Note: Descriptions are shown in the official language in which they were submitted.
1333579
ELECTROCHEMICAL GAS GENERATOR
Field of the Invention
The present invention relates to a device for
electrochemical generation of gases for the transportation
of fluids, lubricants and similar media.
Background of the Invention
It is known to use catalytical and electrochemi-
cal processes to produce pressurized gases for transporting
fluids in technical applications. For example, hydrazine
can be catalytically decomposed into hydrogen and nitrogen
thereby generating a pressurized gas which can be used to
quickly discharge the water filled tanks of submarines.
The gas mixture produced by this reaction contains ammonia
in a concentration which is a function of the nature of the
catalyst. The higher the proportion of ammonia the higher
the temperature of the generated gas. The pressurized gas
may be used, for example, in control units in space appli-
cations.
It is also possible to use oxygen as a pres-
surizing gas. Oxygen gas may be generated by catalyti-
cally decomposing hydrogen peroxide using, for example, a
silver catalyst. This reaction also produces a large
quantity of heat which, in general, requires special heat
management.
With both of the methods described above, the
rate at which gas is released is determined by the diffu-
sion or convection current of the reaction fluid to the
catalyst. Therefore the reaction may only be stopped by
interrupting the current.
It has been proposed to use self-controlled
decomposition apparatuses for hydrazine and hydrogen perox-
ide by using catalysts similar to those used in the tech-
133357~
-- 2
nique of gas diffusion electrodes (valve electrodes aredisclosed in US patent 3 201 282 and German patent 1 542
565). In these systems the rate of reaction is dependent
upon the pressure of the generated gas. By using a diluted
aqueous solution of hydrazine (or hydrogen peroxide) the
rate of gas production may be kept constant provided that
the discharge of water is kept constant.
It is also possible to generate hydrogen gas by
reaction of a metal with a base or an acid. When hydro-
chloric acid is brought into contact with zinc, hydrogen is
generated, and zinc chloride is released into solution.
Hydrogen can also be generated by the reaction of zinc in
an alkaline solution but when using pure zinc almost no
generation of hydrogen can be observed. This is because
the hydrogen minimum overvoltage of zinc is extremely high.
The inertness of zinc in an alkaline medium is also due, in
part, to the generation of a layer of zinc oxide on the
surface of the zinc which passivates the zinc.
It is possible to accelerate the corrosion by
contaminating the zinc with another metal having a much
lower hydrogen minimum overvoltage. For example, it is
well known that when a clean zinc plate is contacted by a
platinum wire Hydrogen gas is generated near the wire and
the zinc corrodes close to the contact area.
It is also known to drill a hole in a zinc block
and to solder the block onto a Molybdenum bar. Again, the
result is a galvanic element. The rate of generation of
hydrogen depends upon the size of the metal surface upon
which the generation of Hydrogen is promoted. The perform-
ance of a short-circuit element such as this depends upon
many random factors relating to how the surfaces influenc-
ing the corrosion are formed. This means that it is noteasily possible to regulate the rate of generation of
hydrogen from outside.
- 3 - 1333~79
Lubricant dispensers which dispense lubricant by
means of a gas produced by a galvanic element and an
electrolytic fluid are well known. To start the generation
of gas, the galvanic element is brought into contact with
the electrolyte by means of a screw. The generated gas
expands a hollow body and moves a piston or a dividing
insert which presses the lubricant out of a grease box to
the lubrication point (German patent 2 139 771 corresp. to
CA 961 420).
Summary of the Invention
The present invention pertains to a device for
generating gases which device is able to produce gases in
an adjustable quantity and/or at exact defined times, which
is compact, easy to manufacture and which prevents the
penetration of electrolyte.
In particular the invention pertains to an elec-
trochemical gas generator comprising a galvanic cell and a
means for completing an electrical circuit between the
electrodes of the cell. The galvanic cell comprises a
sealed housing, a gas generation electrode within the
housing, a counter electrode within the housing but separ-
ated from the gas generation electrode, an electrolyte in
contact with the gas generation electrode and the counter
electrode, an aperture in the housing to release gas
generated at the gas generation electrode, a path by which
gas can flow from the gas generation electrode to the
aperture and a gas permeable, electrolyte impermeable,
membrane blocking the path between the gas generator
electrode and the aperture.
Another embodiment of the device according to the
invention comprises a galvanic cell including an anode, a
cathode and a housing containing an aqueous electrolyte in
_ 4 _ 1333579
which by closing of an external circuit, and, if necessary
by supplying a source of direct current in the external
circuit, a current flows which produces a quantity of gas
corresponding to the current.
The invention is directed to a device for elec-
trochemically generating a hydrogen gas or an oxygen gas in
an adjustable quantity, comprising: a galvanic cell
including an enclosed housing having a base and at least
one side wall integral with said base, a cover, a seal
positioned between and in contact with said cover and said
side wall of said housing, and an opening for releasing gas
from said housing, said galvanic cell further including; a
gas generating electrode forming a part of said housing and
including an electron-conducting porous body contained
within said housing; a counter electrode including an
oxidizable metal or a reducible oxide or nitrate which
serves in countercapacity to said gas generating electrode;
separator means between said gas generating electrode and
said counter electrode; an alkaline electrolyte present in
an amount sufficient to provide reactions in said gas
generating electrode and in said counter electrode; and
means for electrically connecting said gas generating
electrode and said counter electrode, and means for estab-
lishing a current flow between said gas generating elec-
trode and said counter electrode for generating a predeter-
mined quantity of gas by said gas generating electrode, for
release from said opening of said galvanic cell.
The invention is also directed to an actuating
apparatus for means for dispensing solids and fluids, or
transporting mediums, including the device defined, which
also possesses means connecting said opening of said
galvanic cell and said dispensing means or said transport-
ing medium, for supplying gas to said actuating apparatus
for operating said dispensing means or said transporting
medium.
~ 5 ~ 1 333579
The device as defined for generating hydrogen gas
in an adjustable quantity wherein said electron-conducting
porous body of said gas generating electrode forming a part
of said housing may include a hydrophilic, electrolyte-
receiving portion and a hydrophobic, gas-receiving portion,
and a porous hydrophobic layer adjacent to and in engage-
ment with said porous body, for preventing electrolyte from
leaving the galvanic cell; and wherein said counter elec-
trode may include an oxidizable metal which serves incountercapacity to said gas generating electrode; and may
include means for excluding air from said galvanic cell,
and for excluding oxygen from the pores of the gas generat-
ing electrode.
The device as defined for generating oxygen gas
in an adjustable quantity wherein said electron-conducting
porous body of said gas generating electrode forming a part
of said housing may include a hydrophilic, electrolyte-
receiving portion and a hydrophobic, gas-receiving portion,
and a porous hydrophobic layer adjacent to and in engage-
ment with said porous body, for preventing electrolyte from
leaving the galvanic cell; and said counter electrode may
include a reducible oxide which serves in countercapacity
to said gas generating electrode; and means for electrical-
ly connecting said gas generating electrode and said
counter electrode, and for establishing a current flow
between said gas generating electrode and said counter
electrode for generating a predetermined quantity of gas by
said gas generating electrode, for release from said
opening of said galvanic cell.
The invention is also directed to a method for
electrochemically generating a hydrogen gas or an oxygen
gas in an adjustable quantity by means of a galvanic cell
including an enclosed housing having a base and at least
one side wall integral with said base, a cover, a seal
1333579
-- 6
positioned between and in contact with said cover and said
side wall of said housing, and an opening for releasing gas
from said housing, said galvanic cell further including a
gas generating electrode forming a part of said housing and
including an electron-conducting body contained within said
housing, a counter electrode including an oxidizable metal
or a reducible oxide which serves in countercapacity to the
gas generating electrode, separator means between said gas
generating electrode and said counter electrode, and an
alkaline electrolyte present in an amount sufficient to
provide reactions in said gas generating electrode and in
said counter electrode, said method comprising the steps
of: electrically connecting said gas generating electrode
and said counter electrode, establishing a current flow
between said gas generating electrode and said counter
electrode, generating a predetermined quantity of gas by
said gas generating electrode, and releasing said gas from
said opening of said galvanic cell.
In the device as defined, said oxidizable metal
can be selected from the group consisting of zinc, cadmium,
lead and copper. Said reducible oxide can be selected from
the group consisting of manganese dioxide, silver oxide,
mercury oxide and nickel oxide. Said reducible oxide can
be selected from the group consisting of nitrate or ammon-
ium nitrate.
Said gas generating electrode can comprise Raney-
metals of Group VIII of the periodic table or noble metals
selected from the group consisting of Pt, Pd and platinum
containing metals. Said porous body of said gas generating
electrode can comprise a metal from Group VIII of the
Periodic Table of elements selected from the group consist-
ing of platinum, palladium and nickel, alone or in combina-
tion with carbon, said metals having low overvoltage withrespect to the evolution of the gas at said electrode.
1333579
Said gas generating electrode can generate
hydrogen gas and can comprise a Raney nickel powder bound
with a porous foil of polytetrafluoroethylene metal rolled
into a net of nickel, said rolled nickel net being a
current conductor. Charcoal powder can be blended with
said Raney nickel powder.
Said gas generating electrode can generate oxygen
gas and can comprise Raney nickel powder in admixture with
a hydrophobic resin powder selected from the group consist-
ing of polytetrafluoroethylene and polyethylene. Said
oxidizable metal can be zinc powder or zinc gel and said
cell can operate at a voltage of about 0.4V or less. Said
zinc cell can be short circuited via low resistance and can
contain sealing means to prevent entrance of air into said
cell when said cell starts generating gas to actuate said
means for dispensing or transporting.
Said opening in said housing can release gener-
ated gas resulting from electrochemical activity withinsaid cell and air can be excluded from said device by
valves which allow a one directional flow of the generated
gas to actuate. An electrical circuit and adjustable
resistance can be provided to control gas generation.
The device may contain means to open the circuit
provided by direct current upon reaching a predetermined
gas pressure and to close said circuit when said gas
pressure falls below a predetermined value. The generated
gas can be released in pulses.
Said oxidizable metal can be zinc gel containing
said electrolyte situated within said housing. The said
reducible oxide can be in the form of a porous tablet of a
manganese dioxide and can be situated within said housing.
- 8 ~ 13 33 S7 9
Brief Description of the Drawings
Figure 1 is a cross section through a cell made
according to the invention.
Detailed Description of the Preferred Embodiment
It has been found that it is advantageous to
produce hydrogen in a cell having a cathode made of zinc
powder and an anode, which is a hydrogen evolution elec-
trode, in the form of a gas diffusion electrode. In a pre-
ferred embodiment the gas evolution electrode is made of a
metal of the 8th sub group of the periodic table of el-
ements, preferably platinum, palladium or nickel, the
latter in particular in the form of Raney nickel, because
these metals have a low hydrogen minimum overvoltage. The
electrode is preferably made in form of a porous elec-
trode, e.g. as a double-skeleton catalyst electrode accord-
ing to German patent 1 019 361 or in form of a Raney-
nickel structure combined with PTFE (polytetrafluorethy-
lene) or in the form of a Raney-nickel/active charcoal-
structure embedded in the meshes of a net or of an expanded
metal. The cell containing the zinc, electrolyte, a
separator and the hydrogen evolution cathode is closed to
the outside by a foil of PTFE. The PTFE foil allows gas to
pass through its pores. At the same time the high capil-
lary depression in the pores of the unwettable PTFE pre-
vents the exit of electrolyte from the cell.
It has been found that zinc/air cells which are
typically used in hearing aids can be used - against their
proper purpose - for the new object of generating hydrogen
by making a short-circuit via a low resistance and sealing
the cell against entrance of air in the starting phase.
Thus, evolution of hydrogen and flow of a current start.
The evolution of hydrogen is maximized where the electro-
motive force of the potential difference between the
1333579
g
reversible zinc electrode and the reversible hydrogen
evolution electrode, is about 0.5 volts. By selection of
the proper short-circuit resistance it is possible to
calculate the corrosion current and to regulate the rate at
which hydrogen is produced by temporarily changing the
incremental resistance of the galvanic cell.
The cathode normally used in such known zinc/air
cells is not applicable to the new purpose. It is possible
to adapt this element in an optimal way to the new purpose
by exchanging the electrode made of a compound of PTFE and
active charcoal for another made of a compound of Raney-
nickel and PTFE or Raney-nickel and charcoal which compound
is laminated or rolled into a net of nickel and by provid-
ing the layer with a porous foil of PTFE according toGerman patent application 3 342 969. In principle it is
possible to use a massive zinc cup, zinc powder or a gel of
zinc powder, which is normally used in production of
primary cells in the battery industry, as the zinc elec-
trode. In order to reduce the unwanted evolution ofhydrogen by self-corrosion the zinc may be amalgamated.
For discharging the zinc powder or zinc gel
electrode is in contact with an amalgamated metal nail or
a galvanized or cadmium-protected contact element of
relatively high hydrogen minimum overvoltage. This can
also be a part of the housing of the cell made of a corre-
sponding metal, such as zinc or brass. This housing part
is separated by an electrically isolating seal from a
second metallic housing part which includes the gas evol-
ution electrode. The quantity of zinc which is required in
the housing depends upon the quantity of hydrogen to be
produced in the cell. By contrast, the size of the gas
evolution electrode depends only upon the maximum rate of
hydrogen evolution required. For a quantity of 40 Ncm3/h
of hydrogen, corresponding to 100 mamps an electrode having
a surface area of 1 cm2 is sufficient.
lo - 1 3335 7g
The quantity of electrolyte required is propor-
tional to the quantity of zinc. During the reaction water
is consumed, while hydrogen and zinc oxide are produced as
reaction products. The quantity of electrolyte must be
such that after the consumption of the water needed for the
reaction enough electrolyte fluid remains.
In the zinc/air cell oxygen from the air is
absorbed, therefore the volume of the cell increases as gas
generation proceeds. A zinc/air cell must be dimensioned
according to the final volume of the balance of the pro-
duced and consumed materials. The same relates to the
hydrogen producing cell but with the difference that the
hydrogen produced in high volume needs only little space
within the cell. During reaction a reduction of total
volume takes place because the hydrogen produced also
transports vapour out of the housing. To maintain contact
between the phases involved in the reaction, the electro-
lyte may be pressurized, which can be done by introducingit with small overpressure into the wide pores of a hydrop-
hobe body or by using hydrophile absorbent paper in the
important areas. It is also possible to maintain contact
between a zinc electrode and conductor and separator by
means of a spring element, the separator is a hydrophile
and arranged between the zinc electrode and the gas evol-
ution electrode.
Finally, it is possible to enforce the change of
volume from outside by deformation of the cell housing.
The element disclosed may be applied to pressure
pistons used to transport fluids and similar media. Due
to the fact that the internal resistance of such cells is
only some ohms a slow rate of gas generation may be set and
controlled by varying the external resistance. As electro-
motive force the difference in voltage between the zinc
1 3335 79
electrode and the reversible hydrogen electrode may be
regarded which in 6 n (normal) potash lye (KOH) is 0.42
volts. To produce a hydrogen current which is equivalent
to an electric current of 10 mamps an external resistance
of 50 ohms is sufficient. To produce hydrogen equivalent
to an electric current of 1 mamps the external resistance
must be 500 ohms. In this case the variation of the
internal resistance of the cell of about 10 ohms is of no
importance any longer.
The gas generating element according to the
invention is arranged within the space of the piston or in
the pressure chamber. It will be activated e.g. by a
sealed screw which via a chosen resistance produces a
short-circuit between the zinc electrode and the gas
producing electrode. Preferably the housing of the cell
may be formed by deformation of the piston while the gas
evolution electrode is arranged within the housing in a
sealed and pressure-safe manner. To start gas generation,
a short-circuit is made between the zinc electrode and the
gas evolution electrode by a resistance which e.g. is
formed as a layer and comprises a time-scale for adjusting
the process time, which scale shows in which time the
available quantity of lubricant will be dispensed.
In the example mentioned hydrogen will be pro-
duced because the whole active volume of the cell is filled
with a substance which will be oxidized by water. If the
zinc is exchanged for cadmium it is not possible to start
hydrogen generation by contact of a metal with a low
hydrogen minimum overvoltage. However, such an element may
be used for hydrogen evolution by running a current through
the element from an external source and arranging the
cadmium as anode and the hydrogen evolution electrode as
cathode. In this case the cathode is connected to the
negative pole and the cadmium electrode is connected to the
positive pole of the electric source. Also in this cell
- 12 - 1333579
hydrogen is produced which is equivalent to the current.
In contrast to the zinc element described above there is no
danger of hydrogen evolution in the absence of current,
because cadmium is a noble metal with respect to hydrogen
and is not able to displace hydrogen out of the compound
with oxygen.
In some cases it is preferable to block evolution
of hydrogen. In such cases an oxygen producing element may
be used in which oxygen is anodically produced in the
electrodes in the described manner. As a counter electrode
a metal oxide is used, e.g. silver oxide, mercury oxide,
nickel oxide, lead dioxide or manganese dioxide; in such
cases the oxides are reduced - depending on their electro-
chemical behaviour - to metal (silver, mercury etc.) or to
an oxide with lower valency (e.g. Mn304). However, the
current consumption to produce a corresponding quantity of
oxygen is twice that required for generating hydrogen
because the electrochemical valency of a molecule of oxygen
is 4 while the electrochemical valency of hydrogen is only
2.
There is no need to use metal oxides as material
for oxygen electrodes. It is possible to reduce ions of
nitrate into ammonia in a special cell, while the counter
electrode is producing oxygen. Raney-nickel electrodes may
be used as both oxygen and nitrate reduction electrodes.
It is also possible to use ammonia for generation of
hydrogen because under anodic current the ions of ammonia
are oxidized to nitrite resp. nitrate ions in the Raney-
nickel electrodes, while the counter electrode produces
hydrogen.
The common features of the gas producing cells
described are, that they contain the initial state either:
- 13 - 1333579
a) electrochemical oxidizable substances, a
hydrogen electrode and a liquid electrolyte
or
b) electrochemical reducible substances, an
oxygen electrode and a liquid electrolyte
and that they generate under flow of current initiated from
outside hydrogen or oxygen, which is produced in the pores
of a gas diffusion electrode and penetrates through the
pores of a hydrophobe diffusion membrane into an external
chamber, while the electrolyte is retained in the cell
housing due to the high capillary depression of the mem-
brane.
Detailed Description of the Drawinq
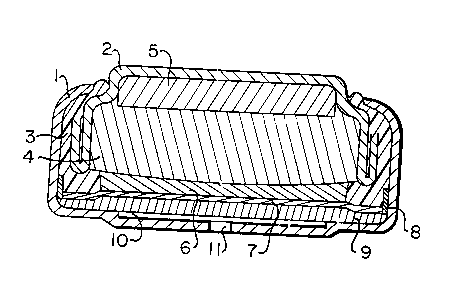
Figure 1 shows a diagrammatic elevation in
section of one embodiment of a cell according to the
invention. The knob cell comprises a cup 1 and a cover 2
which together with a plastic seal 3 form the housing.
Within the cover 2 and in contact with it is an active
substance 4 in form of a zinc gel containing an electrolyte
or in form of a porous tablet of manganese dioxide. 5 is
a compressible porous body which may contain an additional
quantity of electrolyte. 6 is a fleece impregnated with
electrolyte, 7 a separator in form of a ion-exchange foil.
This foil is kept in position by a support ring 8. 9 is
the gas diffusion electrode which is e.g. made of a Raney-
nickel powder bound with PTFE and rolled into a net ofnickel. On the side to the bottom of the cup 1 the gas
diffusion electrode is provided with a PTFE foil. The
metallic support ring 8 is in contact with the gas diffu-
sion electrode 9 and electrically connects the gas diffu-
sion electrode 9 and the cup 1. 10 is a wide-pore fleece
layer which channels the gas generated in the gas diffusion
1333579
- 14 -
electrode to the hole 11 in the bottom of the cup from
where is leaves the cell.
Every zinc atom sets free two electrons, which
are able to reduce one molecule of water to hydrogen. It
is therefore necessary to insert into the cell for every 65
grams of zinc 18 grams of water.
As will be apparent to those skilled in the art
in the light of the foregoing disclosure, many alterations
and modifications are possible in the practice of this
invention without departing from the spirit or scope
thereof. Accordingly, the scope of the invention is to be
construed in accordance with the substance defined by the
following claims.