Note: Descriptions are shown in the official language in which they were submitted.
1 3341 24
- -1- 63293-2883
PROCESS AND APPARATUS FOR PRODUCING HYDROGEN
The invention relates to a process for producing
hydrogen and to an apparatus suitable for carrying out such a
process.
It is well known to prepare a hydrogen-containing gas
such as synthesis gas (which mainly contains hydrogen and carbon
monoxide, and in addition carbon dioxide, nitrogen and
(unconverted) hydrocarbons and steam) by means of steam reforming
or (non) catalytic partial oxidation of a hydrocarbonaceous feed.
It is furthermore known to remove hydrogen from a
hydrogen-containing product gas, e.g. by means of pressure swing
adsorption, thus obtaining substantially pure hydrogen and in
addition hydrogen-depleted off-gas.
It has now been found that said hydrogen-preparation and
-separation steps can be efficiently integrated by employing
energy produced by combusting in a combustion zone hydrogen-
depleted off-gas obtained from the latter step in at least one of
the steps of the integrated process itself, e.g. for the
compression of oxygen-containing gas required in at least the
former step of the process.
The invention therefore provides a method for
integrating hydrogen-preparation and hydrogen-separation steps
comprising the steps of:
a) preparing a gas mixture containing hydrogen and carbon-
monoxide by means of 1) steam reforming in a convective reformer
_1
~IF
1 1 3 3 4 l6234293 2883
containing a reforming zone and a combustion zone at a temperature
from 600C to 1600C and a pressure from 2 to 200 bar or by means
of 2) (non)catalytic partial oxidation of a hydrocarbonaceous feed
in a (non)catalytic partial oxidation zone at a temperature from
600C to 1600C and a pressure from 1 to 250 bar;
b) optionally catalytically converting at least part of the
carbon-monoxide present in the said gas mixture in the presence of
steam at carbon-manoxide conversion conditions into carbon-dioxide
and hydrogen in a conversion zone which is maintained at a
temperature from 180 to 450C and at a pressure from 2 to 200 bar;
c) removing hydrogen from the obtained product gas by
passing the hydrogen-containing gas to a pressure swing adsorption
(P.S.A.) unit from which separately a substantially pure hydrogen
gas stream and a stream of hydrogen-depleted P.S.A. off-gas are
withdrawn; wherein the said hydrogen-depleted P.S.A. off-gas
obtained in step (c) is combusted with an oxygen-containing gas in
at least one of the steps of the said integrated process itself:
in the said combustion zone of the said convective reformer for
convective heating of the said reforming zone or in the combustion
chamber of a gas turbine driving a compressor providing oxygen-
containing gas to the said combustion chamber of the said gas
turbine.
The process according to the present invention will be
elucidated hereinafter with the use of the Figures in which
various preferred options of the process have been incorporated
without having the
1 334 t 24
-- 2 --
intent of limiting said process to those partinllAr Pmho~imP~ts as
depicted in the Figures.
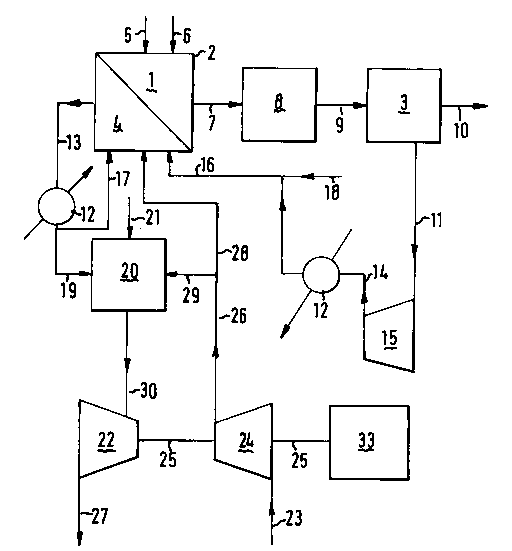
Figure l relates to a ~Lefe-l~d ~rha~imPnt of the present
process wherein h~dloy~-depleted off gas is heat .~ An.~ with
flue gas, before use as fuel gas in a convective rPf~rmin~ zone.
Figure 2 relates to another preferred ~mko~ nt of the
process according to the invention in which off gas is used as fuel
gas in a gas ~lrhinP.
R~Le.~e numerals relating to similAr process steps andtor
equipment are the same in the two Figures.
In Figure l the essential process steps (a), (b) and (c) are
carried out in reforming zone (l) of convective reformpr (2), in
pressure swing adsorption unit (3), and in combustion zone (4),
Le:j~Lively.
A h~Lu~.h).. A~ feed, ~LefeL~bly containing ~rmAlly
liquid and/or ~cy~l~ hydrocArhnn~, in parti~llAr Cl-C4 hydrocArhnn~
such as those present in natural gas, is introduced via line (5)
into L~fo..,;ng zone (l) Loy~Lher with steam introduced via line (6).
In zone (l) step (a) of the present process is suita-hly carried out
at a L~.~eLa~re fl~.l 600 to 1600 C and a pressure from 2 to
200 h~r. The refQrming zone preferably comprises catalyst in order
to ~eLaLe said zone at a relatively low L~.~e1~LuLe from 600 to
1100 C and at a pressure from 5-50 bar.
The reactor which contains said ref~rming zone (l) and option-
ally combustion zone (4) (which may also be spaced apart from the
ref~rming zone and be located outside the reactor) preferably
contains internals in order to improve heat exchange between said
zones and ensure optimal use of catalyst, if any.
The reactor intPrnAl~ suitably comprise double concentric
tubes with catalyst in the annular space between the tubes. The
outer tubes are suitably mLunted s~sL~ILially vertically in a
horizontal inlet manifold for hydrocArhnn/steam feed distribution.
The lower ends of the outer tubes are preferably closed in order to
re~tlse the flow of gas having passed downwardly ~1L~U~11 the
AnmllAr catalyst bed. The inner tubes into which the h~L~y~--
_ 3 _ l 3341 24
containing product gas is sllhse~l~ntly passed, are suitably
connected to a product outlet mAn;fold. AdvantA~e~lcly, the
oombustion gas (having a ~ ~eJ~LuLe of e.g. 900-1200 C) enters
the ,-f~..,;ng reactor below or n~Ar the lower ends of the ~lh~ r
reaction zone and leaves the reactor below the hor;7~ntAl inlet
manifold, situated at the relatively cold (e.g. 500-800 C) upper
part of the reactor. When the concentxic tubes are mDunted in the
above-descr;h~ l,Yu~lel, their hot lower ends can expand freely and
~hPrmAl ~x~An~;~n in the m nifolds is kept to a m;n;mllm.
A gas mixture oon~A;n;ng hyd~uy~ and carbon m~,nx;~ is
L~.JV~ from reform;ng zone ~1) through line (7). In order to
produ oe a~;tional h~koy~n, at least part and ~ere~hly all of
said gas mixture is ~rerelably directed to carbon mnnnx;~
~llv~l~ion zone (8) in which at least part of the carbon mn~nx;~
present in the gas mixture is catalytically O~llV~3d in the
presenoe of steam at the a~lu~iate ~ .x;~P ~ullv~lsion
conditions in one or mDre steps into carbon ~;~x;~. C~l~v~ ion
zone (8) is sui W ly m~intained at a ~ e~a~re from 180 to 450 C
and a pressure from 2 to 200 bar.
H~d~y~,-contA;n;ng product gas obtained from wl~v~ion
zone (8) and/or l~r~ ng zone (1) is directed via line (9) to
pres Æ e swing adsorption unit (3) from which a suLs~ ;A11Y pure
h~koy~. gas stream is wi~h~rAwn via line (10). Unit (3) ~le~lably
comprises a plurality of vessels oont~;n;ng molP~llAr sieve beds
which are sequentially in the adsorption-, desorption- and purge-
stage. Hcwever, it is also poss;hlQ to substitute a liquid
absorption unit (wherein carbon mnnox;~p and/or ~Arbon ~;nx;~P are
selectively Ahsorhe~ by a liquid which is subsequently ~y~ ~la~ed)
or a h~kuy~l pPn~PAhle II~I~LaUle unit for pressure swing adsorption
unit (3) in order to recover h~dl~y~ from the product gas ob~;ned
via line (9).
H~koy~l-depleted off gas (which may still oontain up to 5 or
even up to 30% by volume of h~kuy~l, ~PpPn~;ng on th~ type of
adsorption unit and pressure employed) obtained fl~.l unit (3) is
- \ -
~ 4 ~ l 3341 24
eL~bly directed via line (11) to oompressor (15) and
e~ ntly via line (14) to heat ~x~h~n~r (12) wherein heat is
~x~h~n~e~ with the effltlent gas stream (13) from oombustion
zone (4) which gas stream generally has a higher l~ ~ Lure (e.g.
from 150 to 1000 C) than the off gas. Accordingly, the energy
eff;~ cy of the process according to the invention is
subst~nt;~lly ill~Luv~d, thus Pn~hl;ng optimal use of the
h~L~ -depleted off-gas in one or more process steps.
The heat e~ my~d off-gas is directed via line (16) to
combustion zone (4). As the energy-cvllL~lL of the off-gas is in
many cases not ~lff;c;~nt to employ said gas as the only fuel
saur oe for a cc~bustion zone, additional fuel is ~LereLobly
provided via line (18).
In a preferred ~mho~;m~nt of the pro oe ss according to the
invention as depicted in Figure 1 the combustion zone (4) as
applied in step (c) provides ~h~rm~l energy for the læfo.~
reaction zone (1) of step (a) by means of o~llv~Live heat transfer.
A main a~v~-L~y~ of such an aLl~ J - ~L is that the reaction zone
will as a result he heated substantially lm;formly instead of
risking local overheating by a number of burners located in the
reaction zone, as in previous reform;ng process~s.
~fflll~nt gas from combustion zone (4) ~rpl;f~ in steps (a) and
(c) as ~ se~ her~inhefore is suitably (after heat eXchAn~e)
dil}YiJ2d via line (19) to a separate cc~bustion zone (20) to be
used as ~ LCL gas LoyeL~leL with fuel gas supplied via
line (21). Cp~;on~lly, part of the heat-px~-h~e~ effluent gas is
recycled via line (17) to oombustion zone (4). ~ff~ nt gas
emanating from the. latter oombustion zone (20) is preferably
dlrecbed via line (30) to turbo-~xL~I~Pr (22) wherein the gas is
~x~ to provide mp~hAn;cAl energy to ~ SS oxygen-con~A;n;ng
gas (e.g. air) supplied via line (23) to ~ SS~L (24). In some
cases ~lff;~;,Pnt oxygen is present in the e~x~ded efflu~nt gas
obtained via line (27) from turbo-Px~ , (22) to enable the use
of said gas as oxygen-containing gas for the oombustion zone (not
depicted in Figure 1).
_ ~ 334 1 ~
- 5 -
Thrbo-PxpAn~r (22), ~AI~L~SsoL (24) and ~eraLoL (33) are
pr~r~ y coupled (e.g. by means of axis (25)) and opt;nnAlly
cnmh;nf~ with one or mDre other compressors (15).
Ccmpressed oxygen-contA;n;ng gas (e.g. the gas provided via
line (26)) is pref~rAhly employed in at least one of the steps (a)
and (c) of the present process, in part;~llAr in combustion zone (4)
(via line (28)) _nd via line (29) in oombustion zone (20). me use
of compressed, and LheL~LY preheated, oxygen-containing gas is
~L~feLL~d in the process according to the invention in order to
improve the thPnnAl eff;~;~ncy of the combustion zone(s) and thus
of the entire process.
The process and d~aLaLus which are ~ a~ically depicted in
Figure 2 will be described hereinafter only in so far as features
diffeL~l~ from those depicted in Figure 1 are in~
A 5;gn;f;rAnt differ~e is the use of a catalytic or non-
catalytic partial oxidation zone (31) in the ~mho~;mpnt depicted in
Figure 2. &ch a zone is generally ~eLaLed at a L~l~eLaLure from
600 to 1600 C and preferably at a ~ Lure from 1000 to
1500 C, whereas the pressure in said zone is generally from 1 to
250 bar and ~L~feLably from 10 to 100 bar. Zone (31) constitutes
the reaction zone employed in step (a) as well as a combustion zone
as employed in step (c) of the process according to the invention.
A further difference with the process and a~aLa~us as depicted
in Figure 1 is that in Figure 2 the h~dkoy~l-depleted off gas
obtained from h~L~y~l separation unit (3) through line (11) is
used as fuel gas in combustion zone (20) of a gas ~llrh;n~ instead
of in a ccmbustion zone (4) of a r~form;ng a~aLaL~s. F~ P~
eff~lent gas from turbo-P~pAn~er (22) is opt;~nAlly at least partly
used in a combustion zone (not depicted in Figure 2).
O~ygen-cnnt~;ning gas is advant~qeal~ly provided by oompressor (24)
via line (26) to oombustion zone (20). S~bstantially pure oxygen
gas is supplied via line (34) to compressor (32) and sllhsec~ently
diracbed via line (35) to partial oxidation zone (31).
The invention further relates to an apparatus suitable for
producing h~loy~l which oomprises a reactor having feed inlet
1 334 1 24
-- 6 --
means and product outlet means cYnT~m;cAting with heat ~x~hAn~Pr
reactor intPrnAl~, a combustor which is in heat exchAn~ relation
with said intPrnAl~ a pressure swing adsorption unit cY~T~micAting
with the product outlet means and having separate h~rvy~l and
off-gas outlet means, and a gastllrh;n~ which is in oommunication
with the conbustor and/or the off-gas outlet mPans.
The process according to the invention is ill~sL~aLed hy way
of th~ following Example.
EX~E
me process su~hstA-ntially as depicted in Figure 2 is carried
out by intro~l~;ng 872 tons/day of feed gas (contA;n;ng
su~sLanLially methane) at a L~.~elaLure of 50 C and a pressure of
51 bar in catAlytic partial oxidation zone (31) and reacting the
feed gas with 2490 tons/day of su~LallLially pure oxygen gas
introduced via line (35) at a L~,~elàL~re of 100 C and a ~Les~u
of 48 bar.
The h~dL~yt~ ntA;n;ng synthesis gas ohtained via line (7) at
380 C and 30 h~r is subjected in carbon m~nnx;~ veLsion zone
(8) to a catalytic steam shift together with steam having a
L~,~elaL~re of 380 C and a pres OE e of 61 bar. 2160 tons/day of
mainly h~dLuy~l- and carbon ~;nX;~-contA;n;ng product gas from
zone (8) is led to Pressure Swing AdsoL~Lion unit (3) at a
L~.~elaL~re of 40 C and a pressure of 26 bar; fram unit (3) 200
tons/day of suhs~A~t;Ally pure h~dL~y~n is obtAined at 40 C and 25
bar in addition to 1960 tons/day offgas oontA;n;~ carbon dioxide
and h~dL~y~l as major r~5~ Ls at 40 C and 1.6 bar. & id offgas
is led via line (11) to compressor (15) from which an outlet gas
stream (14) is obtained at a L~,~eLaL~re of 310 C and a pressure
of 17 hur and cnmh;ne~ with 344 tons/day of methane-contA;n;ng gas
at a pressure of 51 bar and a L~~ ature of 50 C having a S;m;lAr
c~m~os;tion as the feed gas to zone (31).
The cnmh;n~ gas stream (16) is directed to a gas tllrh;n~
oomprising oo~bustion zone (20), ccmpressor (24) and turbo ~
(22). In said gas tllrh;n~ 76 Megawatt electric power is y~leLdLed
by generator (33) of which 18 Megawdtt is required for operating
_ 7 _ l 3341 2~
compressors (15) and (32), leaving 58 Megawatt nett power export,
excluding additional electricity y~ ~a~ion by means of waste heat
recovery from the ~X~ f~ efflll~nt gas stream (27).