are
usually refined as followed.
Generally, the main material, such as nickel silicate
ore and the auxiliary material, such as cokes, limestone,
etc., are respectively subjected to an addition of large
amount of water, thereby forming respective slurry
materials. Each of slurry materials is subjected in
sequence to steps, such as wet crushing and mixing and
kneading.
-2-
. ~ . -: : . : ~, -: . : :
; -
-.,
.~ . .
~ 332286
The resultant mixture is dehydrated and then
pelle-tized, into pellets or the like, which do not contain
cokes in an amount necessary to reduce nickel or other metal
components contained therein. Owing to, effecting steps,
such as drying, pre-heating and sintering of the pellets or
thelike using the rotary kiln, an addition of the cokes to
the pellets or the like is necessiated at the instance of
feeding the rotary kiln with the pellets or the like.
Namely, furnishing the rotary kiln with excessive cokes
effects a good sintering process, in which arises a
reduction of nickel or other metal components, and a growth
of reduced nickel or metal as luppe.
The luppe is combined with slag to produce clinker to
be discharged. In a subsequent process, ferro-nickel is
separated and obtained from the discharged clinker.
In this method, since large amounts of water are
required to be added to the main and auxiliary materials to
form the slurry materials before wet crushing, step, water
removal from the slurry mix-ture using a filter or the like
is necessiated after mixing with slurry materials. However,
the conventional drum filter or the like can not effect a
sufficient dehydration of the slurry mixture. In addition,
since the individual slurry materials are mixed in the form
of slurry, it is difficult to uniformly mix the materials.
Particularly, it is difficult to uniformly disperse cokes,
which has a large specific gravity difference, in a large
','. ' ' ~ '
1 33228~
amount and uniformly.
"Nippon Kogyo Kaishi" (Japanese Mining Industry
Bulletin) No. 97-1122 issued in August, 1981, p-p. 792-794.
discloses a prior art method of refining nickel oxide, which
is shown in Fig. 3 as a flow sheet. Nickel ore as the main
material and cokes and limestone as the auxiliary material
are separately crushed by wet crushing in tube mills with
addition of large amounts of water to obtain slurries with
water content of about 50%. The individual slurries are
collected in a storage tank and mixed. Then, a desired
polymer coagulant is added to the mixture, and the mixture
with the polymer is supplied to a drum filter for
dehydration. The degydrated material is then rendered in-to
cakes. The cakes are then supplied to a briquette former to
form briquettes, which are then fed to a rotary kiln with a
grate. In the rotary kiln, briquettes, are subjected in
sequence to steps, such as drying on the grate, preheating
and sintering. However, with a dehydration of some nickel
ores having very inferior dehydration property, by using a
conventional drum filter or the like, the nickel ores can
not reduce their water content upto about 30% or lower. In
the dehydrated nickel ores remains a great amount of water,
and the w&ter content of the dehydrated nickel ores varies
greatly. Consequently, it is very difficult to form the
dehydrated nickel ores in a briquette with a good shape.
Additionally, drying and pre-heating the dehydrated
-4-
r
: '
1 33228~
nickel ores necessiates a great quantity of heat energy.
In a further aspect, the main and auxiliary materials
are mixed in the form of slurry in the storage tank. In
this case, however, segregation of the cokes and so forth is
liable to result and it is difficult to obtain uniform
mixing. This is because the main and auxiliary materials
are greatly different in the specific gravity and viscosity.
This lack of uniformity presents problems at the time of the
reduction of metal components by sintering. Further, since
it is difficult to obtain uniform mixing of materials, only
a small quantity of cokes can be incorporated into briqettes
or the like. Therefore, the coke content is liable to
fluctuate, and the briquettes obtained are fragile and
readily liable to crumble. This leads to the problem of
generation of the so-called ring on the rotary kiln inner
surface. Thus, smooth operation can not be ensured, and it
is extremely difficult to improve the productivily.
Summary of the invention
The invention has been intended to solve the problems
discussed above, and its object is to provide a method of
porducing a ferro-nickel luppe by refining nickel oxide ore,
in whcih at the time of mi~ing and kneading the main
material consisting of nickel oxide ore and the auxiliary
material consisting of limestone and cokes after crushing of
the material, cokes and solvent can be uniformly dispersed
-5-
. .
~ ,. . - .
.
: : . :: ~ - : :
` 1 332286
and incorporated in amount in excess of an amount necessary
for the reduction of nickel and other metal components in
the main material, so as to promote a reduction of nickel
and metal components and a growth of the reduced nickel or
metals as luppe during steps, such as drying step, pre-
heating step and reduction step, rotary kiln with grate or
shaft furnace, thereby permitting improvement of the
operation effeciency and productivity, and by using a high
pressure briquette former, the briquettes can get their
water content kept up a low level, so as to increase the
mechanical strength of the briquettes, and to effect a
reduction of heat energy supplied for drying and pre-heating
in the rotary kiln.
According to this invention, there is provided a
method of producing a ferro-nickel luppe containing iron and
nickel, by using a rotary kiln with a grate, a shaft furnace
or the like, in which said method comprises steps;
dividing a nickel oxide ore into two groups;
wet crushing one group nickel oxide ore, while dry
crushing the other nickel oxide ore;
adding to wet crushed and dry crushed nickel oxide ores
and an auxiliary material such as limestone and cokes in an
amount in excess of an amount necessary for the reduction of
nickel oxide and other metal components;
mixing and kneading informly said wet crushed and dry
crushed nickel oxide ores and said auxiliary material;
- :. : ,: -
:~ .
:.. : : :
~ t332286
forming the resultant mixture into briquettes having
a predetermined shape;
drying said briquettes by using said grate, shaft
furnace or the like; and
sintering said briquettes by using said rotary kiln,
thereby reducing nickel oxide and other metal components
included in said briquettes by action of said cokes and also
promoting a growth of the reduced nickel oxide as luppe.
Further, in the method according to the invention, both
the main material such as nickel oxide ore and auxiliary
material such as limestone and cokes may be crushed by
drying crushing in the crushing step, and the crushed
materials may be kneaded together with adequate quantity of
added water. -~
The constitution and functions of the invention will
now be described in detail with reference to the
accompanying drawings.
sRIEF DESCRIPTION OF THE DRAWINGS
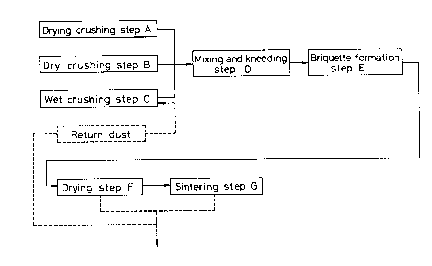
Eig. 1 is a flow sheet illustrating an embodiment of
the method according to the invention;
Fig. 2 is a view showing an example of arrangement of
equipment for carrying out the method according to the
invention;
Fig. 3 is a flow sheet illustrating a prior art method
25 of refining nickel oxide ore; and ~;
- 6a -
: : , : : . :.
1 33228S
Fig. 4 is a graph showing the relation between various
mixing/kneading methods and results of fall test on obtained
briquettes.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
As shown in Figs. 1 and 2, nickel oxide ore as the main
material is classified into at least two groups according to
the dehydration property. One group of ore, i.e., ore 1a,
the dehydration property of which is in~erior, is crushed in
a drying crushing step A. The other group of ore, i.e., ore
1b, which has satisfactory dehydration property, is crushed
in a wet drying step C.
In the drying crushing step A, the ore 1a is crushed in
a dry state. The ore 1a is usually crushed into particles
with diameters around 2mm. Usually, this step is performed
with an arrangement as shown in Fig. 2, in which a crusher
1, e.g., a crushing mill, a cyclon 2 and a bag filter 3 are
coupled together. Dry air is supplied from a hot air
furnace 4 to a crusher 1. Part of the ore usually contains
20 to 40 ~ of water and has a diameter of about -300mm. It
is crushed in a dry state in a crusher 1, to a grain size of
about -2mm, and is then dried until it contains less than 10
% of water. More specifically, hot air is supplied from the
hot air furnace 4 to a crushing section of the crusher 1,
and the crushed material is discharged together with hot
water. By crushing the material in the presence of the hot
air stream, it is possible to obtain a uniform grain size,
control the grain s~ze according to the amount of hot air
supplied and remove water sufficient to obtain satisfactory
.~
, ~ . : - . . . .
}~
,
1 332286
drying.
The dried crushed material is collected in the cyclon 2
and bag filter 3 and temporarily stored in a storage tank
3'.
In the wet crushing step C, ore 1b having satisfactory
dehydration property is crushed in the form of a slurry.
This step may be performed using any arrangement. Usually,
however, an arrangement as shown in Fig. 2 is used, which
comprises a wet tube mill 5 and a plurality of slurry tanks
6, these being coupled to a dry filter 7. The ore 1a having
inferior dehydration property, which also usually contains
25 to 40 % of water and has a diameter of about -300mm, is
crushed together with added water in the wet tube mill 5 to
obtain a slurry containing about 50 % of water. Thus slurry
is temporarily stocked in the slurry tanks 6. Subsequently,
it is progressively dehydrated in the drum filter 7 to
obtain cakes containing 28 to 35 % of water.
The auxiliary material 1c such as cokes and limestone
is usually crushed in the dry crushing step B. These
crushing materials have differenct crushing properties from
that of the ore. Also, they contain less water. Therefore,
the dry crushing step B, unlike the drying crushing step A,
is performed with an arrangement, in which a tube mill 8
which does not require any screening and a storage tank 9
are coupled together. With this arrangement, the auxiliary
material 1c with a diameter of about -100mm is crushed by
dry crushing, i.e., in situ, in the tube mill 8 to a grain
size of about -2mm. The crushed material is temporarily
- 8 ~
. ~
.. . .... ~, , j ~
.
, ... .. . . . . . .
", ., : ., , . ~
.; , ' ' . .
1 33228~
stored in the storage tank 9.
When re-using return dust, it may be added in the next
mixing/kneading step. Further, it may be rendered into the
form of a slurry in the slurry tank 6 in the wet crushing
step C, and the slurry thus obtained may be added to the ore
slurry after the wet crushing, the mixture being then
dehydrated in the drum filter 7 to obtain cakes.
The grain size of each crushed material may be as small
as possible from the standpoint of increasing the crushing
strength of briquettes. For this reason, according to the
invention the material is crushed to a grain size of about
-3mm. If the grain size is above -3mm, briquettes having a
predetermined mechanical strength can not be obtained with a
high yield (over 90 %) even by taking the crushing in the
following mixing/kneading step into consiaerations.
The individual materials with the controlled grain size
of about -3mm are mixed together in adequate quantities and
kneaded in the mixing/kneading step D. At this time, the
water content is adjusted to 10 to 20 %, preferably 15 to 20
%, from the considerations of the moldability of the
briquettes or the like and also the saving of heat energy
for subsequent drying and pre-heating. Further, the cokes
in the auxiliary material should be added in a great amount
in excess of the amount necessary for the reduction of
nickel and other metal components in the total ore,
particularly including an amount of energy which is consumed
in combustion.
More specifically, to obtain predetermined moldabllity
'~'
' ~ :
_ g _
.
. :
. . . ~ . .
,:: : . , ~ . :
l 332~86
and mechanical strength of briquettes or the like in a
subsequent briquette formation step E, a certain amount of
water is kneaded. If the amount of water is insufficient,
the mechanical strength is reduced.
However, if the amount of water is excessive, the
briquette formation property is spoiled. In addition, a
great amount of heat energy is required at the time of pre-
heating drying, so that sometimes sufficient drying can not
be obtained with the exhaust gas produced by reduction
combustion. From this standpoint, the upper limit of the
water content is preferably about 20 %. Further, it is
necessary to incorporate a great amount of cokes in the
auxiliary material. More specifically, the cokes should be
incorporated in an amount in excess of an amount which is
necessary for the reduction of niçkel and other metal
components. It is to be appreciated that the method
according to the invention, unlike the prior art method,
permits formation of briquettes or the like, which contain
cokes and solvent in an amount in excess of an amount
necessary for the reduction of the metal components and have
high mechanical strength. This constitutes one feature of
the invention.
If cokes are incorporated only by an amount necessary
for the reduction of nickel and other metal components, 50
to 80 % of the incorporated cokes is consumed for the
combustion, resulting in the shortage of cokes necessary for
the reduction metal components. For this reason, it is
necessary to incorporate cokes in an amount in excess of the
-- 10 --
~ ' , ~: `" ' ' . . ' . . . ' : .
` . ,.-
~' ' ` ' , ' ' ' ' `: ' '
, ' ' ~
. . . :~
.
.
:
' ' .' ' '. '' .' " ~ '
1 332286
amount necessary for the reduction of nickel and other metal
components. Actually, it is necessary to incorpoxate two to
five times the amount of cokes necessary for the reduction,
although the amount depends on the furnace operating
conditions.
In the mixing/keading step D, cokes are incorporated in
the great amount as noted above, and the water content is
adjusted in the amount noted above. Further, it is
necessary to uniformly disperse the great amount of
incorporated cokes. If water and incorporated material are
not dispersed uniformly, it leads to a reduction of the
mechanical strength of briquettes, which are formed as
pillow-shaped or armond-shaped briquettes with a diameter of
20 to 30mm after the kneading of the material. This means
that the kneading should be carried out more than is done in
the case of the prior art method. For this reason,
according to the invention a mixer 10, e.g., a pug mill, and
kneader 11, e.g., a rod mill, are directly coupled together
as shown in Fig. 2. Particularly, it is preferred to
provide plural kneaders 11 such as rod mills. The materials
are thus preliminarily mixed in the mixer 10 such as a pug
mill, and the mixture is kneader while it is slightly
coarsely crushed in the kneaders 11 such as rod mills.
During this time, the particles of the materials having
already been crushed are further crushed by the rod mill, so
that they are uniformly mixed as they are kneaded. That is,
water and cokes are uniformly dispersed to enhance the
briquette formation property.
- 11 -
,
:, - ~; . .
:- . .: : : : , -
.
: :~ ~ , . . :
. . - .
1 33228n
The use of rod mills not only for the crushing but
also for the kneading is disclosed in Japanese Patent
Publication No. 6256/68. ( Date of filing: March 23, 1964
Application No. 15671/64 Applicant: Yahagi Seitetsu
Kabushiki Kaisha ) In this technique, however, a ball mill
and a rod mill are directly coupled together to prepare
pellets from powdery iron ore, and these crushers are
provided not only with a function of comminution but also a
function kneading. This technique, therefore, is different
from the technique of the method according to the invention,
in which the kneader and crusher are used for the purpose of
dispersing a large amount of cokes in the material. More
specifically, the disclosed technique noted above is based
on the preparation of pellets by comminuting powdery iron
ore and through subsequent steps of kneading, addition of
water, granulation, etc. The disclosed technique permits
improvement of this process by omitting the comminution step
and simultaneously effecting a certain extent of comminution
and kneading with the ball mill and rod mill. In other
words, while the ordinary pellet preparation process
inpcludes a comminution step and a kneading step, in the
disclosed technique the comminution and kneading are
effected with the ball mill and rod mill without
preliminarily comminuting the powdery iron ore. It is a
feature of the disclosed technique that the comminution and
kneading are carried out in a wet state and without use of a
pug mill or like kneader.
In contrast, by the method according to the invention
- 12 -
-- . . ,: . :.
~: . . , . - .
1 3322~6
the materials are preliminarily crushed by drying crushing,
wet crushing, etc. be~ore the mixing and kneading. In
addition, a large quantity of auxiliary material is
incorporated uniformly. Further, as the mixing/kneading
equipment a pug mill ~r like mixer is provided, and a rod
mill or like kneader is provided after and coupled to the
mixer. More specifically, as shown in Fig. 2, kneading is
performed to a certain extent in the mixer 10. By this
operation, the auxiliary material is uniformly disperesed,
that is, the materials are mixed to a considerable extent.
This mixture is further kneaded in the rod mill, which has a
function of coarse crushing. In this kneading step, the
falling of rod rather promotes the kneading.
The uniformly knead material thus obtained is
temporarily stored in a material yard 12 before being
supplied to the next briquette formation step E.
In the above description, the material having been
crushed by wet crushing and material having been crushed by
drying crushing are mixed under the conditions noted above.
However, instead of crushing materials separately by wet
crushing and drying crushing, it is possible to crush all
the materials in the proportions noted above, and kneading
the mixture with10 to 20 % of added water.
In the briquette formation step E, the material is
supplied to a briquette formation apparatus 13' for
formation of briquettes. In this step, the material may be
formed into a pellet-like form. Usually, however, pillow-
shaped or almond-shaped briquettes with a diameter of 20 to
1 3322~6
60mm are formed. Pillow-shaped or almond-shaped briquettes
may be dried very satisfactorily in the subsequent drying
step. In addition, during this drying step no substantial
powdering results. Further, heat energy necessary for the
drying can be greatly reduced.
After the drying step E, the briquettes or the like are
supplied to a sintering step G. The drying step F is
carried out in a grate 13 of a grate type rotary kiln as
shown in Fig. 2. The sintering step G is carried out in a
rotary kiln 15. More specifically, in the grate type rotary
kiln shown in Fig. 2, the briquettes are first charged into
the grate 13 to be dried with exhaust gas led from the
rotary kiln 15 and supplied from above the grate 13. Since
the briquettes are of pillow shaped or almond-shaped, they
are dried very satisfactorily. Also, since an adequate
amount of water is provided, the mechanical strength of the
briquettes is further increased when they are dried, and no
powdering results. More specifically, the briquettes
prepared contain a large amount of cokes as noted above and
contain a comparatively small amount, i.e., 10 to 20 %, of
water. Therefore, as the briquettes are dried on the grate
13, their temperature can be elevated substantially
linearly from normal temperature up to 400 C. In contrast,
briquettes prepared in the prior art method contain much
water and have inferior molding property, so that their
temperature can be elevated only up to about 200 C even
when they are dried on a grate. In addition, a long time of
pre-heating in a rotary kiln is required.
_ 14 -
: . . .- . . . , . - ~ -
1 3322~6
After the drying, the briquettes are charged into the
rotary kiln 15 from an inlet thereof over a shoot 14. In
the rotary kiln 15, the briquettes are pre-heated and
sintered. During the sintering, nickel and other metal
components in the briquettes are~reduced in a state
surrounded by a large amount of cokes. As the briquettes
approach an outlet of the rotary kiln 15, the metal
components grow as luppe ( i.e., metal particles), which are
discharged as clinker contained in slag.
The discharged clinker is cooled and then usually
separated by magnetic separation or other separation means
to be recovered as an alloy of iron such as ferronickel.
The dust produced during drying of the briquettes on
the grate and also during the sintering in the rotary kiln,
which is reduced as will be described hereinbelow, is
returned for re-use to the wet crushing step C as mentioned
before.
The rotary kiln has a conventional construction with a
burner 16 provided at the outlet. At the burner 16, fuel,
e.g., fine powdery coke, heavy oil, gas, etc., is burnt, and
the temperature of the briquettes in the rotary kiln is
elevated by the heat energy thus generated.
Further, in the rotary kiln 15, the temperature of the
briquettes is gradually elevated as they proceed over the
grate 13 from the inlet to the outlet. The briquettes are
heated up to about 400 C and to such an extent that they
hardly contain water. Their temperature is elevated
linearly. When they proceed slightly in the rotary kiln
- 15 -
' : -
: ~ :- . : : . .
- , .
~,
-: 1 3~2286
(e.g., by 35m from the inlet), the metal components are
reduced very satisfactorily mainly because of the presence
of a large amount of cokes. As the reduction proceeds in
this state, the partial pressure of oxygen in the material
5 layer is reduced, and FeO and the like are increased. When
FeO is combined with SiO2, fayalite (2Fe-SiO2) or the like
low-melting compound is produced. Such a compound is liable
to be attached to and grow as a ring on the inner wall of
the rotary kiln. This phenomenon is liable to occur
10 particularly in the presence of a powdery material, and it
constitutes a significant problem in the operation of the
conventional rotary kiln. In the method according to the
invention, the briquettes have a high mechanical strength.
Thus, the generation of powdery material is suppressed, to a
15 small extent if any. The ring thus can be hardly formed, so
that very smooth operation can be ensured.
Heretofore, it has been in usual practice, for pellets
as source of iron, to incorporate as much cokes as possible
and utilize fine powdery coke in place of petroleum system
20 energy. By way of example, "Tetsu-to-Hagane" ( Iron and
Steel ) issued in 1982, No. 15, p-p 2231-2237 and p-p 2238-
2245 discloses pellets, which contain at most about 1 % of
fine powdery coke as means of replacement of petroleum
energy with coal energy to cope with the early oil shocks.
25 In these pellets, the incorporated coke does not serve for
reducing metal components such as iron, but it only serves
as fuel. These pellets are refined as ore in a blast
furnace. The amount of coke is determined such that no
- 16 -
... . . . - ::
- ~
: `
~ 33228~
powdering results in the furnace, i.e., in a range, in which
a crushing strength or falling strength in excess of a
predetermined level can be ensured. For this reason, the
coke is incorporated in an amount of 1 % at most. In
contrast, according to the invention use is made of a rotary
kiln, which serves as a reaction furnace corresponding to
the blast furnace to promote reduction of nickel and other
metal components. In other words, as much cokes as possible
are incorporated to provide a state, in which metal
components and ore particles are surrounded by cokes to
obtain quick and smooth reduction of the metal components by
gas reduction.
Next, this invention will be described in connection
with its examples.
Example 1
Nickel oxide ores A to D having compositions as shown
in Table 1 were each crushed in two groups. At the first
group, 380kg, 50kg and 130kg, in dry weight, of the ores A,
B and D, respectively, were crushed. The average grain size
20 of the ores was less than 300mm (400mm at the maximum).
This crushing was done by drying crushing in the drying
crushing step A shown in Fig. 2. As the second group, 250kg
and 200kg of the ores B and C were crushed. The average
grain size of the ores was the same as that of the ores in
the first group. This crushing was done wet crushing in the
wet crushing step C. The crushing of the first group of
ores was carried out in a ball mill while supplying hot air
supplied from the hot air furnace. The grain size after the
-:
- 17 -
~ 332286
crushing was adjusted at this time by hot air to -2mm, and
the water content was reduced to about 5 % by drying. The
resultant crushed ore was stocked in a stock tank. The
crushing of the second group of ores was carried out by
adding water such that the total water content isabout 50 %
to obtain a slurry and crushing this slurry in a wet tube
mill to a grain size of about -2mm. The resultant slurry
was stored in the slurry tank, and 75 kg of return dust
(having a composition as shown in Table 4) was added to the
slurry. The resultant system was then dehydrated to reduce
the water content to 30 %.
As the auxiliary material, cokes and limestone were
crushed in the dry crushing step B. 130kg of cokes A
(having the composition shown in Table 2 and water content
of 10 %) and 70kg of limestone ~having the composition shown
in Table 3 and water content of 3 %) were continuously
crushed to obtain a crushed material with a grain size of
-2mm and water content of 6.1 %.
Table 1
Composition of nickel oxide ore (wt%)
. '
Ore g.loss SiO2 Fe Al23 Ni ~gO Water Content
Ore A 8.89 46.48 11.73 1.99 2.32 21.25 27.9
Ore B 10.62 41.68 12.68 0.93 2.47 24.11 25.4
Ore C 10.45 43.12 11.92 0.82 2.50 23.80 24.2
Ore D 10.29 42.50 14.96 0.99 2.28 20.36 30.0
,
- 18 -
.. . ~ . ~ , . . . ..
-: . . . :
'.''''- ~ ., :, . ~ . .
:: . . : :.,: ': ' - : .
Table 2 t 332286
Composition of cokes (wt%)
Ash Volatile Fixed Phosphorus Sulfur Kcal/kg
component component carbon
Coke A 13.5 5.9 80.6 0.003 0.16 6500
Coke B 11.7 2.4 85.9 0.030 0.62 7018
* Coke B is a coke sleeve
Table 3
Composition of limestone (wt%)
Cao Ig.loss
54.6 43.3
Table 4
Composition of return dust (wt%)
:
Ig.loss CSiO2 Fe Al23 CaO MgO Ni -~
1.75 11.1736.03 12.00 1.98 2.39 17.08 2.43
The individual crushed materials were mixed and kneaded
in the mixing/kneading step D. At this time, the
.
composition was set such that the water content was 17 to 19 ;~
96, the cokes were 1 30kg, and the limestone was 70kg. The
step D was carried out by coupling together a pug mill and
two rod mills, and water and a large amount of cokes were
uniformly dispersed.
- 19 - ' :~
~:~
::
~` - : : . .- ~ '
:
t 332286
Subsequently, pillow-shaped briqettes having a diameter
of about 40mm were formed by molding in the briquette
formation step E using the briquette forming machine. A
fall test was conducted on the briquettes thus formed by
causing the briquettes to fall repeatedly five times from a
position at a height of 2m. 95% or more briquettes were not
cracked.
The briquettes were then dried in the drying step F
using the grate (with a length 17m). The drying was carried
out by introducing exhaust gas (at 550 C) as opposed stream
from the rotary kiln. At this time, very satisfactory
drying could be obtained with the briquette temperature at
390 C at the outlet of the grate. Substantially no
powdering due to bursting or like cause resulted, and the
amount of recovered return dust was 42kg.
After the drying, the briquettes were charged into a
rotary kiln (with a length of 70m) from an outlet thereof to
be pre-heated and sintered.
At this time, the briquette temperature was elevated
linearly as they proceeded through the rotary kiln. At a
position at a distance of 20m from the inlet (and at a
distance of 50m from the outlet), the temperature reached
700 C, and decomposition of crystalized water began.
Subse~uently, the reduction proceeded, and the temperature
at a position at a distance of 1Om from the outlet was 1,320
C. Further, the same temperature was maintained even in a
luppe formation zone covering 1Om from the outlet, and
sufficient growth of luppe was recognized. The nickel
,
-- 20 --
. - , ' .' ' '
- ,,
.: - , : ,,
,:.
1 332286
reduction ratio was 96.5 %.
As a contrast, the auxiliary material was added to the
ore slurry with the water content of 50 ~ obtained from the
second group of ores through wet crushing in a slurry tank
such that the coke content was 80kg. However, much
segregation occurred in the slurry tank, so that it was
difficult to obtain uniform mixing. Accordingly, the slurry
was dehydrated in the drum filter to reduce the water
content to about 30 % and was then formed into cakes. From
these cakes briquettes with a diameter of 12mm were formed.
Since the water content was high at the time of the
briquette formation, the briquette formation machine was
heated by steam heating to progressively dry the briquettes
on the grating. In this case, the moldability of the
briquettes was inferior, and the water content was up to 15
%. The briquettes were pre-heated and sintered in the -~
manner as described above in the rotary kiln. Since the
amount of incorporated coke was small, 80kg of coke was
externally supplied for pre-heating and sintering of the
briquettes in the rotary kiln. The status in this case is
as shown in Table 5. The nickel reduction ratio was 95.0
- 21 -
: :
Table 5 1 332286
Method according Comparative method
to the invention ¦
Amount of incorporated cokes 130kg 80kg :-.
Amount of externally supplied O 80kgcokes
Water content in briquette 17 - 19% 29 - 32%
.__
Water content in briquette 1 - 1.5% 15%after drying
. _. .,
Gas temperature immediately 150 C 220 C
after drying of briquette
. . .
20m from inlet Temperature of 700 C 600 C
material (C)
Furnace status Deoomposition of Pre-heating
_ crystallized water
30m from inlet Tenperature of 820 C 700 C ~:
material (~C) :~
Furnace status Decomposition of Deoomposition of
.. _ limestone crystallized water ~:
35m from inlet Temperature of 830 C 820 C :~:
material (C)
Furnace status Prcgrees of Deoomposition of :~ :
reduction limestone
50m from inlet Tbmperature of 1060 C 1100 C :~
meterial (C)
Furnace status Start of luppe Process of reduction
. _ formation (generating of ring)
60m from inlet Temperature of 1320 C 1330 C
material (C) ~ :~
Furnace status Formation of luppe Start of luppe ~:
formation
.. .. . . . _
as far as 10m Temperature of 1370 - 1200 C 1370 - 1050 C
from outlet material (C)
Furnace status Grcw~l of lupFe Growth of luppe
- 22 -
. .
: :
.
.... ~, : ~;: :
~ ~ :
.
c~
~--
~:.-. - : : :
1 332286
Further, on the basis of the above results, nickel
oxide ore was actually processed by the method according to
f the invention and the contrast method, and the operation
results per ton of dry ore were as in Table 6 below.
Table 6
Method according toComparative method ;
the invention
Amount of cokes130 kg 160 kg
Lime as fuel 47 kg 78 kg
10 Amount of return75 kg 300 kg
Amount of 262kg 715 kg
incorporated water -~
Amount of supplied 1135 X 103 kcal 1575 X 103 kcal
15 heat energy _
Example 2
In this example, unlike Example 1, the second group of
ores was crushed by drying crushing like the first group of
ores. Auxiliary material was added to crushed materials in
the same way as in Exaple 1. Water was added at the time of
subsequent kneading to obtain kneaded material as in Example
1. Subsequently, briquette formation, drying, pre-heating
and sintering were carried out in the same way as in Example
1. The same results as shown in Table 5 were obtained. ~-
ExamPle 3
When kneading the three different crushed materials
obtained in Example 1 by ad;usting the water content to 18.5
- 23 -
. .
~i
~;,' , ~, ~ :
1 33228~
and coke amount to 130 kg.
(A) a sole rod mill
(B) a sole pug mill
(C) three pug mills
(D) a combination of pug mill and rod mill
were used as kneader, and diffusion of water and cokes and
moldability at the time of briquette formation were
evaluated.
In the case of a sole mill in (A), both water and cokes
could be dispersed uniformly because of a water content of
about 18.5 %. However, the yield of briquette formation was
insufficient.
In the case of a sole pug mill in (B), cake-like
particles remained in the kneaded material due to
insufficient kneading. In the case of using three pug mills
in (C), the molding yield was inferior due to insufficient
kneading due to the same reason.
In the case of use of the combination of pug mill and
rod mill in (D), satisfactory diffusion of water and cokes
and very satisfactory kneading could be obtained because the
materials were mixed in the pug mill and then kneaded in the
rod mill.
Subsequently, briquettes with a diameter of 40mm were
formed from the kneaded materials (all with water content of
18.5 %) obtained in the cases (A) (C) and (D), and a fall
test of these briquettes was made. The results were as
shown in Fig. 4. In the test, the briquette was aliowed to
fall onto a concrete floor from a position at a height of
- 24 -
~;- - . . ... ~
.. .
~, :
.
~-
.~ . .. .
1 33228~
2m five times repeatedly. In Fig. 4, shaped portions show
rates of generation of cracks and, especially, the cross~
hatched portions show rates of generation of 5 mm and less
cracks. ~
From these results, it was found the case of (D) was :
most effective with a yield of 96 % obtained.
- 25 -
" .': ' : , .
`~` ` ' :
.;