Note: Descriptions are shown in the official language in which they were submitted.
~1333219
-- 1 --
MICROTUBER PROPAGATION OF POTATOES
Field of the Invention
The present invention relates generally to the
production of potatoes and particularly to the use of
microtubers in the production of potatoes. The present
invention is specifically related to inducing a high rate
of microtuber shoot formation, wherein each shoot will
form a new tuber.
Background of the Invention
The potato is one of the world's most economically
important agricultural crop plants. A member of the
Solanaceae family, potatoes are conventionally propagated
clonally by subdividing tubers, i.e., the underground
stems of the plant, into sections which are then planted.
The potato is a premier example of a crop where the
control of diseases in the propagation phase is essential
for consistent and high yields. The vigor and value of
the crop depends in large part on maintaining the source
tubers as virus and disease-free as possible. One way of
achieving this goal is to produce the certified potato
stocks in disease-free areas. However, such environments
are not always available.
An alternative to the use of normal tubers for cloning
potatoes for production is the micropropagation of
- 1333219
microtubers, which are produced in completely disease-free
environments. Microtubers are small in vitro produced
tubers that are usually about the size of a pea. They are
produced in sterile culture under controlled conditions.
Microtubers were first reported in the scientific
literature by Barker (1953) Science 118:384-5. Until
recently, the expense involved in the production of
microtubers preventeA the commercial exploitation of
microtubers in the potato crop industry. As a result,
microtubers were used primarily as a physiological tool to
investigate the process of tuberization. Mingo-Castel, et
al. (1976, Plant Physiol. 57:480-485) reported on the
effects of carbon dioxide promotion and ethylene
inhibition on the tuberization of potato explants cultured
in vitro. Mauk and Langille (1978, Plant Physiol.
62:438-442) reported on the influence of temperature and
photoperiod on the incidence and changes in a cytokinin in
a potato plant tissue, and the effect of the cyto)cinin on
in vitro tuberization. Palmer and Smith (1969, Nature
221:279-280) and Stallknecht (1972, Plant Physiol.
50:412-413) also reported on the effects of cytokinins and
coumarin on in vitro tuberizat;on of potato plants.
Wang and Hu (1982, American Potato Journal 59:33-37)
were the first to report on the use of microtubers for the
production of potatoes in the field. Subsequently,
Wattimena, et al. (1983, American Potato Journal 60:27-33)
compared the plant growth, the yield, and the tuber
quality of two cultivars of potatoes grown from
transplants generated from microcuttings or microtubers
with potatoes grown from seed tubers. Both studies used
(1) stem cuttings as the explant source for creating
microtubers in vitro, (2) Murashige and Skoog (MS) mineral
salt medium (Murashige and Skoog, 1962, Physiol. Plant
15:473-497), (3) high sucrose levels (6-8%), (4) low
temperatures (15 C - 20 C), and (5) a synthetic
cytokinin to induce tuberization. The procedures differed
significantly in the tuberization photoperioA, the method
of multiplication, and agitation.
13332I9
Su~sequent publications have been based on both the
stationary system (Bourque, et al., 1987, In Vitro
Cellular & Developmental Biology, 23/5:381-3~6; Rosell, et
al. 1987, Potato Research 30~ 116 Ortiz-Montiel, et
al 1987, American Potato Journal, 64:535-544; Slimmon and
Souza-Machada, 1~87, American Potato Journal, 64:458, and
Hussey and Stacey, 1984, Annals. of Botany, 53:565-578)
varying only in synthetic hormones, the photoperiod and
the temperature; and the shaker system (Estrada, et al.,
1986, Plant Cell, Tissue and Organ Culture, 7:3-10) where
chlorocholine chloride was used in addition to benzyl
amino purine (BA) for inducing tuberization.
As mentioned above, the commercial use of microtubers
has been limited by the high cost of producing
microtubers. This cost is in large part determined by the
high input of labor necessary to produce microtubers based
on the methods reported above. The labor demands are
great because while every node of an in vitro grown shoot
has the theoretical potential to form a microtuber, in
~eneral, only one microtuber will form on a multi-node
shoot axis. Thu~l in order to achieve a high rate of
tuber formation, the nodes have to be separated from each
other by manual manipulation, thus requir$ng a significant
input (i.e., cutting and culturing) for each shoot to
culture each microtuber.
An alternative approach to achievin~ high tuber
numbers is the production of a large number of
independently-growing shoot axes, each of which will form
one or more microtubers. However, this approach has not
heretofore been successful on potatoes since the
commonly-used hormonal stimulants, such as cytokinins, for
shoot multiplication are not particularly-effective for
potato shoot cultures grown under standard conditions.
Summary of the Invention
According to an aspect of the present invention
a novel and commercially-applicable method of
1333219
increasing the number of potato shoots which will
subseqently lead to microtuber formation, is provided.
Itis a further aspect of the present invention to
provide a method for inducing a high rate of microtuber
formation from a potato shoot in in vitro culture.
The present
invention describes a method of induclng a high rate
of microtuher formation beginning from a single potato
microtuber. The method includes inducinq a multiple shoot
complex formation from the single shoot or shoots produced from the
microtuber by the induction of shoot-tip necrosis on the
growing shoot. By shoot-tip necrosis, it is meant that
the condition of the tip of the potato shoot ceases to
grow and dies, encouraging axillary branching and
formation of a complex of subsidiary shoots. The modified
shoot complex is then placed in a growth environment under
conditions suitable for increasing the length of the shoot
and allowing a separate root structure for each subsidiary
shoot. The shoot complex is then placed under conditions
suitable for allowing the tuberization of the shoots. The
multiple shoot complex initially formed from one
microtuber will then produce a multitude of microtubers.
The present invention is also directed to a
microtuber-shoot complex produced by the above-described
process.
Because the economical use of the potato transplant
depends in large part on the number of times that the
transplant must be handled, the method of the present
invention is advantageous due to its low labor
requirements. For example, only one manual manipulation
of the plant tissue is required, i.e., at harvest.
Further, no manual separation of the tuberization centers
is necessary. Further still, any changing of the liquid
media between the above-described steps can be easily and
readily achieved by automated techniques as required
_ 5 _` ~ 3~1fi~
Detailed Description of the Invention
The microtuber multiplication system of the present
invention has three interconnected stages: (1) the
formation of the microtuber-shoot complex; (2) the
elongation of the shoot axes; and (3) the tuberization of
the shoot axes.
Formation of Microtuber-Shoot Complex
In this stage, initial shoot axes are initiated from
previously developed "seed" microtubers. The initial
source explant is preferably a preselected sterile
microtuber, grown in a dark, sterile environment, that has
clearly broken dormancy and has visible sprouting. These
shoots are more responsive to subsequent in vitro
manipulations than, for example, shoot-culture derived
shoots. The sterile microtubers have been generally
stored at temperatures of about 5 C in the dark for
approximately 24 to 36 weeks.
The initial source shoots which are emerging from the
microtuber explants are induced to extensively branch and
form a mass of short shoot "initials", thus providing the
basis for multiplication of the propagules. To achieve
this formation, the microtubers are placed in an
environment and nutrient medium which supports potato
shoot growth. One suitable nutrient medium is a Murashige
and Skoog (MS) (supra) mineral salt medium supplemented
with vitamins, 3% (w/w) sucrose and 1.5 g/L gel rite.
Cytokinins may optionally be added to the medium in order
to enhance development of the shoots. Cytokinins, i.e.,
thidiazuron, are added at a concentration of approximately
l.O micromolar (uM). Suitable examples of cytokinins
which may be added to the nutrient medium are benzyl
adenine (BA) or thidiazuron (TDZ). Preferably, the medium
contains no or, at the very most, low levels of calcium.
1333219
The microtuber is allowed to grow at a temperature of between 20and 25C
(preferably 22C) in continuous light at 20-40 microEinsteins (Ein~2 sec~l) of
fluorescent light. The longer the conditions are kept under these conditions, the more
shoot axes will be formed.
A preferred example of the nutrient medium for use on "Red Pontiac"
microtubers is a MS medium which does not contain any levels of calcium, but
contains 1.0 uM TDZ. The microtubers are allowed to grow at a temperature of 22GC
and under constant 20-40 microEinsteins fluorescent light.
It is within the scope of the present invention to allow the shoot axes to
multiply by stimulating them with only cytokinins and the above-described mediumwith normal calcium levels; however, the rate of multiplication may be lower and the
uniformity of shoot multiplication less. Generally, the microtuber shoot complexformation increases by the induction of shoot tip necrosis at low calcium levels.
Maximum shoot axis stimulation is achieved with low calcium in combination with
the cytokinin TDZ.
The basis of the shoot axis multiplication is in the injury to the shoot tip, i.e.,
shoot tip necrosis, which releases axillary buds from apical dominance. Sha, et al.
(1985) J. Amer. Soc. Hort. Sci. 110(5):631-634. Under an in vitro environment,
multiple shoot axes formed by the growth of these axillaries. The axillary or
subsidiary shoots may be similarly effected and branch heavily, which further increases
the number of shoot axes formed. This mass of short shoot axes with the
accompanying mother microtuber is termed "microtuber-shoot complex".
The use of cytokinins in microtuber formation has been extensively studied.
Cytokinins have been found to either act cooperatively to amplify the effects of a low
calcium media, or independently to stimulate axillary branching of shoots emerging
from the microtubers. The latter is a unique response for potatoes and differentiates
microtuber-derived shoots from the more commonly used shoot cultures, where
7 1333219
cytokinins are not particularly effective in stimulating axillary-based shoot
multiplication.
Elon~ation of the Shoot Axes
Each of the shoots of the newly-formed microtuber-shoot complex is now
allowed to develop into an independent axis. The shoot complex is thus preferably
transferred to a medium and environment which promotes the rapid growth of the
potato shoots. This transfer may be accomplished by physically transferring the
microtuber-shoot complex to a different culture vessel or most simply by ch~nging the
medium in the vessel. Although other methods are known to the art, such as a
stationary bottle and a shaker bottle, the preferred environment is a roller bottle, also
known to the art for providing a growth environment for other types of cell cultures.
Roller bottles are rolled at a slow rate, i.e., 1/2 revolutions per minute (rpm), so that
a film of a growth medium is constantly m~int~ined on the surface of the bottle. This
process allows for the complete bathing of the developing shoots in the liquid medium
while m~ g adequate gas exchange and nutrition. Further, the bathing of the
shoots in the medium facilitates the subsequent tuberization during the next stage. The
developing shoots are kept close to the sides of the bottle by the insertion of a coarse
plastic mesh into which the shoots grow. The plastic mesh is preferably positioned
as a liner on the interior of the roller bottle. This may be done by inserting a pliable
plastic insert inside of the mesh placed in turn inside the bottle to hold the mesh
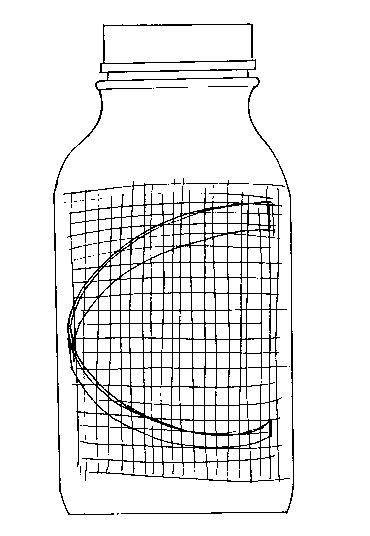
against the inside of the bottle, as shown in the attached drawing figure. As the bottle
turns, the microtubers are alternately immersed in medium and air. This prevents the
shoot from having an orientation bias and also bathes the entire shoot in the medium.
The preferred environment is a liquid con~i~ting of MS mineral salt medium
supplemented with 3% (w/w) sucrose at a temperature of about 22C. The shoot
complex is kept in the dark during the development stage.
The process continues until the individual shoots are at least two centimeters
- 1333219
- 8 -
(cm) in length. Further, the individual shoots may have independently derived roots
even though they may still be attached to the original microtuber shoot axis and thus
the mother microtuber. The entire process takes approximately two weeks.
The first week of the elongation stage may be
benefited by the addition of cytokinins to the medium if
the microtuber-shoot complex formation stage used no
cytokinins in the process.
Tuberization Stage
The tuberization stage is that stage when numerous
shoot axes have developed to a point where they become
physiologically independent of each other while still
being continuously and totally bathed in a growth medium.
The elongated shoots emerging from the shoot complex are
tuberized by one of the known in vitro tuberization
procedures. A routine including a growth medium of MS
mineral salts supplemented with 8% w/w sucrose and 3.2 uM
cycocel in roller bottles at a temperature of 15 C will
induce microtubers in "Red Pontiac" within about two
weeks. The microtubers will reach convenient harvest size
in one to two months. The tuberization procedure is also
well adapted to be used with the roller bottles.
Thus the method of the present invention utilizes
three basic steps. First, culturing a shoot from a
microtuber and then inducing shoot tip necrosis in the
apical shoot, resulting in a proliferation of axillary or
subsidiary shoots. Secondly, culturing the microtuber
shoot complex thus produced to elongate the multiple shoot
axes. Thirdly, inducing microtuber formation from the
multiple shoots. The result is that the original "seed"
microtuber is multiplied by the procedure resulting in up
to ten or more daughter microtubers from the original
one. Because the steps in the procedure involve simply
changing media and environment conditions such as lighting
and temperature, the process is well adapted for automated
use. No human manipulation of the shoots or the culture
!
' 133321g
9 _
need be required between inserting the original microtuber
and removing the multiple microtuber mass for harvest.
Thus the multiplication of microtubers for potential
commercial use in the creation of aseptic microtubers for
cultivation becomes practical.
The following examples are illustrative of the process
of the present invention and show two examples of the
microtuber multiplication system with two different potato
cultivars.
Examples
Example 1
Sterile "Red Pontiac" microtubers were stored in a
stationary storage container in air in the absence of any
growth medium or sucrose, and grown at 5 C in the dark.
After approximately six months the sterile microtubers
were harvested and nine microtubers, which had clearly
broken dormancy as evidenced by visible etiological
sprouts, were utilized in the example.
The sprouts were sterilized in a 15% liquid bleach
solution at room temperature, and rinsed twice with
sterile distilled water. The surface disinfected
microtubers were then transferred to a sterile growth
medium consisting of MS medium supplemented with 3% (w/w)
sucrose. No cytokinins were used. The medium contained
no calcium. The microtubers were kept in this growth
medium without agitation at a temperature of 22 C under
constant 20-40 Ein sec light for approximately 7
1/2 weeks to allow multiplication of the shoot axes on the
developing microtuber shoot complex.
After the formation of the microtuber shoot complex,
the complex was transferred to a MS growth medium with
normal calcium chloride, 3% (w/w) sucrose and 1.0 uM
thidiazuron, and incubated in the dark at 22 C in a
roller bottle rotating at 1/2 RPM for two weeks. After
the two week period, the growth medium was replaced with
fresh MS growth medium again containing calcium chloride,
1333219
-- 10 --
but omitting thidiazuron. The microtuber shoot complex
was incubated for an additional week under the
aforementioned conditions. By this time, the shoot axes
of the microtuber shoot complex were at least two
centimeters long and ready for tuberization.
The tuberization process used consisted of replacing
the spent growth medium with fresh MS growth medium
supplemented with 8% (w/w) sucrose, 170 uM coumarin and
3.16 uM cycocel. The microtuber shoot complex was then
incubated in the dark at 15 C in a roller bottle
constantly rotating at 1/2 RPM. After two weeks, the
growth medium was replaced with fresh MS growth medium
supplemented with 4% sucrose, but no hormones.
Approximately 2 1/2 weeks later, the tubers reached a size
convenient for harvesting.
The effect of Experiment 1 resulted in an average
multiplication of 4.78 microtubers per tuber for each of
the nine sterile microtubers initially processed in this
experiment. Tuber multiplication for the nine replicates
ranged from 1 to 11 fold.
Example 2
Example 2 was conducted using "Superior" microtubers
in place of "Red Pontiac" microtubers. The experimental
procedure was similar to the same as that conducted in
Experiment 1 with the following exceptions. Three
sterilized and washed microtubers were transferred
directly to a sterile growth medium consisting of the MS
without calcium growth medium supplemented with 3% (w/w)
sucrose and 1.0 uM thidiazuron, and incubated at 22 C in
the light for approximately ten weeks. The microtuber
shoot complex was then transferred to a fresh MS growth
medium supplemented with 3% (w/w) sucrose, but no
hormones, and incubated in a roller bottle rotating at 1/2
RPM at 22 C in the dark. After approximately two weeks,
the growth medium was replaced with fresh MS growth medium
supplemented with 8% (w/w) sucrose and 3.16 uM cycocel,
and incubated in the dark at 15 C under roller bottle
1333219
-- 11
conditions. Approximately four weeks later the tubers
were harvested for observation.
The results of three replications of "Superior"
sterile microtubers show a 2, 4 and 5 fold multiplication
of tubers on the original microtubers.
It is understood that the invention is not confined to
the particular construction and arrangement herein
described, but embraces such modified forms thereof as
come within the scope of the following claims.