Note: Descriptions are shown in the official language in which they were submitted.
3~6653
~ETHOD AND APPARATUS FOR TREATING THIN SAMPLE
ON A SURFACE EMPLOYING CAPILLARY FLOW
The present invention relates to apparatus and
methods for treating samples such as histology,
5 cytology, or hematology specimens immobilized on a
suitable flat surface such as a microscope slide with
liquids such as: (1) chemical staining solutions or (2)
dissolved reagents such as (a) antibodies or (b) labeled
DNA or RNA probes, such reagents being used,
respectively, for detection of antigens or nucleic acid
sequences present in the immobilized sample.
In the present art of histology, cytology, and
hematology, most clinical or research laboratories
employ manual staining procedures which require many
hours of technician time to perform. These procedures
are usually cost effective because large batches of
slides can be stained simultaneously in a single
sequence of staining events by an individual
technician. Both manual and automated staining systems
in current use sequçntially immerse a holder containing
parallel slides with tissue or cellular smears
immobilized on one planar surface of each slide in an
identical series of liquid reagents such as aqueous
reagents or organic solutions of dyes or stains in a
routine or programmed fashion. Exemplary manual
staining systems for histology, cytology, and hematology
specimens are well known to the art of histo- and
cytopathology, and protocols for their performance can
be found in any laboratory performing staining on
immobilized specimens. Exemplary automated systems
include those sold by Technicon Instruments, Shandon
Southern and Fisher Scientific (see pages 426-427 of the
Fisher 86 Catalog for a description of the Fisher
Histomatic~ Slide Stainer Model 172).
Capillary action has been used in the following
prior art patent in an attempt to develop bulk automated
slide staining procedures. U.S. Patent 4,199,613 to
Johnson (1980) describes a system wherein a stack of
__
1 336653
parallel slides are engaged near both ends by a series
of generally parallel shims. The shims are between
corresponding ends of adjacent slides being stacked in
parallel so as to space the facing planar surfaces of
5 adjacent slides by the thickness of the shims. Such
thickness (e.g., 0.008 inch or 0.2 mm) provides a
spacing between such opposite planar faces of adjacent
slides suitable for capillary flow. In use, a set of
slides (e.g., 50) is held in a vertical stack; and a
continuous stream of liquid (e.g., staining solution)
flows over adjacent edge portions of the slides
(starting with the top slide in the vertical stack) and
fills successively the thin gaps between adjacent
slides. The filling is by capillary flow in a
horizontal direction. Excess liquid over that required
to fill the thins gaps flows off of the bottom slide.
This system is intended to stain a multiplicity of
slides with an identical series of reagents which is the
same strategy used in manual and automated staining
procedures noted above.
In the field of trapping liquid specimens in a
microscopic viewing space, which field is not admitting
to be analogous with the treatment of immobilized
samples by liquid stains and reagents, capillary flow is
often used. Generally, as in U.S. Patents 4,501,496 to
Griffin (1985) and 3,961,346 to White (1975), liquid
sample is introduced onto a bottom plate and migrates by
capillary flow into a thin gap defined by a viewing
surface of the bottom plate and an overlaying clear
plate. In U.S. Patent 4,308,028 to Elkins (1981),
however, a device called a strip is immersed vertically-
extending into a sample such as a centrifuged urine
sample in a tube. As described at col. 4, line 53 -
col. 5, line 14 (see Figures 6 and 7 of Elkins), a
particulate-rich aliquot from the bottom fraction of the
sample flows by capillary action into a chamber
(identified as 14 in the Figures of Elkins). Elsewhere
in Elkins, the construction of the strip by lamination
--3--
1 336653
of multiple layers (one middle layer being short and of
defined thickness, at least one other layer being long
and transparent) is described. Col. 7, lines 3-45. At
the completion of the method, the sample in chamber 14
5 of approximately the defined thickness is viewed
unstained and untreated as indicated by Figure 22 of
Elkins through a portion of a long transparent layer
which extends beyond the end of the short middle
layer.
BRIEF DESCRIPTION OF THE DRAWING
Figure lA is a side elevational view of a slide
assembly according to a first embodiment of the present
invention.
Figure lB is a front elevational view taken along
lines lB-lB in Figure lA.
Figure lC is a front elevational view, in section
taken along line lC-lC in Figure lA.
Figure 2A is a side elevational view of a
disasssembled slide pair according to a second
embodiment of the present invention.
Figure 2B is a view similar to Figure 2A of the
same slide pair assembled within a holder portion into a
slide assembly.
Figure 2C is a top view of the slide assembiy in a
holder taken in section along line 2C-2C in Figure 2B.
Figure 2D is a view similar to Figure 2B of a
dissassembled slide assembly according to a third
embodiment of the invention.
Figure 2E is a view similar to Figure 2B of the
slide assembly of Figure 2D in a holder.
Figure 3A is a side elevational view, taken in
section along line 3A-3A in Figure 3B, of an array of
slide assemblies above a droplet holder device, each
according to the second embodiment of the present
invention.
Figure 3B is a plan view of the droplet holder
device shown in section in Figure 3A, taken along line
3B-3B in Figure 3A.
- - 1 3 3 6 6 5 3
Figure 3C is a magnified view of one slide assembly
contacting one droplet, from an angle similar to that of
Figure 3A, showing liquid being drawn vertically into
the thin gap by capillary flow according to the methods
5 of the present invention.
Figure 3D is a view, similar to that of Figure 3C,
of liquid being drawn vertically out of the thin gap by
capillary flow into an absorbent material.
Figure 4 is a front elevational view in section,
similar to that of Figure lC, of a slide assembly
according to a fourth embodiment of the present
nvent lon .
Figure 5 is a perspective view of an inverted slide
holder, partially filled with slide pairs, according to
a fifth embodiment of the present invention, differing
from the embodiment shown of Figure 2A, 2B, 3A, 3B, 3C
and 3D only in that the array is three rows of ten slide
pairs rather than five rows of five slide pairs.
Figure 6 is a plan view of an array of stations for
either a manual or an automated multistep process
employing the slide pairs array of Figure 5.
Figure 7 is a persepective view of a partially-
filled droplet holder according to the embodiment of
Figures 5 and 6.
SUMMARY OF THE INVENTION
The various methods and apparatus provided in the
present invention enable multistep treatment of a thin
sample or material immobilized on a flat surface with
the advantage of either conservation of expensive
liquids, flexibility in varying the treating liquids for
concurrently-treated samples or materials, minimization
of cross-contamination between samples, safety in
preventing toxic reagents from contacting laboratory
personnel or some combination of these factors. In the
present method, such advantage or advantages are
achieved: by the use of a thin capillary gap in front of
the surface containing the immobilized sample,
especially when the gap extends vertically, by contact
1 336653
of an edge of the gap with a discrete aliquot of the
treating liquid, especially at the base of the
vertically-extending gap, or by the subsequent removal
of the liquid by contacting an edge of the gap with an
5 absorbent material, especially the bottom edge of a
vertically-extending gap, or, especially, by
combinations of these features. Such features offer
particular advantages over the method of U.S. Patent
4,199,613, which cannot concomitantly treat individual
slides with unique reagents and which employs, b~
contrast, a horizontally-extending gap, introduction of
liquid as a continuous stream and removal of liquid by
spinning the entire slide assembly.
Although the present invention may be used for bulk
staining wherein a multiplicity of slides are exposed
serially to a single sequence of liquid reagents, it has
particular advantages over the prior art when used as a
discrete analyzer in which individual slides have their
own unique series of reagents applied concomitantly to
them.
Accordingly, the present invention provides, in one
form, a method for applying liquid to a thin sample on a
first surface which comprises the steps:
a) maintaining a second surface substantially
parallel to and spaced by a first distance from the
first surface, thereby providing a gap between the first
and second surfaces, and
b) contacting an edge of the gap with a discrete
aliquot of liquid,
the first distance being sufficiently small to
cause liquid to migrate by capillary action within the
gap into contact with the thin sample.
The present invention further provides, in a second
form, a method for treating a thin sample on a first
face with a series of treating liquids which comprises
the steps:
a) drawing a first treating liquid by capillary
flow in a gap between a sample-bearing first surface and
_
1 336653
a second surface of a facing element to at least the
position of the sample immobilized on the sample-bearing
first surface,
b) retaining the first treating liquid by
5 capillary action in the gap in contact with the sample,
c) removing the first treating liquid from the gap
by capillary flow, and
d) drawing a second treating liquid by capillary
flow in the gap to at least the position of the sample.
The present invention further provides, in a third
form, an apparatus for treating a thin sample on a first
surface which comprises:
a) engagement means for holding a first member
having a sample-bearing first surface a fixed distance
from a second surface of a facing element, with the
first surface and second surface being maintained
substantially in parallel and with first and second
edges of the two surfaces extending in parallel and
being separated by substantially the first distance, and
b) contacting means for contacting the space
between the first and second edges with a discrete
aliquot of a liquid,
the first distance being sufficiently small for
liquid to migrate from the space by capillary action
between the first and second surface into contact with
the sample.
The present invention further provides, in a fourth
form, an apparatus for treating a thin material on a
planar surface which comprises:
a) engagement means for holding a material-bearing
planar surface in a vertically-extending position a
first distance from a surface of a facing element, the
engagement means maintaining alignment between the
facing planar surfaces such that the lower edges of the
material-bearing planar face and the facing planar
surface are horizontally extending and substantially
parallel, and
b) contacting means for contacting the space
1 336653
-
between the lower edges of the material-bearing planar
surface and of the facing planar surface with liquid,
the first distance between the material-bearing
planar surface and the facing planar surface being
5 sufficiently small for the liquid to migrate upwardly by
capillary action between the facing planar surfaces to
at least the height of the thin material.
In each of the first four forms of the present
invention, the second surface (or surface of the facing
10 element) may also bear a thin sample or material which
is contacted by the same treating liquid as is the thin
sample or material on the first surface (or material-
bearing planar surface). Furthermore, or alternatively,
an array of multiple pairs of surfaces may be arranged
so that liquid is drawn by capillary action into the gap
between each pair of surfaces simultaneously,
concurrently or concomitantly.
The present invention further provides, in a fifth
form, an array of slide assemblies comprising:
a) a plurality of vertically-extending slides,
each having a vertically extending face,
b) a plurality of vertically-extending cover
members, each having a vertically-extending face,
each face of a vertically-extending slide beiny
spaced by a first distance less than 0.5 mm from a face
of a vertically-extending cover member, and
c) engagement means for holding the vertically-
extending slides and vertically-extending cover members
adjacent to their upper ends in a fixed array with the
sample face of each slide being a first distance from a
substantially parallel face of a vertically-extending
cover member and with the lower edge of each slide
extending horizontally and being spaced from a
substantially parallel horizontally-extending lower edge
of a cover member by the first distance,
the space between the horizontally-extending lower
edges being open.
The present invention further provides, in a sixth
~ -8- 1 3 3 6 6 5 3
form, a device for holding a horizontal array of
discrete aliquots of treating liquid comprising:
a) a horizontally-extending rigid base,
b) a horizontally-extending elastomeric member
5 having a substantially planar horizontally-extending
upper surface, and
c) a plurality of recesses formed in the
elastomeric member, each recess opening to the
horizontally-extending upper surface,
the elastomeric member having at its upper surface
a material sufficiently incompatible with the treating
liquid for a discrete aliquot of treating liquid in a
recess to form a convex shape extending above the plane
of the adjacent upper surface of the elastomeric member.
Although above-described systems, such as that of
Johnson, are capable of applying a specific sequence of
identical reagents to a set of flat surfaces such as
microscope slides, such prior art systems do not have
the flexibility to concommitantly process individual
slides with unique reagents. In addition, the volumes
required to immerse the slides in a vessel of aqueous or
organic stain are too great to economically perform
specific steps of more sophisticated analyses of tissue
or cellular bound antigens or genetic sequences, by
antibody-directed detection technology or nucleic-acid-
hybridization methodologies, respectively. Any multi-
step process involving such specific steps can only be
automated by the prior art systems by performing the
other steps, disassembling the slide array to perform
the specific steps manually, and then reassembling the
slide array to perform the subsequent steps
automatically. Such disassembly/reassembly defeats the
advantages of automation for such sophisticated
analyses. Therefore, there is a need, met by the
present invention, for either manual or automated
methods that perform simultaneous, multiple, and
discrete analyses on separate tissues or cellular smears
immobilized on individual slides using only microliter
1 336653
.
quantities of expensive antibodies or nucleic acid
probes.
In a further embodiment of the lnvention there i6
provided an apparatus for treating a thin sample on a
first surface which comprises:
a) engagement means for holding a first member
having said first surface containing a sample thereon a
fixed distance from a second surface of a facing element,
~ with the first surface and second surface being
maintained substantially in parallel and with first and
second edges of the two surfaces extending in parallel
and being separated by substantially the first distance,
and
contacting means for contacting the space between
the first and second edges with a discrete aliquot of a
liquid,
the fir6t distance being sufficiently small for
liquid to migrate from the space by capillary action
between the first and second surface into contact with
the sample, wherein said contacting means comprises:
(bl) liquid holding means comprised of the first
member and the facing element for holding a liquid
aliquot below the first and 6econd lower edges, and
(b2) moving means for moving the liquid holding
means vertically relative to the first member and facing
element.
DETAILED DESCRIPTION OF THE INVENTION
Such methods would have a wide spectrum of
applications in both clinical or research laboratories
that presently perform the analysis of discrete
antigenic or genetic information by individual manual
procedures.
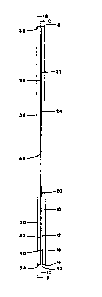
A first embodiment of slide pair assembly is shown
in Figures lA, lB and lC. Referring to Figure lA, the
sample-bearing microscopic slide 10 has a sample-bearing
front surface 12, a first lower edge 14, a back surface
16 and a top edge 18. A thin sample 20, such as a 5-10
,
_ -9a- 1 336653
micrometer thick histology specimen, is provided on a
lower portion of the front surface 12. Assuming that
the slide is 75 mm high, 25 mm side and 1 mm thick
(standard dimensions for a microscope slide), the sample
can be a 20 mm x 20 mm square located at least 1.0 mm
(e.g., 10 mm) mm above the first lower edge 14.
Attached to the upper portion of the front surface
12 of the first slide 10 is a shim 22, shown in this
first embodiment as two-sided adhesive tape of thickness
0.2 mm (200 micrometer). One sticky side 24 of the shim
22 adheres to the top portion of front surface 12,of
first slide 10. The opposite sticky side 26 of shim 22
adheres to a facing surface 32 of facing element or
slide 30. In this embodiment, facing slide 30 is also a
75 mm x 25 mm x 1 mm microscope slide. The shim 22
holds facing slide 30 in alignment with first slide 10
such that: facing planar face 32 of facing slide is
parallel to front surface 12 and spaced therefrom by the
thickness of shim 22 (200 micrometers), second lower
edge 34 of facing slide 30 is coplanar with first lower
edge 14 of first slide 10, back surface 36 of facing
slide 30 is parallel to surfaces 32, 12 and 16 and top
edge 38 of facing slide 30 is coplanar with top edge 18
of first slide 10.
The spacing of 200 micrometers is substantially
--10--
`- 1 336653
constant from between the inner edges of top edges 18
and 38, along the vertical lengths of front surface 12
and facing surface 32, and to the inner edges of first
and second lower edges 14 and 34. Assuming that the
5 tape is 25 mm high (its width can be the full 25 mm
width of slides 10 and 30, or can be less, e.g., 22 mm
as shown), then a gap 40 is formed between the front
surface 12 and the facing surface 32. This gap 40,
which is 50 mm high, 25 mm wide and 0.2 mm (200
10 micrometers) thick, is the capillary gap terminating in
lower end 42. The sample 20, being only 5-10
micrometers thick, has no significant impact upon the
thickness of the gap 40, even at the height of the
sample 20. Similarly, other imperfections, entrapped
particles, angling of the two slides toward or away from
parallel, or other factors that affect the gaps 40 by
less than 20% (i.e., cause the 200 micrometer thick gap
to remain between 160 and 240 micrometers in thickness)
have no adverse impact, and even slightly larger
variations would have no significant adverse impact.
Furthermore, while the basic or average thickness of the
gap in this first embodiment is 0.2 mm (200
micrometers), gaps as small as 0.05 mm (50 micrometers)
or as large as 0.5 mm (500 micrometers) are permissable,
with other dimensions (such as height) adjusted as
described below in relation to Figure 4. Under
appropriate circumstances, thickness of the gap still
less than 50 micrometers or more than 500 micrometers
may also be appropriate.
Figure lB shows the same slide pair assembly from
the front. The facing slide 30, with its back surface
36 on front, completely covers the first slide 10, from
the top edge 38 to the bottom edge 34 of the facing
30. Sticky side 26 of shim 22 can be seen under the top
portion of facing slide 30; and sample 20, which is
immobilized on sample slide 10, can be seen centered
under the lower portion of facing slide 30. The precise
vertical alignment shown in Figure lB, wherein neither
_
1 336653
-
side of first slide 10 extends beyond the corresponding
side of facing slide 30, is not critical. Misalignment
in such direction of 2 mm, or even 5 mm, is of no
significant adverse impact. Furthermore, as indicated
5 above, the widths need not all be equal (e.g., 25 mm).
Figure lC shows the same front view as Figure lB,
but now in section so as to look behind facing slide
30. The front face 26 of shim 22 occupies the top 25 mm
of the visible surface. The bottom 50 mm x 25 mm of
front surface 12 of first slide 10 (below lower end 44
of shim 22) is now visible; it is this 50 mm x 25 mm
that is exposed to the capillary gap 40. The sample 20
occupies a 10 x 10 mm portion centrally located within
this 50 mm x 25 mm portion of sample-bearing surface
12. The height of the gap can be adjusted by using
shorter or longer pieces of tape as shim: e.g., 25 mm
wide and 20, 30, 40 or 50 mm long (high) tape.
Figures 2A, and 2B and 2C illustrate a second
embodiment of slide pair assembly. First slide 10 with
first lower edge 14, front surface 12 and sample 20
thereon is identical to corresponding elements in Figure
lA. The facing slide 130 is also a 75 mm x 25 mm x 1 mm
microscope slide, with facing surface 132 and second
lower edge 134, but now the shim 122 is a 40 mm x 25 mm
(or 22 mm) x 0.15 mm glass cover slip having a lower end
144. The first 40 mm x 25 mm surface 124 of shim 122
faces (and, when assembled in Figure 2B abuts against)
the upper portion of front surface 12 of first slide
10. The second 40 mm x 25 mm surface 126 of shim 122 is
glued to the upper portion of facing surface 132 of
facing slide 130.
Along the back surface 136 of facing slide 130 are
provided upper and lower elastomeric protuberances 146
and 148, shaped as O-rings, compressible flat springs or
rollers or solid discs, which may have beveled upper
portions (not shown).
In Figure 2B, the slide pair of Figure 2A is
assembled by placing slides 10 and 130 together in
1 336653
parallel and slipping their upper ends into a recess of
dimensions 30 mm high, 26 mm wide and 2.4 mm thick
formed in holder 150. The recess opens downwardly and
has, on its top, a vertically-extending aligning face
5 156. Top edges 18 and 138 of first slide 10 and facing
element 130 abut against aligning face 156.
Protuberances 146 and 148 are engaged within a
vertically extending, downwardly-opening slot 152 within
the back wall of the recess formed in holder 150, so as
to force the upper portion of facing element 130 and all
of shim 122 against the upper portion of first slide
10. This combination of engagement means causes the
first slide 10 and facing slide 130 to be aligned in
parallel, with a gap the thickness of shim 122 (0.15
mm), the width of slides 10 and 130 (25 mm) and the
height (35 mm) not covered by shim 122. Lower edges 14
and 134 are at the same height and are spaced from each
other by substantially the same distance as the
thickness of shim 122, i.e., 0.15 mm.
Figure 2C is a top view of Figure 2B taken along
line 2C-2C in Figure 2B. In this sectional view,
protuberance 148 is seen inside its slot 152 which is
cut into the slide holder 150 as a downwardly open slot
in the recess. Protuberance 148 presses against slot
152 and compresses shim 122 which is glued to the
opposite side of facing element 130. This in turn
exerts pressure on the upper portion of the first slide
10 which is held in place by holder 150. In this manner
the upper portion of the facing slide 130 and the first
slide 10 are kept in contact and suspended vertically
below. Since slot 152 is downwardly open, the facing
slide 130 and the first slide 10 may be easily inserted
into and removed from the recess in the holder 150 by
the guiding action of slot 152 on protuberances 146 and
3S 148.
Figure 2D and 2E illustrate a third embodiment
differing from that of Figure 2A in that the
protuberances 146' and 148' are now located on the
- - 1 3 3 6 6 5 3
interior of the recess within the holder 150' rather
than on the back surface 136 of facing element 130.
Referring to Figure 2D, the sample-bearing
microscope slide 10 has its sample bearing front surface
5 12 facing a second sample bearing microscope slide 130'
and its sample-bearing surface 132'. Thin sample 20 on
sample bearing microscope slide 10 is present opposite
sample 120' on the opposite sample-bearing slide 130'.
Referring to Figure 2E, sample-bearing slides 10
and 130' are held in place in the recess in holder 150'
by the pressure of the elastomeric protuberances 146'
and 148' pressing against their upper portions. Shim
122' is sandwiched in between their upper portions.
Sample 120' immobilized on sample bearing surface 132'
of the second sample bearing slide 130' is held in the
gap 40 produced by the close apposition of the sample-
bearing surfaces held in place across and on the
opposite side of the gap 40 from sample 20 by the
pressure of protuberances 146' and 148' and the holder
on the upper portions of the two sample bearing slides
10 and 130' against shim 122'.
Figures 3A and 3B show how an array of twenty-five
slide pairs can be aligned and used in accordance with
the present invention. Referring to Figure 3A, one row
of five slide pairs is shown. Each pair of first slide
(lOa, lOb, lOc, lOd, lOe) is spaced from a second or
facing slide (230a, 230b, 230c, 230d and 230e) by a
shim. Vertical alignment is maintained by the upper
edges (256a, 256b, 256c, 256d and 256e) of five recesses
formed in the bottom face of holder 250.
Thus vertically-extending gaps of the thickness of
the shim are formed in each slide pair, as described
above in relation to Figures 2A and 2B, terminating in
lower spaces 42a, 42b, 42c, 42d and 42e between,
respectively, aligned first and second lower edges of
the first and facing slides lOa/230a, lOb/230b lOc/230c,
lOd/230d and lOe/230e. All sets of lower edges are in a
common horizontal plane a fixed distance below ~he lower
_
1 336653
face of holder 250.
A droplet holder is located below this horizontal
plane, consisting of a rigid base 62 and a horizontally-
extending elastomeric member 64. As shown in Figure 3A,
5 five holes 66a thru 66e are formed in and through
elastomeric member 64, and these holes are filled with
discrete aliquots or droplets 68a through 68e,
respectively, each of defined volume, e.g. 150
microliters. As described more fully below, each
10 droplet 68a-68e projects above the top face of
elastomeric member 64. The alignment is such that, when
the slide holder 250 is lowered, lower spaces 42a-42e
are contacted by the upper portions of droplets 66a
through 66e, respectively. The droplets are normally
introduced from above (e.g., by a micropipetting
device), but can also be introduced from below by means
of a narrow passage formed in rigid base 62. A
perspective view of an analogous droplet holder is shown
in Figure 7.
Referring to Figure 3B, the top of elastomeric
member 64 can be seen with five double rows of droplets
68a-68y and 69a-69y. Looking at the profiles of slides
lOa-lOe, with facing slides 230a-230e, it can be seen
that they will contact droplets 68a-68e and 69a-69e,
with, for example, lower space 42a contacting droplets
68a and 69a near the two ends of lower space 42a.
Just as the one row of slide pairs lOa/230a through
lOe/230e contacts droplets 68a-68e and 69a-69e, four
additional rows of five slide pairs each can be aligned
within holder 250 so as to contact, respectively: 2)
droplets 68f-68j and 69f-69j, 3) droplets 68k-680 and
69k-690, 4) 68p-68t and 69p-69t, and 5) 68u-68y and 69u-
69y. Because the lower edges of all first slides,
facing slides and thus lower spaces can be held in
precise alignment within a common horizontal plane, and
elastomeric member 64 holds the entire array of droplets
in precise alignment within a common horizontal plane,
one can reproducibly contact each lower space between
1 336653
first and second lower edges of a first and facing
slide, respectively, with two droplets. Furthermore, as
discussed below, the discreteness of droplets 68a-68y
and 69a-69y enables flexibility in treating samples on
5 each first slide either similarly or differently than
each other first slide as to the treating liquid
applied.
Referring now to Figure 3C, the effect of space 42a
(between first lower edge 14a and second lower edge 234a
10 of slides lOa and 230a) being contacted by a droplet in
hole 66a can be seen. A capillary column of liquid 70a
rises in the capillary gap 240 (similar to gap 40 in
Figure lA) by capillary action. This effect is enhanced
by the relative incompatability of the liquid with the
surface of elastomeric member 64, e.g., because the
aqueous droplet is repelled by the hydrophobic surface
of elastomeric member 64. Such incompatability
(evidenced by beading of the treatment liquid if it were
placed on a flat surface of elastomeric material used
for member 64) also causes the droplets to stand above
the top surface of member 64.
After the capillary column 70a has risen as far as
capillary action will take it (typically about 30 to 40
mm in the indicated gap of 0.15 mm), the slide assembly
can be lifted by holder 250 away from elastomeric member
64. Each slide pair (e.g., lOa/230a) will hold, by
capillary action, the treating liquid received from the
droplets (e.g., 68a and 69a) with which its lower space
(e.g., 42a) has been contacted. After the liquid has
remained in the gap for a desired time period, the slide
assembly is now lowered onto an absorbent material 72 as
shown in Figure 3D. Since the liquid is more compatible
with the absorbent material 72 than with the surfaces of
slides lOa and 230a, now the capillarly column 70a will
descend, with the treating liquid spreading downward and
outwardly as a liquid front 74a within absorbent
material 72. Within a matter of seconds, the slide pair
will be evacuated essentially completely of liquid by
-16
~ 336653
such capillary action, except perhaps for minute amounts
that may adhere to the sample or to other hygroscopic
surfaces along the slide gap 240 or lower edges 14a and
234a. Once the liquid is evacuted from the slide gap
5 240, the slide pair may now be moved to another droplet
holder, or to a sheet or bath of treating liquid for the
next step.
Figure 4 illustrates, in a view similar to that of
Figure lC, an embodiment of the invention wherein three
10 vertically-extending sample-bearing surfaces are formed
on one 75 mm x 25 mm slide. The slide extends
horizontally with its 75 mm lower edge 314. Two outer
shims 322 of 25 mm height, 2 mm width and 0.25 mm
thickness extend vertically on the front (75 mm x 25 mm)
face. Two inner shims 322' have similar 25 mm x 2 mm x
0.25 mm dimensions, and are equally spaced from and
parallel to end shims 322. Such shims 322 and 322' can
be formed by applying a thermosetting material (e.g.,
epoxy or silicone) to the face of a glass slide. The
uncovered and isolated faces are therefore 312a, 312b
and 312c, each extending upwardly 25 mm from lower edge
314, and each approximately 22.33 mm in width. A facing
slide can be placed over this first slide, so that gaps
of 0.25 mm thickness, 25 mm height and 22.33 mm width
will form over faces 312a, 312b and 312c. By contacting
the lower space of each such face which is adjacent to
lower edge 314 by a treating liquid and then by an
absorbent material, liquid reagent can be drawn into and
out of each gap as described above. Such a slide pair
can be applied to droplets or to a bath or sheet of
treating liquid manually.
Alternatively, a series of such horizontally-
extending slide pairs, each with three vertically-
extending capillary gaps, can be held within a holder
using, for example, the slide rack shown in Figure 1 of
U.S. Patent 4,199,613 of Johnson, with such modification
as is required to leave lower edges 314 of each sample-
bearing slide available for contact by droplets or
1 336653
sheets of treating liquid. The "shims" of Johnson in
this embodiment would not be positioned between a sample
slide and its companion facing slide to help form the
capillary gap between them, but would rather be located
5 at both laterial ends and on the outer surface of the
facings sample bearing slides, forcing them together by
compressing the facing slide and the sample bearing
slide against shims 322 described above. In this
embodiment, shims 322 and 322' in Figure 4 would be the
lO only parts defining the first distance of the capillary
gap between the facing and sample bearing slide.
The thickness of the side walls of the recess in
the holder would then define a second distance
separating parallel pairs of facing and sample bearing
slides. This second distance is not designed for
capillary action and separates sets of slide pairs so
that liquid reagents can be drawn up into them through
the capillary gap from discrete droplets as in Figures
3A and 3B. This second distance can be any thickness
greater than 2 mm, which is significantly thicker than
the 200 microns of ~ohnson's shims or the shims
described in this patent. The preferable length of this
second distance and, therefore, the preferable thickness
of the side walls forming the borders of any downwardly
open slide recess in the slide holder, ranges from 5 to
7 mm. Using this range, the greatest number of slides
can be engaged into a slide holder for the purpose of
drawing up, incubating and removing liquid reagents from
the capillary gaps between adjacent slide pairs.
This second distance range allows adjacent
capillary ga~s such as 42a and 42b in Figure 3A to be
maintained from 7 to 9 mm apart. At this distance,
individual droplets in the droplet holder such as 68a
and 68b and 69a and 69b pictured in Figure 3B can be
maintained apart without contaminating each other by
inadvertantly overcoming the incompatibility of the
surface of elastomeric member 64 and the individual
droplets in the droplete holder. Such advantage would
18-
1 336653
not be possible with the slide rack of Johnson where 200
microns is too close to stably separate adjacent reagent
droplets on the droplet holder. Therefore, the slide
rack of Johnson would have to be completely and
substantively modified from its original description to
5 achieve the advantages of the present invention.
To cause the liquid to rise 15-20 mm above lower
edge 314, the gap (thickness of shims 322 and 322') may
be thicker than the 0.15 - 0.20 mm thickness most
preferred in the earlier embodiments, where liquid was
10 intended to rise 25-45 mm above lower edge 14. Through
routine experimentation, the gap can be adjusted (by
varying shim thickness) to achieve the desired vertical
rise of liquid for any sample-bearing slide surface.
Figure 5 shows a holder partially filled with slide
15 pairs according to a fifth embodiment of the present
invention. It differs from the second embodiment shown
particularly in Figures 3A and 3B in providing three
rows of ten slide pairs rather than five rows of five
slide pairs.
The main body 450 of the slide holder shown in
Figure 5 is shaped as a rectangular solid with, as
described below, a series of slots formed in its lower
face for receiving slide pair assemblies.
Alternatively, the slide pairs may be held in a
holder where the series of slots formed at its lower
face are collapsible and can be tightened upon the top
portions of the slide pair assemblies using, for
example, a substantial modification of the slide rack of
Figure 1 of U.S. Patent 4,199,613 of Johnson in which
the ~shims" are significantly thicker and used to
separate slide pair assemblies and not to produce
capillary action.
Because the slide holder is inverted in Figure 5,
compared to its configuration in use, for the insertion
of slide pairs, this bottom face appears on top. In the
following description, relative positions in use (e.g.,
slots in the bottom face) will be described.
--19--
1 336653
A plate 451 is above main body 450 (as a flange) in
both horizontal directions so as to cover a larger
rectangular cross-sectional area than the rectangular
cross-sectional area of main body 450. An arm 476
5 extends vertically upward from one side of plate 451,
with two angled portions 478 and 480. A similar arm
476, with angled portions 478 and 480, extends
vertically upward from the opposite side of plate 451,
but is hidden from view. A horizontal bar 482 connects
the two arms 476.
Formed in the bottom face of main body 450 are ten
long slots, each extending vertically and in a
horizontal direction 90 relative to horizontal bar
482. These ten long slots are each divided by
partitions into three slots, for a total of thirty
slots. The nearest three slots are designated 455j,
455t and 455dd in Figure 5, each such slot being at the
near end of a row of ten slots. Sample-bearing slides
lOa, lOk and lOu are shown extending out of the slots at
the far end of each of the three rows. As illustrated
by facing slide 430u, a facing slide is inserted with
each sample-bearing slide in a common slot. The bottom
edges of each sample-bearing slide and the adjacent
facing slide defines a lower end of a gap, shown as
lower end 442a, 442k and 442u for slides lOa, lOk and
lOu, respectively. Each individual slide pair appears
in cross-section substantially as shown in Figure 2B.
If thirty sample-bearing slides are to be treated,
then the remaining slots shown in Figure 5 (up to slots
455j, 455t and 455dd) are filled and the entire slide
holder assembly inverted. To keep track of the various
slides, either visually- or machine-readable indicia may
be present or applied (e.g., on a frosted portion of
each slide remote from the sample) so as to be read
before and after treatment, or (if the indicia are
properly placed, e.g., just above the sample location)
also while the slides are in the holder. Additionally,
the holder may be indexed numerically to ease the
.._
-20-
1 336653
localization of individual slides without taking them
out of the holder and to ease reagent handling by having
corresponding numbers denoting the specific holes in the
droplet holder pictured in Figures 3B and 7 with which
5 the slide pair assembly interacts.
The holder is then lowered into a bracket the width
of horizontal bar 482 along angled portions 478 of arms
476 until the slide assembly is held and aligned
(vertically and horizontally) by the engagement of the
10 bracket with horizontal bar 482 and arms 476. The
machine can now conduct the assembly through a series of
stations as described below. Alternatively, the
holder's horizontal bar 482 may be engaged manually and
thereby advanced.
Figure 6 shows a plan view of the interior of an
automated system for practice of the present
invention. It resembles the interior of a HISTOMATIC~
Slide Stainer (Model 172) as illustrated on page 426 of
the Fisher 86 Catalog (Fisher Scientific 1985),
modified for practise of the present invention.
Figure 6 illustrates an array of stations into
which the slide array of Figure 5, once completely
assembled, can be dipped by sequential operation as
described below. Stations 1-6 (numerals 501 through
506) contain, in this arrangement, staining vessels of
the general type previously used with the HISTOMATIC~
slide stainer, Model 172 (115V, 60 Hz version). See
Fisher 86 Catalog, pp. 426-27 (Fisher Scientific 1985).
Each vessel holds a pool of liquid (xylene, ethanol,
ethanol/water mixtures or distilled water, as indicated)
of top cross-sectional area being larger than the array
of lower edges of slide pairs in Figure 5. Such
geometry permits the array to contact each pool without
hitting a vessel edge. Similarly, stations 8 (numeral
508), 10 (numeral 510), 12 (numeral 512) and 17 (numeral
517) contain staining vessels of composition indicated
below.
Station 7 (numeral 507) is a wet chamber maintained
-21-
1 336653
._
at 37C I 5C (once enclosed as described below) by a
standard electric heater, kept saturated by water vapor
because a pool of water is placed in the chamber below
the height reached by the lowermost horizontal surface
5 of the slide array. The top of the wet chamber is of
horizontal dimensions (rectangular or square) larger
than the slide array in Figure 5, but smaller than the
flange 451 in Figure 5. Accordingly, when the slide
array 450 in Figure 5 is lowered into the wet chamber in
station 7 (numeral 507), the flange 451 (shown in Figure
5) completes the enclosure of the wet chamber.
Stations 9 and 11 contain dry blotters such as
paper, cotton or super-absorbent gauze pad with top
surfaces sufficiently high and level to simultaneously
contact lower spaces (see 42a in Figure 2D) of the slide
array when the array is lowered into the appropriate
station. The slide array may, in such case, compress
the blotter material down a short distance.
Station 13, 14, 15 and 16 (numerals 513, 514, 515
and 516) contain droplet holders similar to elements 62
and 64 in Figures 3A and 3B except that the holes and
droplets are arranged in three double rows of ten.
Thus, in station 13 (numeral 513), the top row of ten
slides will contact, simultaneously, droplets 468a-468j
and 469a-469j in the same manner described above for
droplets 68a-68e and 69a-69e as shown in Figures 3A and
3B. The second double row, beginning with droplets 468k
and 469k, will be contacted simultaneously by the lower
spaces of a second row of ten slide pairs. The third
double row, beginning with droplets 468u and 469u, and
ending with droplets 468dd and 469dd, will be contacted
simultaneous by the third row of ten slide pairs when
the slide pair array is lowered into station 13 (numeral
513).
In similar fashion, stations 14 (numeral 514), 15
(numeral 515) and 16 (numeral 516) each contain a
droplet holder, each holding in precise alignment three
double rows of ten droplets (sixty droplets in each
- _ 1 3 3 6 6 5 3
station). The lower row in Figure 6 is identified as
droplets 569u thru 569dd in station 14, 669u thru 669dd
in station 15 and 769u thru 769dd in station 16.
Station 18 (numeral 518) is empty in the array shown in
5 Figure 6. If additional treating steps are desired, it
can contain a staining vessel, droplet holder or
temperature bath, as appropriate, similar to another
station described above.
Washer 519 is the standard unit for washing slide
10 arrays provided with the HISTOMATIC~ Slide Stainer,
Model 172. It is equipped either for once-through flow
of rinsing liquid or recirculation of treating liquid.
The latter mode is generally used in the present
invention. In actual work, this unit has been modified
by a solenoid to provide for recirculating flow of
rinsing liquid only when the slide array is in the
washer and to provide no drainage instead of a
continuous drainage as when the machine is operated in
the flow-through mode. The dryer 520 is a station
generally not used in the present invention (because of
the use of blotting stations 509 and 511), but
preferably present so that the instrument can also be
- used for conventional staining of slides arranged
vertically-extending and separated one from another by a
distance greater than 0.5 mm (e.g., 2.0 mm) when using a
standard 40-place slide holder provided commercially
with the above Model 172 Slide Stainer.
The flexibility of this invention is illustrated by
the fact that all the staining vessels, droplet holders,
and wet chambers are completely removable and
interchangeable at the discretion of the user.
Therefore, for example, the droplet holders of Station
13, 14, 15 and 16 (numerals 513, 514, 515, 516 of Figure
6) can be easily replaced even with the instrument
running, with a shallow common reagent tray to treat all
slide pair assemblies with an identical reagent or an
additional blotter to evacuate them or a wet chamber to
incubate them. The flexibility of the present process
-23-
- - 1 3 3 6 6 5 3
is further illustrated by the following illustrative
process for staining tissue sections with a respect to
antigenic sites for antibody. The following staining
procedure log refers to numerals in Figure 6, as
5 described above. Following the log are a discussion of
several of the individual steps and a discussion of how
the procedure would be modified to use three different
types of tags: avidin biotinylated horseradish
peroxidase complex, alkaline phosphatase linked to goat
10 anti-mouse antibody and, as the initial probe, either a
primary biotinylated heterologous primary antibody, an
unlabeled monoclonal antibody, or a DNA or RNA strand
linked to biotin in the manner of EPA 63,879 of Ward,
Wildrop and Langner (November 3, 1982, based on U.S.S.N.
255,223) or PCT 84/04970 of Ward, Leary and Brigati
(December 20, 1984, based on U.S.S.N. 503,298), both
assigned to Yale University. See also Proc. Nat. Acad.
Sci. vol. 80, pp. 4045-49 (1983); Virology, vol. 126,
pp. 32-36 (1983).
The staining procedure begins with thin (e.g., 5
micrometer thick) slices of tissue which are cut from
blocks of tissue that have been formalin fixed and then
wax embedded in, e.g., a Histomatic~ Model 266 MP Tissue
Processor (Fisher Scientific) (see U.S. Patent 4,141,312
to Louder, issued Febrary 27, 1979). Each event is
described below by number, station (and corresponding
numeral in Figure 6), time and solution or other
treatment.
Station (Fig. Time Solution or
Event6 Numeral) (min.) Other Treatment
LA 1 (501) 1.0 Xylene
lB 9 (509) 0.6* Blot
2A 1 (501) 1.0 Xylene
2B 9 (509) 0.6* Blot
353A 1 (501) 1.0 Xylene
3B 9 (509) 0.6* Blot
4A 2 (502) 0.6 Xylene
-
--24--
1 336653
4B 9 (509) 0.6* Blot
5A 3 (503) 0.2 Reagent Alcohol or
Absolute Alcohol)
5B 9 (509) 0.6* Blot
6A 3 (503) 0.2 Reagent Alcohol (or
Absolute Alcohol)
6B 9 (509) 0.6* Blot
7A 4 (504) 0.6 95% Ethanol
7B 9 (509) 0.6* Blot
1 0 8A 12 (512) 5.0 Acid Alcohol
8B 9 (509) 0.6* Blot
9A 5 (505) 0.2 30% Ethanol
9B 11 (511) 0.6* Blot
lOA 6 (506) 0.2 Triton~ X-100 (0.1%) in
distilled water
lOB 11 (511) 0.6* Blot
llA 6 (506) 0.2 Triton~ X-100 (0.1%) in
distilled water
llB 11 (511) 0.2 Blot
12A R (519) 1.0 Buffer (O.lM Tris HCl,
O.lM NaCl, pH 7.5,
0.01% Iriton~ X-100) in
the recirculating n~de
12B 11 (511) 0.6* Blot
13A**13 (513) 0.6 Enzyme Digestion
Solutions**
13B** 7 (507) 2.0 37C Wet Chamber
13C** 9 (509) 0.6* Blot
14A** R (519) 2.0 Buffer
14B**11 (511) 0.6* Blot
15A 14 (514) 0.6 0.25% Gelatin in O.lM
Tris HCl, O.lM NaCl! pH
7.5
15B 7 (507) 2.0 37C Wet Chamber
3 5 15C 9 (509) 0.6* Blot
16A R (519) 0.6 Buffer
16B 11 (511) 0.6* Blot
17A 15 (515) 0.6 Primary Antibody
-25- 1 336653
(Biotin-labeled)
17B 7 (507) 60 37C Wet Chamber
17C 9 (509) 0.6* Blot
18A R (519) 2.0 Buffer
5 18B 11 (511) 0.6* Blot
l9A R (519) 1.0 Buffer
l9B 11 (511) 0.6* Blot
20A 16 (516) 0.6 Avidin & Biotin-Alkaline
Phosphatase Cbnjugate
20B 7 (507) 10 37C Wet Chamber
20C 9 (509) 0.6* Blot
21A R (519) 0.6 Buffer
21B 11 (511) 0.6* Blot
22A R (519) 2.0 Buffer
22B 11 (511) 0.6* Blot
23A 17 (517) 0.6 BCIP & INT (Enzymatic
Reagents)
23B 11-(511) 0.6* Blot
24A 17 (517) 0.6 BCIP & INT
24B 7 (507) 10 37C Wet Chamber
24C 9 (509) 0.6* Blot
25A 17 (517) 0.6 BCIP & INT
25B 7 (507) 10 37C Wet Chamber
25C 9 (509) 0.6* Blot
26A R (519) 2.0 Buffer
26B 11 (511) 0.6* Blot
27A 8 (508) 6.0 Hematoxylin Stain,
Harris Mbdified
27B 9 (509) 0.6* Blot
28A 10 (510) 0.6 Triton8 X-100 (0.01%) In
Distilled Water
28B 9 (509) 0.6* Blot
29A 12 (512) 0.1 Acid Alcohol
(Differentiates
Hematoxylin)
29B 11 (511) 0.6* Blot
30A R (519) 2.0 Buffer (blues Hematoxylin
at pH 7.5)
__
-26-
1 336653
30B11 (511) 0.6* Blot
31 6 (506) 0.6 Triton X-100 In Distilled
Water
*for each indicated blotting step, 0.6 minutes (36
5 second) was used due to a machine limitation. With
reprogramming, most of the blotting steps will be
reduced to 12 or 18 seconds.
**steps 13A-14B are required only in those
procedures where a protein digestion step (e.g., with
pronase, trypsin or pepsin, each with appropriate
buffers and cofactors) is needed to expose the desired
antigenic sites of the tissue. In the work with thin
tissue samples, such steps were not generally needed
and; therefore, these steps were omitted and the drop
holder in station 13 was replaced with a flat pan
holding 1% Bovine Serum Albumin in 0.1 M TrisHCl, pH 7.6
with 0.1 M NaCl.
In considering the above overall process, events 1-
7 and 9-12 involve removing the wax and converting to an
aqueous buffered medium. In those instances wherein
frozen samples have been sliced into thin samples, step
1-7 and 9 are unnecessary (since no wax is present).
The surfactant was included in steps 10, 11 and 12 to
facilitate capillary flow of the more viscous fluids
that follow. Step 8 is the step used to block
endogenous alkaline phosphatase activity in the
tissue. If another enzyme were used (i.e., in step 20),
a different endogeneous enzyme blocking treatment would
be used. For peroxidase as the enzyme in step 20,
absolute methanol with 0.9% hydrogen peroxide might be
used as the solution in station 18 for step 8. Acid
alcohol in station 12 would still be used in step 29.
For processing frozen sections, the slides are first
fixed in cold acetone for 10 min. and then exposed to
0.01% Triton X-100 in distilled water for 0.6 min
(station 10); blotted for 0.6 min. (station 11), treated
with acid alcohol to block endogenous alkaline
phosphatase enzyme activity (station 12) and then
-27-
1 336653
proceed through the remaining stain program depicted
above, beginning at step 13.
Steps 13 and 14, as indicated above, have not
generally been needed for most antigens of interest in
5 tissue, but would be used for hard-to-access antigenic
markers such as tissue bound immunoglobulins, keratin,
viral antigens such as Cytomegalovirus, Adenovirus, and
Hepatitis B virus surface and core antigens, and for
procedures employing nucleic acid probes.
Step 15 involves applying a general protein to
adhere to the non-specific protein binding sites found
in most tissue specimens. Failure to block these sites
will give undesired background levels due to non-
specific adherence of the primary antibody or avidin or
15 biotin-enzyme conjugate in steps 17 and 20. When a
secondary antibody is used in step 20 (e.g., alkaline
phosphatase conjugated goat-anti-mouse immunoglobulin
antibody in cases where the primary antibody is
unlabeled mouse monoclonal antibody) instead of an
avidin-biotin alkaline phosphatase complex, the blocking
action of non-specific proteins such as gelatin in step
15 may be insufficient to preclude non-specific binding
of the secondary antibody. Accordingly, one can use
normal (unsensitized) serum of the same species as the
secondary antibody used in step 20 (i.e., unsensitized
goat serum in the illustrative case). For DNA probe
work, it may be desirable to apply non-specific DNA as
well as protein in step 15.
Step 17 provides the primary antibody used to
target the antigenic sites of interest. Generally, it
is biotin labeled, but if a secondary antibody is used
in step 20, then unlabeled antibody may be used in step
17. Alternatively, the primary antibody may be
radioactively or fluorescently labeled. DNA or RNA
probes (e.g., biotin-labeled) may also be used in step
17, provided that adequate pretreatment steps have
occurred. In such case, after application (step 17A),
the slide assembly should be placed in a chamber at
-28-
1 336653
_.
temperatures high enough for denaturation (e.g., 100C)
for a few minutes before placement in the 37C Wet
Chamber (step 17B) for rehybridization. The washing
steps represented by steps 18 and 19 in the above
5 procedure may be significantly expanded in number and
duration and variety of liquids for DNA probes. See,
e.g., U.S. Patent 4,533,628 to Maas (August 6, 1985),
and references cited therein.
Step 20, as shown, involves the crosslinking of the
10 biotin chemically bound on the primary antibody to a
second biotin moiety chemically bound to the detection
agent such as an enzyme by the tetravalent egg white
binding protein, avidin. Because of its improved
stability, avidin (egg white Avidin from Vector Labs)
15 was used rather than streptavdin. Provided that the
proper pretreatments were used, other biotin-labeled
detection systems could be used: e.g., horseradish
peroxidase (HRP) or beta-galactosidase conjugate with
biotin. HRP has the advantage of creating chromophoric
enzymatic reaction products (e.g., polymerization
products of diaminobenzidine tetrahydrochloride) which
are more securely anchored in the tissue than are the
chromophoric enzymatic reaction products produced with
alkaline phosphatase [e.g., 3 bromo, 4 chloro, 5 indolyl
phosphate (BCIP) and either Iodonitrotetrazolium (INT)
or Nitro Blue Tetrazolium (NBT)]. The adherence of the
alkaline phosphatase chromophores can be enhanced by
omitting the Triton X-100 in stations 6 and 10, and by
programming the instrument to go directly into an extra
two rinse cycles in distilled water. (Station 10
followed by Blot Station 11). The slides are then
transferred to Station 18 where a shallow tray of
ammonia water is placed. The slides are then directly
mounted in polyvinylpyrrolidone (PVP-40) at 400 mg per
ml in O.lM Tris HCl, pH 7.5, with O.lM NaCl. HRP has
the disadvantage, however, that the enzymatic reactants
that would be required in steps 23-25 are unstable to
light and are suspected carcinogens. Therefore, if HRP
-29-
- -- 1 3 3 6 6 5 3
is used, then the program is preferably stopped at step
21 or 22 until fresh reagent is made up and placed in
station 17. The program is then manually restarted.
Such time is compensated for by a shorter incubation
5 time in steps 24B and 25B. Furthermore, the enzymatic
product is sufficiently insoluble for the slides, after
step 31, to be taken back through stations 6, 5, 4, 3
and 2 (the reverse order of steps 1-7 and 9), with
multiple contacts at some station and a blot after each
10 contact. The resultant stained samples are now coated
with xylene and ready for dry mounting, e.g., with
PermountX mounting medium.
One may alternatively use a fluorescent tag in step
20, e.g., avidin-fluorescein conjugate. In such case,
steps 23-26 are not needed.
Steps 23-25 supply enzymatic reagents (BCIP plus
INT) appropriate to produce insoluble chromagens with
the enzyme tag (alkaline phosphatase) introduced in step
20. Step 27, 29 and 30 represent application and
development of hematoxylin as a counterstain for nuclear
visualization of the tissue in which the labeled
antigenic sites are found.
In the above procedure, steps 17 and 20 employ
particularly expensive regents and are therefore
performed with droplet holder in stations 15 and 16,
respectively. Such droplet holders would normally be
used to conserve these reagents, even when all droplets
are the same, so as to treat all samples identically in
this step. In many cases, however, individualization is
required, particularly with respect to the primary
antibody in station 15, in these droplet holders. The
partially-filled droplet holder shown in Figure 7
illustrates how different liquids can be supplied as
droplets in any desired pattern.
A rigid horizontally-extending base 462 supports a
horizontally-extending elastomeric member 464. Sixty
holes are provided through member 464 in three double
rows of ten. The first double row is filled with twenty
-30-
1 336653
droplets of a first treating liquid, includiny 468a,
468j, 469a and 469j. The second and third double rows
of holes, including holes 466k, 466t, 466u and 466dd are
empty. They can be filled, if desired, with a second
5 and third treating liquid, to be applied to different
slide pairs while the first row of droplets is being
applied to a first row of slide pairs.
When enzyme digestion is employed in step 13, a
droplet holder would also be used in station 13 (513 in
10 Figure 6). Individualization in this step can be
employed where it is desired to vary digestion type or
degree (e.g., some droplets being buffer without pepsin,
some with) at this point. Similary, in step 15, when
more expensive blocking agents than gellatin are
employed in station 14, or if the degree or type of
blocking is a desired variable, then a droplet holder
would be used in station 14.
While stations 8 and 17 are shown as trays, droplet
holders may be used to provide individualization in
steps 23-25 and 27 as well. Where adequate slides and
specimens are available, it may be desirable to achieve
a different color level of the enzyme-generated stain
and of the counterstain for replications of equivalent
samples so as to create a range of contrast levels from
which to choose.
Even as to those steps where trays are used to
apply moderately expensive treating liquid (e.g., the
hematoxylin stain) the present invention uses less
liquid then that the system of Johnson, et al. (which
fills the majority of the 75 mm x 25 mm capillary space)
because only a portion (approximately 30-40 mm x 25 mm)
is filled in the present process. Drainage,
furthermore, is greatly facilitated by blotting rather
than spinning.
It is preferred to use absorbent materials of
sufficient absorbent capacities and to use a sufficient
number of absorbent material stations (stations 9 (509)
and 11 (511) in Figure 6) to absorb all of the various
- 1 336653
liquids to be drained from the slide gaps during the
entire process. Alternatively, at a convenient point in
the process (e.g., during Event ltB) each absorbent
material may be replaced by a fresh absorbent material
(in Stations 9 and 11); or, while one absorbent station
is being used (e.g., Station 11 during steps 9B-14B) the
absorbent material in the other station (Station 9) may
be changed.
In preferred forms of the invention, the gap
10 between the two surfaces is maintained in the vertical
position and a discrete aliquot of liquid reagent
contacts the space produced between the parallel lower
edges of two facing surfaces, such as two glass
microscope slides, and flows upwardly by capillary
action to cover, in total or in part, the inner surface
of the gap. After treatment, the liquid reagent can be
removed from between the planar faces by contacting the
space at any point with an absorbent material. In less
preferred forms suction apparatus or similar liquid
extraction systems may be used. Such method is
particularly useful in streamlining complex treatment
regimens that involve treating a large number of
immobilized samples with a series of liquid reagents and
require the sequential application and removal of one
liquid reagent from the sample analytes prior to the
subsequent exposure of the same analyte to next liquid
reagent in the process. Such is also extremely useful
when it is desirable to use minimal volumes of precious,
hazardous, or expensive liquid reagents such as
dissolved tagged or untagged antibodies, nucleic acid
probes, radioactive materials, or biohazardous materials
where it is desirable to minimize human contact.
There are other embodiments of the invention
wherein the parallel surfaces, and thus the gap there
between, are not vertical, but rather are inclined
upwardly or are even horizontally extending. In each
such case, the advantages of the present invention,
attributable either to the contact of an appropriate
. _
-32-
1 336653
edge of the gap by a discrete aliquot of treating liquid
(permitting individualization), or to the removal of
liquid from the gap by capillary action (e.g., by
contact of the edge of the gap by an absorbent material,
5 permitting multistep processing with rapid drainage of
each liquid), or both, can be obtained in similar
fashion to embodiments described above. Similarly, the
substantially parallel surfaces need not be planar, but
may, for example, be curved as in cylindrical or conical
10 sections.
Both the vertical and horizontal embodiments of
this invention have the same uses and advantages over
prior art in manual stain technology as practiced
routinely in clinical and research laboratories that
15 presently perform the analysis of discrete antigenic or
genetic information by individual manual procedures.
These applications include, but are not limited to, the
detection of antigens of diagnostic prognostic
importance in human, plant, or animal tissues, cellular
smears, or extracts immobilized on solid surfaces such
as a glass microscope slides, nitrocellulose or
cellulose acetate membrane filters, or flat
organoplastic support. These applications further
include screening of identical human, plant, or animal
tissue and tissue extracts by nucleic acid hybridization
technology for their specific genes and their RNA
transcripts. These methods would also have application
in special stain techniques wherein a laboratory would
stain a single tissue for several different
histochemical markers, such as but not limited to
mucicarmine, silver, Gram, Giemsa, Papanicolaou, or
other histologic, hematologic, or cytologic stains.
Alternatively, tissues from many different anatomic
sites and species may be stained with a single series of
reagents especially in situations where the reagents
employed are expensive or available in only microliter
quantities. The low volume requirements of such systems
as the screening of a single tissue type with monoclonal
. -33-
- 1 336653
antibodies direct from limited supernatants or ascitic
fluids are ideal uses for a method and apparatus
designed to treat a thin sample immobilized on a planar
surface employing capillary flow in either a vertical or
5 horizontal position.
__