Note: Descriptions are shown in the official language in which they were submitted.
~,
~ 2 7 ~ ~
METHOD FOR PURIFICATION OF WASTE WATER
BACKGROUND OF THE INVENTION
Field of the Invention:
This invention relates to a method for the
purifica~ion of waste water. Particularly, this invention
relates ~o a method for the purification of waste water
containing chemical oxygen-demanding substance~ (hereinafter
referred to as "COD component") by wet oxidation. More
particularly, this invention relates to a method ~or
effective purification of waste water containing a COD
component, i.e. harmful oxidizable organic or inorganic
substances (herein after referred to 'limpurity"), which
method effects the purification o~ the waste water by
subjecting the waste water to wet oxidation in the presence
of molecular oxygen thereby converting the organic
~ubstances into harmless compounds ~uch as carbon dioxide,
water, and nitrogen.
Description of the Prior Art:
Among the methods currently available for the
treatment of waste water, the bioohemical method called the
aotivated ~ludge method and the wet oxidation method called
the Zimmermann method have been renowned.
For the wet oxidation method, use of a varying
oxidizing oatalyst ~or the purpose oP heightening the
reaotlon rate has been proposed. Further, ~or the wet
oxidation method, no matter whether a oatalyst is absent
~rom or present in the reaotion site, the slngle cylinder
type reaotion column is used as the reaotion vessel.
The aotlivated sjlud~e method oonsume~ a long time ~or
the deoomposition of organic substances and requires the
waste water to be diluted to a concentration fit ~or the
growth of algae and bacteria and, ther~fore, has the
disadvantage that the facilities for the treatment of
aotivated sludge oocupy a large floor space. Further, in
reoent years, the handling of the surplus grown sludge has
been entailing an immense expenditure particularly in urban
" :~
:~ ' ':
':
~3~2~
districts. The Zimmermann method comprises oxidatively
decomposing organic substances in an aqueous solution by
introducing air into the aqueous solution of the organic
substances under a pressure in the range o~ 20 to 200
atmo~pheres at a temperature in the range o~ 200 to 370C.
Since the reaction rate is low and the decomposition
consumes a long time, this method necessitates a large
reaction ve~sel made o~ a highly durable material and
attains no real economy because of expensive equipment and
expensive operation. Since the liquid phase within the
reaction ve~sel cannot be retained when the reaotion
temperature is elevated by the heat of reaction, this method
has the disadvantage o~ being incapable o~ e~ectively
treating waste water whose COD component has a high
calorific value. Also for this method, use of a varying
oxidizing catalyst for the purpose o~ heightening the
reaction rate has been proposed. For the use of such an
oxidizing catalyst, none o~ the conventional methods Por
wa~te water treatment is speci~ically devised to relieve the
reaction vessel of the heat o~ reaction.
Particularly in the treatment o~ highly concentrated
by the conventional wet oxidation method, the amount o~ the
heat generated by the reaction i9 conspicuously large.
Since the liquid pha~e within the reaction ves~el cannot be
retained when the temperature thereo~ i9 elevated in
consequance o~ the increase in the heat o~ reaction, it
becomes necessary to curb the amount o~ generated heat by
diluting the waste watar under treatment. The dilution has
as a problem the liability to inorease the amount o~ wa~te
water to be treated. Even where the waste water has a low
COD content and the amount o~ the heat generated by the
reaction is small, the heat generated still goes to
elevating the liquid temperature and inevitably requires the
reaction pressure to be unduly increased for the purpose o~
keeping the waste water in a liquid state. Thus, the
treatment involving the use o~ an oxidizing catalyst attains
~3327~L~
no real economy because of an addition to the expense of
equipment and that of operation.
An object ~f an aspect of this invention, `
therefore, is to provide an improved method for the
purification of waste water.
An object of an aspect of this invention is to
provide a method capable of effective purification of
waste water containing a COD component, i.e. harmful
organic or inorganic substances, by subjecting the waste
water to wet oxidation in the presence of molecular
oxygen thereby converting the harmful substances into
such harmless compounds as carbon dioxide, water, and
nitrogen.
SUMMARY OF THE INVENTION
The objects described above may be accomplished by
a method ~or the purification of waste water by the use
oP a heat-exchanger type reaction vessel composed of a
plurality of inner tubes and a shell defining jointly
with the outer peripheries of the inner tubes a passage
for the flow of a heat transfer medium, which method
comprises passing the waste water through the inner
tubes and, at the same time, feeding a molecular oxygen-
containing gas to the flow of the waste water thereby
establishing contact between the waste water and the
feed gas and consequently effecting wet oxidation of the
impurities present in the waste water.
Another aspect of this invention is as ~ollows:
A method Por puri~ying waste water comprising, ~
providing a heat-exchanger type reaction vessel having a
shell and plurality of tubes wlth outer paripheries,
said shell dePining jointly with the outer peripheries
oP the tubes a passage for the flow of a heat transfer
medium around the outer peripheries of said tubes, (2)
passing said waste water through said tubes and, at the
same time, (3) feeding molecular oxygen-containing gas
to the flow of said waste water thereby establishing ;
- 3 -
:, ': ';
~; ~ ~.`''''''
k
~ 3.~27~
contact between said waste water and said molecular
oxygen-containing gas to effect wet oxidation of
impurities present in said waste water, said wet
oxidation being carried out in the presence of a
catalyst for oxidation of said impurities, and passing
said heat transfer medium through said passage, said
heat transfer medium being in contact with said outer
peripheries of said tubes and not in contact with said
waste water~
The conventional wet oxidation (Zimmermann) method
using the single cylinder type reaction column and not
involving use of any catalyst has been incapable of
effectively treating waste water containing a COD
component in a high concentration because it pays no due
consideration to relieving the reaction column of the
heat of reaction as pointed out previously as the
problem confronting this method. In ~act, when the
waste water subjected to the treatment is in a highly `;
concentrated form, the amount of the heat generated by
the reaction is so large khat the temperature of the
liquid phase with in the reaction column is elevated
conspicuously and the water therein is suffered
', ! ; I ~ ~ ! `
-3a-
' :
to pass wholly into the vapor phase and the reaction can no
longer be continued. Further, the reaction of this wet
oxidation by nature suffers the reaction rate to increase in
proportion as the reaction temperature is elevated. When
the elevation of the reaction temperature is large,
therefore, the reaction itself is acceleraked possibly so
much as to render the control of the reaction dif~icult.
We have continued a diligent stud~ to ~ind that the
use o~ a heat-exchanger type reaction vessel as a reactor
con~igured to ensure thorough removal of the heat o~
reaction is highly effective in the treatment o~ waste
water.
This heat-exchnager type reaction vessel itself
shares a common type with reaction vessels ~requently used
in various vapor-phase oxidation reactions. It has not been
employed, however, for the wet o~idation method. A reaction
system which combine the heat-exchanger type reaction vessel
and the single cylinder type reaction veqsel has never been
adopted for the operation o~ the wet oxidation method~ We
have found, however, that the use o~ the heat-e~changer type
reaotion vessel as a container for the wet oxidation
reaokion brings about a notable improvement in the capaoity
~or waste water treatment as desoribed hereina~tor.
Fir~t, sinoe the use o~ thi~ heat-~xohanger type
reaotion vessel permits thorough removal o~ the heat oP
reaotion ~rom the highly ooncentrated waste water whioh the
oonventional single oylinder type reaotion ves~el has been
unable to treat e;~Peotively, the treatment o~ the waste
water oan be attained by this reaotion vessel without
applioation o~ unduly high pressure. The upper limited of
the COD oonoentration in the waste water subjeated to the
treatment, there~ore, oan be inoreased ~rom the conventional
level of 8% up to 20%. Even when the COD content is low and
oonsequently the amount o~ the heat of reaction is small,
the method o~ this invention obviate~ the ne~e~sity for
unduly hei~htening the reaction pressure in due
-4-
13 ~ 2 ~
consideration o~ the elevation of the liquid temperature.
Further, the amount of the heat to be removed from the
reaction vessel can be finely controlled as by adjusting the
amount o~ the heat transfer medium being ciroulated for
cooling within the heat-exchanger part of the reaction
system in proportion to the COD concentration in the waste
water and the amount of the waste water under treatment.
The heat of reaction recovered from within the
reaction ves~el may be reclaimed in the form o~ steam by the
use o~ a steam generating boiler through the medium o~ a
heat medium or ef~ectively recovered and used ~or preheating
the wa~te water awaiting the treatment. This recovery of
the heat of reaction, there~ore, proves a generous cut in
the expense of equipment and that of operation.
In the wet oxidation reaction, the reaction rate is
increased by the elevation of temperature and, in the
meantime, the pressure ~or the retention of the liquid phase
i9 inevitably increased. In the oonventional reaction
vea~el, since the temperature at the inlet part is low and
the temperature at the outlet part i~ high becau~e of the
generation o~ heat by the reaction, the adjustment of the
pressure for the retention of the liquid phase ha~ been
e~e¢ted at the portion of the reaction vessel assuming the
highe~t temperature. A~ a result, the temperature is low
and the reaotion ratio is proportionately low in the former
hal~ part o~ the reaotion ves~el relative to the magnitude
o~ the pre~sure ~o adjusted. It has been a~oertained to the
inventors that the u~e o~ the heat-exohanger type reaction
vessel enables the entire~ reaction ve~el to be controlled
at a fixed temperature set in advance and allows the
reaotion to proceed efficiently at a fixed reaction rate
throughout the entire interior of the reaction vessel
without requiring application of unnece~ary excessive
pressure.
BRIEF DESCRIPTION OF THE DRAWINGS
.
~ 3 ~
Fig. 1 is a flow ~heet illustrating one embodiment
of thi~ invention,
Fig. 2 iq a flow sheet illustrating a conventional
method 7 and
Fig. 3 is a flow sheet illustrating another
embodiment of this invention, and
EXPLANATION OF THE PREFERRED EMBODIMENT
The reaction vessel to be used in thi~ invention i.~
a shell-and-tube heat-exchanger type reactor whi¢h compriseq
a 3hell and a plurality o~ inner tubes di~po~ed inside the
~hell and utilize~ the empty space de~ined by the inner
~urface of the shell and the outer peripheries of the inner
tube~ a~ a pa~sage ~or the flow o~ a heat tran~fer medium.
The reaction ves~el of thi~ type permits ~implification of
the configuration of reactor, facilitaters design and
maintenance of the reaotor and, at the same time, allows a
decrease in the amount of highly corrosionproo~ material for
use in the reactor part owing to the passage only through
the inner tubes of the waste water possibly containing a
corrosive ~ubstanoe, and there~ore warrant~ a reduction in
the oost of the reaotor.
The heat-exohanger type reaotion vessels
oharaoterized by the shell-and-tube oon~i~uration are
broadly dlvided into the horizontal tube type and the
vertical tube type. From the standpoint of the e~ioiency
of vapor-liquid oontaot, the vertical tube type i~ believed
to be more pre~erable ~or thi3 invention. A~ clas~i~ied by
the bundlin~ pattern o~ kubes, the heak-e~changer type
reaction ve~sel3 characterized by the shell-and-tube
oonfiguration fall under the three kinds, the ~tationary
tube plate type, the U-~haped tube type, and the loose head
type. The method of this invention manifest~ its effeot
invariably with the reaotion vessel o~ any one o~ the~e
kinds. ~s regards the direotion of the flow of the waste
water inside the inner tubes and that of the flow of the
heat tran~er medium on the ~hell ~ide, the two
6 A
combination~, i.e. the counter~low and the parallel flow,
are conceivable.
The choice between these two combinations ~orms no
critical problem. A~ the heat transfer medium to be pa~sed
on the shell side, any of the conceivable commonly
conceivable materialis such as water, steam, heat medium
quality oil, and molten ~alt may be u3ed. The circulating
rate and the temperature o~ the heat tranis~er medium are
isuitably selected, depending on the COD concentration in the
waiste water under treatment.
The inner tubeis of the reaction veissel to be used in
the present invention have an inside diameter in the range
of 10 to 100 mm, pre~erably 15 to 80 mm. If the in~ide
diameter i~ less than 10 mm, there arises an unfavorable
consequence that the reactor grows in structural complexity
and the disadvantage due to an increa ed cost of the
reaction ve~sel more than o~etis the advantage due to the
removal o~ the heat o~ reaction. Conversely, if the inside
diameter exceedis 100 mm, the removal of heat from within the
inner tubeis, particularly in the central part of the inner
tube occurs with inferior e~ficiency, the ~low o~ the
molecular ox~gen-containing gas Ped to the interlor~ o~ the
inner tubes tends to de~lect 9 and con~equently the
e~iclency Or the vapor-liquid oontact iis degraded pos~ibly
to the extent of enkailing a decline in the reaction rate.
The number o~ the inner tubes depends on the in~ide diameter
oP the inner tube~, the ~low volume o~ the waste water
~ub~ected to the treatment, and the like and is requlred to
be plural.
Thl~ lnvention is further characterized by ef~eoting
the treatment o~ waste water which, on being Qubjected to
wet oxidation, exhibit~ a calori~ic value exaeeding 20 kcal
per liter of wa3te water. The treatment is su~iciently
ef~eotive even when the calorific value is lesis than 20
kcal. Where the waste water has a calorific value o~ le~s
than 20 kcal, however, the nece~ity ~or employing the
~ 3 ~
method of this invention loses significance because `the
demerit due to the increase in the cost of the reaction
vessel excels the merit of the removal of heat by the use of
the heat-exchanger type reaction vessel. For the purpose of
improving the effect due to the temperature control of the
reaction vessel and taking and advantage of the merit due to
the rsmoval of heat, the waste water subjected to the
treatment is preferable to exhibit a calorific value
exceeding 50 kcal per liter of waste water. More
preferably, this treatment is given to waste water which
calorific value exceeds 100 kcal per liter of waste water.
In this case, the amount of the heat of reaction to be
recovered is to large as to emphasize all the more the
advantage of this heat-exchanger type reaction vessel. When
the heat exchanger type reactor of the present invention is
used, even if a temperature of the waste water to be fed is
lower than the reaction temperature, it is heated by the
heat transfer medium at the side of the reaction tube inlet
and reache~ rapidly to the de~ired temperature. This
increases u~e efficiency and reaction efficiency of the
reactor and decreases load of the heat-exchanger for
preheating and also decrea~es co~t of the device.
When the calorific value of the waste water exceeds
600 kcal per liter o~ wa~te water, the inner tube~ oP the
reaetion ve~sel to be used for the treatmenk are pre~erable
to po~se~s an in3ide diameter in the range o~ 10 to 30 mm.
IP the inside diameter o~ the inner tubes exceeds 30 mm
where the ealoriPlc value i~ not le~s than 600 koal per
liter o~ was~e water, since the amount o~ heat~a generated
inside the inner tubes is so large relative to the amount of
heat removed that the reaotion is liable to proceed
violently and is continued only with difficulty. For the
reaction to be continued smoothly, the reaction ve~el is
required to have inner tubes 10 to 30 mm in inside diameter.
When the inner tubes of the heat-exchanger type
reaction ves~el to be u~ed in thi~ invention are packed with
-8-
~ 3 3 ~
a catalyst, the reaction vessel particularly o~ this kind
exhibit~ an outstanding per~ormance in the removal o~ the
heat of reaction locally generated in consequence oP the
improvement in the reaction rate brought about by the
catalyst. The use of the catalyst in this manner promises a
change for the better such as compaction oP the reaction
vessel.
The catalyst which are usable ~or this purpose
include those which are obtained by having such metals as
manganese, iron, cobalt, nickel, tungsten, copper, cerium,
silver, gold, platinum, palladium, rhodium, ruthenium, and
iridium or the compounds thereof insoluble or sparingly
~oluble in water deposited severally on a carrier o~
alumina, activated carbon, silica-alumina, zirconia,
titania, diatomaceous earth, silica-titania, silica-
~irconia, titania-zirconia, ~or example. The catalyst to be
u~ed herein i~ in the Porm o~ pellets, beads, or honeycombs.
The wet oxidation oontemplated by this invention is
preferable to be carried out at a temperature in the range
oP 120 to 370 C under a pressure high enough ~or the waste
water under treatment to remain in it~ liquid ~tate. For
the retention o~ the liquid pha~e this temperature range
must be observed because the critioal temperature oP water
i~ 370 C.
Tha molecular oxygen-containing gase~ whlch are
u~able h~reln inolude air, pure axygen, and ox~en-enriched
air, ~or example~
This invention manifests its out~kandin~ ePPect to
still better advantage by eP~ecting the wet oxidation using
the shell-and-tube heat-exchanger type reaction vessel in
the first ~tage and the single cylinder type reaction vessel
in the second stage. The conception of this ¢on~iguration
is based on our new knowledge that the wet oxidation
reaction of thi~ invention is such that the greaSer part o~
t~lis reaction oocurs in the part approximating the inlet to
g
,,,
the reaction vessel and the generation of the heat of
reaction is also concentrated in this part.
Sinc~ the shell-and-tube heat-exchanger type
reaction vessel to be used in the first stage of the wet
oxidation reaction has been alrea~y described, the single
cylinder type reaction vessel to be used in the second stage
o~ the reaction will be de~cribed hereinafter.
As the single cylinder type reaction vessel ~or the
second stage, and in~ulated reaction vessel is used. The
reaction ves~el~ o~ this type are roughly divided into the
horizontal tube type and the vertical tube typeO From the
standpoint o~ the efficiency of vapor-liquid contact, this
invention pre~ers the reaction vessel to be o~ the vertical
tube type. The reaction tube~ are pre~erable to have an
in~ide diameter in the range of 50 to 2,500 mm, pre~erably
150 to 1,500 mm, and a length in the range of 1 to 20 m,
preferably 1 to 10 m. The inside diameter and the length
depend on the residual COD concentration oP the waste water
at the outlet of the reaction vessel in the ~irst ~tage, ~or
example.
Similarly to the reaction vessel in the ~ir~t stage,
the reaction vessel in the second stage i~ allowed to use a
oatalyst. The amount o~ this catalyst can be ~reely
selected, depending on the oonoentration of the wa~te water,
~or example. The packln~ o~ the catalyst improves the
rea¢tion rate and permits compaction o~ the rea¢tion ve~el.
Further, in the ~e¢ond ~tage oP the reaction, the heat o~
reaotion po~e~ no problem beoause the re~idual calorifio
value of the wa~te water is ~mall.
Though the waste water at the inlet to the second
~tage ha3 a small calori~ic value, the waste water with the
progreqs o~ the decompo~ition comes to allow persi~tance o~
sparingly de¢omposable substanoes and accumulation o~ the
product Or decomposition and requires muoh time ~or
deaomposition. Preferably, there~ore, the reaction vessel
in the se¢ond ~tage is provided in the inlet part thereo~
-10
with a nozzle for introduction of air and consequently
enabled to introduce air therein and enhance the e~iciency
of contact between the waste water and the air and expedite
the reaction. Since the reaction vesqel o~ the second stage
is not of the heak-exchanger type and in consideration of
the generation of heat by the reaction, the calorific value
of the waste water at the outlet of the reaction vessel in
the ~irst stage is pre~erable to be less than 20 kcal per
liter o~ waste water.
Now, the present invention will be desoribed more
specifically below with reference to working examples. It
~hould be noted, however, that this invention is not limited
to these example
Example 1
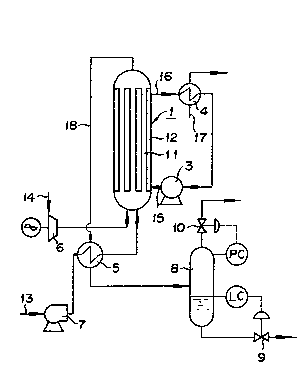
Fig. 1 is a schematic diagram o~ an apparatus for
working the method of this invention for the puri~ication o~
waste water. A reactor 1 u~ed herein had 10 reaction tubes
(inner tubes) 11 measuring 50 mm in inside diameter and 6 m
in len~th di~posed in a ~hell 12. The reaction tubes 11
were ~illed with cataly~t pellet~ (0.5% by weight o~ Pt `
supported on titania-zirconia carriar) 5 mm in average
diameter, each to ~orm a cataly~t layer 5 m in length.
distribution plate Por air (not shown) is provided under the
rea¢tion tubeY,
First, waste water having a COD (Cr) concentration
o~ 120 g/liter and a calori~io value o~ 400 koal per lit~r
of waste water brought in through a line 13 wa~ impelled by
a wa~te water ~eed pump 7 to be preheated in a heak-
exchanger S and then fed to the reactor 1. In the meantime,
the air supplied through a line 14 was given increased
pre~ure by a oompressor 6 and then fed into the reaction
tubes 11 o~ the reactor 1. ~ heat transfer medium impelled
by a oirculation pump 3 through a line 15 to the outside of
khe inner tubes inside the reactor 1 to remove the heat o~
reackion generated during the course o~ reaction, Then, the
heat transPer medium which had fulfilled the part, o~
-11- ,
;
~. 3 ~ 2 P~
removing the heat was discharged through a line 16 and
cooled in a heat-exchanger 4 with the cooling water brought
in through a line 17. The heat deprived of the hot heat
transfer medium was recovered consequently. The waste water
which had been treated in the reactor 1 was ~ischarged
through a line 18, cooled in the heat-exchanger 5, and then
fed to a ga~-liquid separator 8. There to be separated into
a harmless gas and water. In this gas-liquid ~eparator 8, a
liquid level controller LC detects the liquid level and
actuates a liquid level control valve 9 to keep the liquid
level constant and, at the same time, a pressure controller
PC detects the pressure and actuates a pressure control
valve 10 to keep the pressure constant.
In this case, the reaction in the reactor 1 was
carried out at a reaction temperature o~ 250 under a
reaction pressure o~ 75 kg/cm2. G, with the ~low volume of
the waste water through each of the reaction tubes fixed at
llter/hr and the ~low volume o~ the air at 7,200 N.
llter/hr (the total volume of the waste water at 150
liters/hr and that of the air at 72 Nm3/hr throughout khe
entire reactor). In this treatment, the maximum temperature
within the catalyst bed was 267C and the conversion o~ COD
wa~ 99.4%.
Control 1
Fig. 2 is a sohcmatio diagram o~ an apparatus using
a single oylinder type reaotor. A reaotor 21 used herein
mea~ured 50 mm in inslde diameter and 6 m in length. It was
~illed with cataly~t pellat~ (0.5% by weight o~ Pt supported
on titania-z~roonia carrier) 5 mm in average particle
diameter to ~orm a catalyst bed 5 m in length. The single
o~linder o~ the reactor was covered with an insulating
material 39.
The air given increased preAsure by a compressor 26
introduoed waste water into the cylinder of the single
oylinder type reactor 21 packed with the catalyst and not
ve~ted with a ~unction ~or the removal o~ heat, there to
.,
-12-
: ` `; `i. : ~
~3.~7~
estabLish contact and induce reaction between the air and
the waste water. Then, the resultant mixture was passed
through a heat-exchanger 25 and led to a ga~-liquid
separator 28, there to be separated into a harmless gas and
water. At this time, the catalyst bed was tested for
temperature distribution. The conditions such as of the
cataly~t and the treatment used herein were the same aY
tho~e o~ Example 1. In the treatment, the maximum
temperature in the catalyst layer reached 400 C and the
reaction could not be continued.
In Fig. 2, the reference numerals which are the sum
of the reference numerals used to denote component parts in
Fig. 2 severally plus 20 denote the identical component
parts shown in Fig. 1.
Examples 2 to 7
In a similar reactor~ to Example 1 vested with a
function for exchange of heat and provided with reaction
tubes of varying diameters, waste water of varying
concentration was treated under varying conditions. The
reaction conditions, the maximum temperature o~ catalyst
bed, and the conversion of COD involved in each o~ the
experiments were a~ ~hown in Table 1. The volume o~ wa~te
water and that of air indicated in thi~ table were eaoh the
value par reaotion tube.
",
, ! ' ' , , :
-13- ~
' ,:
f~
~ 3 ~ 6 ,1~
~ _ _ _ _
,~ U~ ~ o o L~ o
o o ~ C~ o~ ~ o o~
~.... __~ __ _
td U~ O co N =l ~r)
~d ~ ~D D lfl IS~ U~ Ll~
~dF~ j>oC~ ~I ~I N ~`J ~I ~I "
Cl. o
, _ _ _ _ _ . _ _ _ _
~ 3 ~ ~ rl n '' L'' ~ ~ ~ u~ U~
O ~ ~ Q) O ~
3~ ^_ _ _ ___
a ~ --~ ,C O co oo O O N
a) . ~ .~ .~ .~ ~ .
9~ LY~ ~ _ _ _ __
~ N
Jo CO O L~ I~n L~ L~ Oo O
ad a ~ ___ _
~ a)
O O O O O O
~d E3 ~ EU t~J E~l ~1 ~1 E~l
E~ ~ ~ s~ ~
_ __ _ . . _ _
rl ~o a~ h ~d O 0 8 o o o
~d 4~ ~ h ~ ~d ~ ~ h ~1 N U') ~ 00 Ir~
_~) h~ ,,~ _ ___ __
R I ~j a)
~ ~ 8 ~ ~ --' co co ~- ~
o .,, ~ ~o
c~ C) ~ 3 3e ~
_____ ___ _ _
a) ~ ?~ ~i' E3 ~ , i
-1 0 ,~ -- -- ~ :'
a~ a~
a~ ~ o o o u~ o o
~r~ E3 ~r~ a) ~ c0 11'1 Ir~ ~I oo co
R ~d ~ ~ E~
a) _ _ _ _ _ _
S U~ ~LO ~
, ~W_,___ , _ ,, _ _
- 14 -
: ` ~
~1 3~7~
Example 8
Fig. 3 is a schematic diagram illustrating another
apparatus for working the method o~ this invention. Thi~
apparatus uses a shell-and-tube heat-exchanger type reactor
in the first stage and a single cylinder type reactor in
the second stage. A ~irst heat-exchanger type reactor 41a
and 10 reaction tubes (inner tube~) 51 measuring 50 mm in
inside diameter and 4 m in length disposed in a shell 52.
The reaction tube~ 51 were packed with catalyst pellets
(0.5% by weight o~ Pt supported on titania-zirconia carrier)
5 mm in average diameter, each to form a catalyst layer 3 m
in length. A second single cylinder 41b mea~ured 250 mm in
inside diameter and 2 m in length. It was filled with
catalyst pellets ~0.5% by weight of Pt supported on titania-
zirconia carrier) 5 mm in average particle diameter, to ~orm
a catalyst layer 1.2 m in length. The single cylinder was
covered with an insulating material 59.
First, wa~te water having a COD (Cr) concentration
o~ 120 g/liter and a calori~ic value of 400 kcal per liter
o~ waste water and brought in through a line 53 was impelled
by a wa~te water ~eed pump 47 to be preheated in a heat-
exchanger 45 and then fed to the first reactor 41a. In the
meantime, the air brought in through a lina 54 was given
increa~ed pressure by a oompressor 46 and ~ed into the
reaotion tubes 51 o~ the ~irst reaotor 41a. In the
meantime, a heat transfer medium was ~orwarded by a
oiroulatlon pump 43 through a line 55 to the outside o~ the
inner tubes o~ the reaotor 41 a to e~Pect removal o~ the heat
o~ reaotion. Then, the heat trans~er medium used ~or the
removal o~ hqat wa~ discharged throu~h a line 56 and oooled
in a heat-exohanger 44 with the oooling water ~ed through a
line 57. Thus, the oooling oP the hot heat tran3~er medium
and the reoovery of the heat o~ reaotion were attained. The
waste water treated in the ~irst reactor 4la was then fed to
khe ~eoond reactor 41b to be treated therein. It wa~
disoharged through a waste water line 58, oooled in a heat-
-15-
~3~7S~ :
exchanger 45, and then fed to a gas-liquid separator 48
there to be separated into a harmless gaq and water. In the
gas-liquid separator 48, a liquid level controller detects
the liquid level and actuated a liquid level control valve
49 to keep the liquid level con3tant and, at the same time,
a pres~ure controller PC detected the pre~ure and actuated
a pressure control valve 50 to keep the pressure constant.
In thi~ treatment, the reaction temperature in the
first reactor 41a wa~ 250C and that in the second reactor
41b wa~ 255C and the pre~sure wa~ 75 kg~cm2.G. The flow
volume oP the waqte water per reaction tube wa~ 15 liter3/hr
and that of the air 7,Z00 N liter/hr (the total volume of
the wast water wa~ 150 liters/hr and that oP the air 70 N
m3/hr through out the entire reactor). As the result, the
maximum temperature in the catalyst layer of the fir3t
reactor 41a wa~ 266~ , and that of the second reactor 41b
was 255~ C. The conversion of COD was 96% at the output part
of the first reactor and 99.5% at the outlet of the second
reactor.
,' ' ! I , j , ,
-16-
~"~