Note: Descriptions are shown in the official language in which they were submitted.
13~671S
Deacription~
8-(4-Piperidinyl)-4H-l-Benzo~ an-4-One Derivatives and
Their Use
The present invention relates to the use of 4H-l-
benzopyran-4-one derivatives, to novel 4H-l-benzopyran-
~-one derivatives, and to pharmaceuticalg cont~i n i ng
them.
Benzopyran derivatives have already been described in European
Patent Specification No. 0,137,193 (published 04 Apr 85) and in
German Offenlegungsschrift 3,612,337 (published 15 Oct 87). The
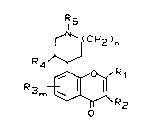
latter discloses compounds of the formula a~.
~I(CH2)n
R4~
o
in which the substituents R~ to Rs and m and n are as
defined; the compounds have an antiinflammatory analgesic
and immune-modifying action.
Surpri~ingly, it has now been found that certain 4H-1-
benzopyran-4-one derivatives inhibit oncogene-encoded
kina~es and are therefore suitable for controlling
tumoral disea~es.
The expre~sion of oncogenes in a mammalian cell entails
transition from the norm~l cell type to the transformed
cell type, which then becomes a cancer cell. The trans-
formation was caused by infecting a cell with a retro-
virus. A well known example was the infection of chickenswith Rous-fiarcoma virus, and these chickens then
developed cancer. The corre~ponding oncogene, which was
-
1~
- 2 - 1 33 6 7~ ~
responsible for the malign transformation, was named
"SRC n ( sarcoma) gene (J-S- Brugge, R.L. Erikson; Nature
269, 346-348 (1977)). A characteristic feature of many
oncogenes known to date is the expression of a protein
having kinase activity. The enzymes catalyze the transfer
of the terminal phosphate group of ATP to an amino acid.
In contrast to many other protein kinases which transfer
the phosphate group to a seryl radical or threonyl
radical, most of the oncogene-encoded kinases phospho-
rylate a tyrosyl radical of the protein chain. Besides,it is known that products of oncogenes, namely those of
the v-mos, v-mil and v-raf oncogenes, have serin/-
threonin-specific protein kinase activity. (R. Molling et
al., Nature (London) 312, 558-561 (1984); B. Singh et
al., Journal of Virology 60, 1149-1152 (1986)).
Tyrosin kinase activity is also expressed in growth
factor receptors; new findings now show that the growth
of many tumors is dependent on the presence of growth
factors, such as Epidermal Growth Factor (EGF), Trans-
forming Growth Factor ~ (TGF~) or Platelet Derived GrowthFactor (POGF) (A.S. Goustin, G.D. Shipley, H.L. Moses,
Cancer Research 46, 1015-1029 (1986). Once the growth
factor is bound to its receptor, tyrosin kinase, which is
an intrinsic component of the growth factor receptor, is
stimulated.
A tyrosin kinase inhibitor and possibly a serin/threonin
kinase inhibitor might therefore inhibit the growth and
~preading of tumors, and it could be employed in tumor
therapy.
The present invention therefore relates to the use of 4H-
l-benzopyran-4-one derivatives of the formula I for
inhibiting oncogene-encoded kinases and growth factor
receptor tyrosin kinases and for the control of tumoral
diseases. Formula I reads
~ 3 ~ 1336715
~ ~ (CH2)1~
O
and is defined as follows: R1 is hydrogen, alkyl having 1
to 6 carbon atoms, aryl-C1-C4-alkyl, substituted C1-C6-
alkyl, C3-C6-cycloalkyl, a C3-C~-heterocyclic ring having
1, 2 or 3 hetero atoms, ~uch as N, S, O or any combina-
tions thereof, or else C3-C6-cycloalkyl-C1-C4-alkyl, C2-C6-
alkenyl, C2-C6-alkynyl, aryl including polycyclic rings,
or aromatic heterocyclic radicals, substituted aryl,
carboxyl or an aldehyde or COO-C1-C4-alkyl group, a
primary amino, alkylamino, aralkylamino, dialkylamino,
arylamino or diarylamino group, or -CH2O-C1-C4-alkyl;
R2 is hydrogen, alkyl having 1 to 6 carbon atoms, aryl,
nitro, amino, di-C1-C4-alkylamino or a halogen, or
hydroxyl, alkoxy, -COOH, -COO-C1-C4-alkyl, -CHO, -CH2OH or
-CH2O-C1-C4-alkyl;
R3 is hydrogen, C1-C4-alkyl, substituted Cl-C4-alkyl,
hydroxyl, carboxyl, C1-C4-alkyl, nitro, amino, a C1-C4-
alkylamino or di-C1-C4-alkylamino group, or halogen,
o
-CHO, -CH2OH, -CH2O-C1-C4-alkyl, R-N-C-O-, where
R
R is H, C1-C6-alkyl, cycloalkyl and aryl;
R4 is hydrogen, hydroxyl, C1-C4-alkoxy, Cl-c4-~lk~noyloxy~
C1-C4-alkoxycarbonyl, aryloxy or amino, or a Cl-C4-alkyl-
amino or di-C1-C4-alkylamino group, or is R-N-~-O-,
where R i~ H, C1-C6-alkyl, cycloalkyl or aryl;
R5 is hydrogen, C1-C6-alkyl, aryl-C1-C4-alkyl, C3-C6-cyclo-
alkyl, C3-C6-cycloalkyl-C1-C4-alkyl, alkylamino, C1-C4-
~lk~noyl or aroyl, where the aryl group is unsubstituted
or mono- or polysubstituted phenyl;
m is an integer between 0 and 3 and 133 6 71~
n is an integer between 0 and 2,
and pharmacologically acceptable acid addition salts
thereof.
The compounds according to the invention have two centers
of asymmetry, one where the heterocyclic ring contAining
nitrogen is fused to the benzopyran moiety (C-4'), the
other at the R4-substituted carbon atom (C-3'), which
means that two pairs of optical isomers are possible. The
definition of the compounds according to the invention
embraces all possible stereoisomers and their mixtures.
It very particularly embraces the racemic forms and the
isolated optical isomers having the specified activity.
The two racemates can be resolved by physical methods,
such as, for example, fractional crystallization. The
individual optical isomers can be obtAine~ from the
racemates by conventional methods, such as, for example,
salt formation with an optically active acid followed by
crystallization.
Examples of alkyl groups which are suitable for Rl to R5
are straight-chain or branched radicals having up to 6,
preferably up to 5, carbon atoms, for example methyl,
ethyl, propyl, isopropyl, t-butyl, pentyl or isopentyl
groups.
Examples of substituted alkyl groups which are suitable
for R1 to R5 are haloalkyl, such as trifluoromethyl,
hydroxyalkyl, such as hydroxyethyl, or carboxyalkyl, such
as carboxyethyl.
Suitable examples of a cycloalkyl group which has 3 to 6
carbon atoms and is represented by Rl and R5 are cyclo-
propyl, cyclobutyl, cyclopentyl or cyclohexyl. Cyclo-
propylmethyl is an example of cycloalkylalkyl.
An example of an aralkyl group which is represented by R
and R5 is a phenylalkyl group in which the phenyl group is
1336715
unsubstituted or monosubstituted or polysubstituted by
substituents such as halogen, Cl-C4-alkyl, Cl-C4-alkoxy or
nitro or by a trifluoromethyl group, amino group and
substituted amino group.
An example of an aryl group which i8 represented by Rl and
R5 is a phenyl group which is unsubstituted or monosub-
stituted or polysubstituted by substituents such as
halogen, Cl-C4-alkyl, Cl-C4-alkoxy, hydroxyl, carboxyl,
nitro or trifluoromethyl, amino, Cl-C4-alkylamino, di-Cl-
C4-alkylamino, aromatic heterocyclic groups such as
pyridyl groups, and polycyclic aromatic radicals, such as
naphthyl groups.
A suitable example of an alkylamino group which is
represented by Rl and R5 is (CH2)n-NR6R7, where n is 1 to 3
and R6 and R7 are alkyl and are as defined as above in the
case of alkyl R1 to R5; moreoever, R6 and R7 together with
the nitrogen atom to which they are bonded can be a
heterocyclic ring having one or more hetero atoms.
Suitable examples of heterocyclic rings which are formed
by R6 and R7 together with the nitrogen to which they are
bonded are piperidine, pyrrolidine, morpholine,
piperazine or imidazole, all of which can be
unsubstituted or substituted in one or more positions by
Cl-C4-alkyl, Cl-C~-alkoxy or aryl or by a hydroxyl or amino
group.
Suitable examples of salts of the compounds according to
the invention with inorganic or organic acids are
hydrochloride, hydrobromide, sulfate, phosphate, acetate,
oxalate, tartrate, citrate, maleate or fumarate.
The use of compounds of the formula Ia
- 6 - 1 336715
R5
HC~ ~ R1 Ia
OH O
in which Rl is hydrogen or Cl-C3-alkyl, naphthyl, aryl,
substituted aryl or a C3-C8-heterocyclic ring,
R2 is hydrogen or Cl-C3-alkyl,
R5 is Cl-C3-alkyl or C3-C5-cycloalkyl, or
C3-C5-cycloalkyl-Cl-C4-alkyl,
or of pharmacologically acceptable acid addition salts
thereof, is preferred.
The use of compounds of the formula Ia as claimed in
claim 2, wherein Rl is phenyl, thienyl, pyridyl, chloro-
phenyl, dichlorophenyl, methylphenyl, aminophenyl,bromophenyl, hydroxyphenyl or naphthyl,
R2 is hydrogen and
R5 is methyl,
or of pharmacologically acceptable acid addition salts
thereof, is very particularly preferred.
Also part of the subject-matter of the invention are the
novel compound~ of the formula Ib
R5
~ ( CH2 )n
~ Rl Ib
~W R2
in which at least one of the substituents has one of the
meanings below, while the other substituents in each case
can be as defined in claim 1: R1 is C3-C6-cycloalkyl, a
C3-C8-heterocyclic ring having 1, 2 or 3 hetero atoms,
such as N, S, 0 or any combinations thereof, or is C3-C6-
cycloalkyl-C1-C4-alkyl, or polycyclic rings, aromatic
~ 7 ~ 1336715
heterocyclic radicals, substituted aryl, a primary amino,
alkylamino, aralkylamino, dialkylamino, arylamino or
diarylamino group, or -CH2O-C1-C4-alkyl;
R2 is aryl, hydroxyl, alkoxy, COOH, COO-Cl-C"-alkyl, -CHO,
-CH2OH or -CH2O-Cl-C4-alkyl;
R3 is carboxyl, a halogen, -CHO, -CH2OH, -CH2O-C1-C4-alkyl
or R-N-C-O- group, where R is H, C1-C6-alkyl, cycloalkyl
and aryl;
R4 is R-~l-C-O-, where R is H, Cl-C6-alkyl, cycloalkyl or
aryl;
R5 is aroyl, where the aryl group is monosubstitued or
polysubstituted phenyl, and
m is an integer between 0 and 3 and
n is an integer between 0 and 2,
and pharmacologically acceptable acid addition salts
thereof. These compounds can be prepared, for example, as
described in European Patent 0,157,193 or in
DE 3,612,337.
Examples of preferred compounds according to the
invention, or of compounds which are particularly
suitable for the use according to the invention, are:
(~)-cis-5,7-dihydroxy-2-(2-thienyl)-8-[4-(3-hydroxy-1-
methyl)piperidinyl]-4H-l-(benzopyran-4-one,
(-)-cis-5~7-dihydroxy-2-phenyl-8-[4-(3-hydroxy-1-methyl)-
piperidinyl]-4H-l-benzopyran-4-one hydrochloride,
(+)-cis-5,7-dihydroxy-2-phenyl-8-[4-(3-hydroxy-1-methyl)-
piperidinyl]-4H-l-benzopyran-4-one hydrochloride,
(+)-cis-5,7-dihydroxy-2-phenyl-8-[4-(3-hydlciAy-l-methyl)
piperidinyl]-4H-l-benzopyran-4-one hydrochloride,
(-)-cis-5,7-dihydroxy-2-(2-pyridyl)-8-[4-(3-hydroxy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one hy~oc:hloride,
(~)-cis-5,7-dihydroxy-2-(2-pyridyl)-8-[4-(3-hydroxy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one hydrochloride,
(+)-cis-5,7-dihydroxy-2-(3-pyridyl)-8-[4-(3-hydroxy-1-
- 8 - 1 ~ 36715
methyl ) piperidinyl ] -4H- l-benzopyran-4 -one hydrochloride,
(+)-cis-5,7-dihydroxy-2-(4-pyridyl)-8-[4-(3-hydroxy-1-
methyl ) piperidinyl ] -4H- l-benzopyran-4 -one hydrochloride,
( - ) -cis-S, 7-dihydroxy-5- ( 2-chlorophenyl ) -8- t 4- ( 3-hydroxy-
1 -methyl ) piperidinyl ] -4H- 1 -benzopyran-4 -one
hydrochloride,
( + ) -c is -5, 7 -dihydroxy-2 - ( 2 -chlorophenyl ) -8- [ 4 - ( 3-hydroxy-
1 -methyl ) piperidinyl ] -4H- l-benzopyran-4 -one
hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 2-chlorophenyl ) -8- [ 4- ( 3-hydroxy-
1 -methyl ) piperidinyl ] -4H- l-benzopyran-4 -one
hydrochloride,
( + ) -cis-5, 7 -dihydroxy-2 - ( 2, 5-dichlorophenyl ) - 8 - ~ 4 - ( 3 -
hydroxy-l-methyl ) piperidinyl ] -4H-l-benzopyran-4-one
hydrochloride,
( ~ ) -cis-5, 7-dihydroxy-2- ( 2, 4-dichlorophenyl ) -8- [ 4- ( 3-
hydroxy- l-methyl ) piperidinyl ] -4H- 1 -benzopyran-4 -one
hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 4-methylphenyl ) -8- [ 4- ( 3-hydroxy-
1 -methyl ) piperidinyl ] - 4H- 1 -benzopyran- 4 -one
hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 3-methylphenyl ) -8- [ 4- ( 3-hydroxy-
1 -methyl ) piperidinyl ] -4H- l-benzopyran-4 -one
hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 4-aminophenyl ) -8- [ 4- ( 3-hydroxy-
l-methyl ) piperidinyl ] -4H-l-benzopyran-4-one
hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 3-bromophenyl ) -8- [ 4- ( 3-hydroxy-
l-methyl ) piperidinyl ] -4H- l-benzopyran-4-one
3 0 hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 2-naphthyl ) -8- [ 4- ( 3-hydroxy-1-
methyl ) piperidinyl ] -4H-l-benzopyran-4-one hydrochloride,
( + ) -cis-5, 7-dihydroxy-2- ( 4-hydroxyphenyl ) -8- [ 4- ( 3-
hydroxy-l-methyl ) piperidinyl ] -4H-l-benzopyran-4-one and
( ~ ) -cis-5, 7-dihydroxy-2- ( 2-hydroxyphenyl ) -8- [ 4- ( 3-
hydroxy- l-methyl ) piperidinyl ] -4H- 1 -benzopyran-4 -one .
Other 4H- l-benzopyran-4-one derivatives according to the
invention or compounds according to the invention which
- 9 - 1336715
are particularly suitable for the use according to the
invention are listed in Tables 1 and 2 below together
with their physical data. Table 1 relates to formula Ic
l l
R4 ~ Ic
RgO ~ R2 X
OR8
and Table 2 relates to formula Id
R4 ~ R 1 o
OR8 Id
133671s
Table 1
Verb. Rl R2 R4 R8 Rg X Melting point Optical
(C) rotation
H H OH H H - 298 (decompJ (+)
2 H H OH CH3 CH3 HCl, 1,5H2O 173-175 (+)
3 CH3 H OH H H HCl, 237-240 (+)
4 CH3 H OH H H HCl, 241-43 (+)
CH3 H OH H H HCl, 241-42 (-)
6 CH3 H OH H CH3 HCl, 230-232 (+)
7 CH3 H OH CH3 CH3 2HCl, 2H2O 236-239 (+)
8 CH3 H H H H H2O 232-233 (+)
9 C2H5 H OH H H HCl, 1.5H2O 230-233 (+)
C2H5 H OH CH3 CH3 HCl, 1.5H2O 240-242 (+)
11 n-c3H7 CH3 OH H H - 191-192 (+)
12 n-c3H7 H OH H H HCl, 190-192 (+)
13 n-c3H7 H OH H H HCl, 0.5H2O 197-200 (+)
14 n-c3H7 H OH H H HCl, 0.5H2O 198-201 (-)
n-c4H9 H OH H H HCl, H2O 157-159 (+)
16 CH3 CH3 OH H H H2O 232-233 (+)
17 2-Pyridyl H OH H H HCl, 0,5H2O 229C (+)
18 3-Pyridyl H OH H H 2HCl, 2H2O 278-280 (+)
19 4-Pyridyl H OH H H 2HCl, 1.5H2O 236-238 (+)
CO2H H OH CH3 CH3 H2O
21 2-Thienyl H OH H H 2H2O 243-244 (+)
22 2-Thienyl H OH CH3 CH3 - 207-208 (+)
23 2-Pyridyl H OH H H 2HCl,2H2O 220-228 (-)
24 ~-Styryl H OH H H HCl, 1.5H2O >300 (+)
1-Naphthyl H OH H H HCl, H2O 195-200 (+)
26 2-Naphtyl H OH H H HCl, 0.5H2O 280-282 (+)
27 (2-Chloro' H OH H H HCl 270-275 (+)
phenyl)methyl
1336715
Table 2
Verb. R1o R8 Rg X Melting point Optical
(C) rotation
28 H H H HCl, 2H2O 273-275 (+)
29 4-No2 H H HCl, 3H2O 249 (decomp~ (+)
4-NO2 CH3 CH3 HCl, 2H2O 257-260(decomp~(+)
31 2-Cl H H HCl, H2O 198-200 (+)
32 2-Cl CH3 CH3 1.5HCl, H2O 190-191 (+)
33 4-NH2 H H 2HCl, 2H2O 240-242 (+)
31 3,5-Dimethoxy CH3 CH3 2HCl, 2H2O 180-182 (+)
32 4-Br H H HCl, 2H2O 215 (+)
33 4-Cl H H HCl, 1.5H2O 225 (+)
34 2,4-Dichlor H H HCl, 2.5H2O 165-166 (+)
4-F H H HCl, H2O 285-287 (+)
36 2-F H H HCl, 2H2O 263-265 (+)
37 4-Methyl H H HCl, 1.5H2O 247-49 (+)
38 3,5-Dihydroxy H H HCl, 3H2O 300-302 (+)
39 3-Cl H H HCl, 2H2O 288-290 (+)
3-Methyl H H HCl, 2H2O 268 (+)
41 2-Methyl H H 204-205 (+)
42 2-Cl H H HCl, 2H2O 190-192 (+)
43 H H H HCl 269-271 (+)
44 3-Br H H HCl, 2H2O 285 (+)
3-CO2Me CH3 CH3 1.5HCl, 3H2O 235 (+)
46 2,5-Dichloro' H H HCl, H2O 251-252 (+)
47 3-COOH CH3 CH3 HCl, 1.5H2O 270 (+)
48 2-Cl H H HCl, 1.5H2O 190-194 (-)
49 H H H HCl, O.5H2O 266-269 (-)
H H H HCl 270-271 (+)
51 4-OH H H H2O > 340 (+)
52 4-Phenyl H H HCl 240-242 (+)
53 2-Br H H 1.5H2O 250-252 (+)
54 2-OH H H HzO 265-270 (+)
- 12 - 1336715
The preparation of some of the compounds which can be
used according to the invention and the preparation of
the necessary starting materials are described in detail
in German Offenlegungsschrift 3,612,337, to which
reference is made at this point. Another subject-matter
of the present invention is a process for the preparation
of the novel compounds of the formula as claimed in claim
4. In this process, a compound of the formula II
R5
~ ( C H2 ) n
R4/~ II
o
where R4 is hydroxyl or acetoxy and R3, R5, n and m are as
defined, is reacted, for example with an alkali metal and
the alkyl ester of an acid of the formula Rl-COOalkyl,
where Rl is as defined in formula I, to give a diketone of
the formula III
R5
(CH2 )n
O III,
lS and the resulting compound is cyclized by reaction with
a mineral acid to give a compound of the formula Ib,
where Rl, R3, R5, m and n are as defined, R4 is the
hydroxyl or acetoxy group and R2 i8 hydrogen, and, if
appropriate, a compound of the formula Ib where R5 is CH3
is reacted with cyanogen bromide after the hydroxyl
groups have been protected, and the resulting compound is
reacted under acid or alkaline conditions to give a
compound of the formula Ib where R5 is hydrogen, and, if
appropriate, a compound of the formula Ib where R5 is
hydrogen is reacted with suitable electrophilic reagents,
- 13 - 133 67 15
such as halides, acid chlorides, tosylates or enones, to
give compounds of the formula Ib where R5 is unsubstituted
or substituted C1-C6-alkyl, aryl-C1-C4-alkyl, C3-C6-cyclo-
alkyl or C3-C6-cycloalkyl-C1-C4-alkyl, and, if appropriate,
S a compound of the formula Ib where R2 is hydrogen is
reacted with a secondary amine hydrochloride and para-
formaldehyde to give a compound of the formula Ib where
R2 is dialkylaminomethyl, or, if appropriate, a compound
of the formula Ib where R2 is hydrogen is nitrated to give
a compound of the formula Ib where R2 is NO2, and, if
appropriate, a compound of the formula Ib where R2 is NO2,
is hydrogenated to give a compound of the formula Ib
where R2 i8 the amino group.
The conditions for the individual reaction steps are as
described in German Offenlegungsschrift 3,612,337. A
particularly preferred method for the preparation of
compounds according to the invention i8 the reaction of
a compound of the formula IV
l l
R-O/~ IV
H3CO~ OH
~ CH3
H3C0
in which R i8 -COCH3 or H,
with a compound of the formula
Rl-COO X
in which X is hydrogen or halogen, preferably hydrogen or
chloride. This esterification takes place under generally
known conditions, as described, for example, in Organikum
[Laboratory Practice in Organic Chemistry], VEB,
Deutscher Verlag der Wissenschaften, 15th edition, Berlin
1977, Chapter D7. The resulting esters are treated in an
inert atmosphere with bases, for example sodium hydride,
or preferably in aprotic ~olvents, for example tetra-
hydrofuran, dioxane or N,N-dimethylformamide, this giving
diketones which are usually not isolated. On ~tirring
- 14 - 1336715
with a mineral acid, for example HCl, the ketone cyclize,
and benzopyran-4-one derivatives of the formula Ib are
formed. The process is widely applicable and is
particularly useful for the preparation of compounds such
as formula Ib where Rl is aryl and heteroaryl groups. The
above reaction will be illustrated in greater detail in
the Examples.
The compounds according to the invention have
pharmacological properties; in particular, they inhibit
oncogene-encoded kinases, such as tyrosin kinase,
serin/threonin kinase and growth factor receptor tyrosin
kinase, and it can therefore be expected that they
inhibit growth and spreading of tumors and that they can
be used in the therapy of tumors.
Further subject-matter of the invention are therefore
also pharmaceuticals for controlling tumors, which
contain at least one compound of the formula I as claimed
in claim 1 or at least one of its pharmacologically
acceptable acid addition salts, and the use of a compound
as claimed in claim 1 for the preparation of a
pharmaceutical having an antitumoral action.
The 4H-l-benzopyran-4-one derivatives are used according
to the invention in the generally known fashion which is
known to the expert. For pharmaceuticals, an effective
amount of the active substance mentioned is employed
either per se or preferably in combination with suitable
- pharmaceutical auxiliaries in the form of tablets, coated
tablets, capsules, suppositories, emulsions, suspensions
or solutions, the active compound content being up to
about 95%, preferably between 10 and 75%.
The expert will know which auxiliaries are suitable for
the desired formulation of the pharmaceutical because of
his expert knowledge. Besides auxiliaries for tablets, or
solvents, gel formers, ba~es for suppositories, and other
excipients for the active substance, it is possible to
- 15 - 1336715
use, for example, antioxidants, dispersants, emulsifiers,
defoamers, flavor corrigants, preservatives, solubilizers
or colorants.
The active substance can be administered orally,
parenterally, intravenously or rectally, intravenous
administration being preferred. For a form of oral
administration, the active substance may be mixed with
other compounds together with the additives which are
suitable for this purpose, such as excipients,
stabilizers or inert diluents, and customary methods can
be used for bringing it into suitable administration
forms, such as tablets, coated tablets, hard-gelatin
capsules, and aqueous alcoholic or oily suspensions or
solutions. Examples of inert excipients which can be used
are gum arabic, magnesia, lactose, glucose or starch, in
particular corn starch. In this context, the formulation
can be prepared as dry granules or moist granules.
Examples of suitable oily excipients or solvents are
vegetable or animal oils, such as sunflower oil or
codliver oil.
For subcutaneous or intravenous administration, a
solution, suspension or emulsion of the active substance
is formed, if appropriate using substances which are
conventional for this purpose, such as solubilizers,
emulsifiers or other auxiliaries. Examples of suitable
solvents are water, physiological sodium chloride
solution or alcohols, for example, ethanol, propanol or
glycerol, and also sugar solutions, such as glucose
solutions or mannitol solutions, or a mixture of the
various solvents which have been mentioned.
The dose of 4H-l-benzopyran-4-one derivatives which is to
be administered can cover a wide range. The dose to be
administered daily is to be selected to suit the desired
effect. About 50 to 1,000 mg are preferably administered
daily per patient, preferably intravenously. If required,
higher or lower daily doses can also be administered, but
- 16 - 1336715
a maximum amount of 2,000 mg should only be exceeded for
- a short time.
The pharmacological properties of the compounds mentioned
are confirmed by the pharmacological assays which follow
and which have been carried out with compounds according
to the invention and their salts; the results obtained
are listed in Table III.
Test methods
Tyrosin ~i n^~^ inhibition assays
Testing procedure:
In the case of tyrosin kinase activity, the starting
material was the rat tumor cell line RR 1022 (ATCC CCL47)
which had been grown in RPMI 1640 medium + 10% of FCS.
This cell line has been transformed with RSV (Rous
sarcoma virus) and contains the oncogene product PP60~rC,
which has tyrosin kinase activity.
The cells were grown until nearly confluent, washed with
PBS (phosphate-buffered saline), scraped off from the
culture bottle and repeatedly washed twice (0.85%
strength NaCl solution) and centrifuged (200 x g).
100 ~1 of buffer (10% of glycerol, 25 mM of Tris-HCl
pH 7.4, 10 mM of RCl, 1 mM of EDTA, 1% of ~Triton X-100,
surfactant manufactured by Rohm & Haas, Philadelphia,
USA, 2 mM PMSF (phenylmethyl sulfonyl fluoride), 100
kallikrein-inactivating units of aprotinin/ml, 2 mM of
dithiotreitol) were then added per 1 x 106 cells in order
to lyze the cells. After 5' at 4C, the lyzate was
centrifuged at 10,000 x g for 10', and the supernatant
was used as a starting material for tyrison kinase
activity.
Tyrosin kinase activity of the lyzate was measured with
- 17 - 1336715
poly(Glu, Tyr), 4:1, as a substrate. The inhibitor was
preincubated with cell lyzate, substrate ( 2 mg/ml) and
Mg2+ (10 mM) in 100 mM of HEPEA (N-2-hydroxyethyl-
piperazin-N'-2-ethanesulfonic acid), pH 7.2, and the
reaction was initiated by adding 7_32p ATP (40 ~M). After
15' at 30C, the substrate was precipitated using 10%
strength TCA (trichloroacetic acid), filtered over a
Millititer Filtration Plate (Millipore Corporation,
Mass., USA), washed and dried. The incorporation of 32p
was determined by means of a liquid scintillation
counter.
Results:
The substances were tested at a maximum concentration of
45 ~g/ml and diluted stepwise in the ratio 1:10. ICSo
denoted the concentration at which 50% of the initial
enzyme activity were inhibited (see Table III).
A~ay for 3~5~-cAMp-derQn~nt protein ~in~ inhibition
p~ re:
The catalytic sub-unit of cAMP-dependent protein kinase
(Sigma) was reconstituted as described by Sigma (Sigma
Chemical Co., St. Louis, MO, USA). The enzyme activity
was measured with kemptide (Sigma) (Leu-Arg-Arg-Ala-Ser-
Leu-Gly) as the substrate. The inhibitor was preincubated
with the enzyme, substrate (190 ~M), Mg2+ (5 mN), 0.25
mg/ml of BSA and 3.75 mM of mercaptoethanol in 50 mM of
MOPA (4-morpholinopropanesulfonic acid), pH 6.9. The
reaction was initiated by adding ~_32p ATP (40 ~M). After
15 minutes at 30C, an aliquot amount was transferred to
p81 ion exchange paper (2 x 2 cm; Whatman Paper Ltd.,
Great Britain), the paper was immersed in 75 mM H3PO4,
washed and dried, and the incorporation of 32p was
determined by means of a liquid scintillation counter.
- 18 -
1336715
RRsult~:
The results are expressed as % inhibition of the initial
enzyme activity at an inhibitor concentration of 45 ~g/ml
(see Table III).
133671S
Table I I I
Formula lc
where Rz = R8 = H and R4 = OH
Rl X Sign of theICso in% inhibition
tif A 1 ~g/TIll of ~_
rotationpp60 v-src ~r''~'"
pmtein kinase
at an inhibitor
,~nn~" 1,, 1; flr~ of
45 ~,g/ml
2-thir~h~nyl (+) 1,7 14
2_h~ yl - (+) 22,3 63
(2-chlo~u~yl)- HCl( + ) >45 ~ 69
~1
phen~1 HCl (-) 5,7 46
2{:hlo~phenyl HC1 (+) 9,2 27
2-fluor~l HC1( + ) 28,0 0
4~thylphe~1 HCl( + ) 2,7 55
3-pyridyl HCl( + ) 10,6 0
2-pyridyl HCl (+) 34,8 0
Eih~rl HCl( + ) 0,7 13
4-pyridyl HCl( + ) 4,2 12
n-pmE~l HCl (_) 45, O O
2-chlo~u~lyl HCl (+) 3~3 29
ethyl HCl (+) >45,0 0
p~nyl HCl (+) 1,9 48
4-br ~ rhf-nyl HCl (+) 3,4 65
4--hi~ yl HCl (+) 39,0 37
n-butyl HCl (+) 0,0 n.b.
2-pyridyl HCl (-) 7,8 6
2-nAphthyl HCl (+) 3 ~ 4 0
4-f~ enyl HCl (+) 2,7 25
4-chluLu~yl HCl( + ) 1,6 37
n-pr ~ 1 HCl (- ) >45,O S
The Example below illustrates the invention without
restricting its scope.
- 19a - 1336~15
SRC tumor test:
Single cell suspensions (from cell culture A 549 or
disaggregated human tumor xenografts LXF 529) in Beriplast
(Behringwerke AG) were diluted 1:2 with RPMI 1640
supplemented with 15 ~ FCS to a final cell concentration of
107 cells/50 ~l. This suspension was sucked quickly into 50
~l glass capillaries (diameter 1.5 mm), of which the inner
walls had been moistered with a thrombin/CaCl2 solution (500
units thrombin in 1 ml 40 mmol CaCl2).
After solidification of the cell-fibrin mixture (about 5 min
at RT) the fibrin clot has been removed from the glass
capillary into a petridish by air pressure. The fibrin clot
was cut into 2 mm pieces (s~ 5 x 105 cells per piece) and the
pieces were stored in RPMI 1640 supplemented with 15 ~ FCS
until implantation.
A single fibrin piece was transferred under the renal
capsule of a nude mouse and, subsequently, two perpendicular
diameters of the implanted piece were measured by microscope
with an ocular micrometer (day 0). The tumor size was
calculated according to
V = a x b
V = tumor size
a = largest diameter
b = diameter perpendicular to a
The substance was given i.v. or p.o. daily on day 2 - 15
with the maximal tolerable dose (MTD) and a dose 2/3 of
the MTD value according to the pretests. 5 mice/group were
used.
1336715
_ 19b -
On day 21 after implantation the animals were sacrificed, the
kidney was exteriorized and the tumor size was measured
again. The effectivity of the test drug was calculated from
the tumor growth inhibition.
The relative tumor size VR was calculated according the
formula
Vt
VR
Vo
Vt = tumor size at the end of experiment (day 21)
Vo = tumor/fibrin size at day of implantation
Subsequently the median relative tumor size of the treated
group (VT) was related to the corresponding median relative
tumor size of controls (Vc) according to the formula
T/C ~ = - x 100
VC
The statistical significance ( p 0.05) of the antitumoral
effects was calculated using the Wilcoxon U test.
Table IV shows the test results.
19c 1336715
Table IV
compound tumor dose schedule T/C
(mg/kg/day) (days) (~)
broncho-
genic
carcinomas
(Exmp. 17) LXF 52925 i.v. 2-15 81 n.s.
35 i.v. 2-15 67
A 549 35 i.v. 2-15 70
100 p.o. 2-15 73
(Exmp. 9) LXF 529 2 i.v. 2-15 59
4 i.v. 2-15 51
(Exmp. 19) LXF 529100 i.v. 2-15 98 n.s.
150 i.v. 2-15 80
n.s. = not significant (P 0.05)
- 20 - 1336715
Example 1
( +/- ) -Ci8-5,7-dimetho y-2-(2-thienyl)-8-t4-(3-acetosy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one
2-Thiophenecarboxylic acid (2.73 g) was added at 0C to
a æolution of (~)-cis-3-acetoxy-1-methyl-4-(3-acetyl-4,6-
dimethoxy-2-hydroxy)phenylpiperidine of the formula IV,
R = COCH3, (3.0 g) in dry pyridine (30 ml), and POCl3
(2.2 ml) was then added. The reaction mixture was stirred
for two hours at room temperature. Water (50 ml) was
added slowly to the reaction mixture, and the reaction
solution was later rendered alkaline by adding sodium
carbonate (pH 8). The reaction mixture was extracted
using ethyl acetate (3 x 40 ml). The combined organic
extracts were washed with water, dried over anhydrous
sodium sulfate, and the solvent removed in vacuo. The
residue was chromatographed on silica gel (4% of MeOH in
CHCl3); yield 2.7 g of (+)-cis-3-acetoxy-1-methyl-4-(3-
acetyl-4,6-dimethoxy-2-(2-thienyloxy)phenyl)piperidine;
melting point 153-154C.
This compound was taken up in dry dioxane (50 ml), sodium
hydride (5 equivalents) was added, and the mixture was
stirred for four hours at 40C. MeOH (10 ml) was added to
destroy the excess sodium hydride, and dry HCl gas was
passed in until the pH of the solution was clearly acid.
The reaction mixture was worked up by adding ice-cold
sodium carbonate solution, and the resulting solid
product was separated by filtration. Further purification
was by means of column chromatography (silica gel, 2% of
MeOH, 1% of NH40H in CHC13), and (+)-cis-5,7-dimethoxy-2-
(2-thienyl)-8-[4-(3-acetoxy-1-methyl)piperidinyl]-4H-l-
benzopyran-4-one were obt~i n~A . Yield 2.50 g, melting
point 207-208C.
The following compounds were prepared analogously to
Example 1:
13~6715
Example 2
(+)-cis-5,7-Dihydroxy-2-(phenyl)-8-[4-(3-hydroxy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one hydrochloride.
Example 3
(-)-cis-5,7-Dihydroxy-2-phenyl-8-t4-3-hydroxy-1-methyl)-
piperidinyl]-4H-1-benzopyran-4-one hydrochloride.
~ample 4
(+)-cis-5,7-Dihydroxy-2-phenyl-8-[4-(3-hydroxy-1-methyl)-
piperidinyl]-4H-1-benzopyran-4-one hydrochloride.
~sample 5
(-)-cis-5,7-Dihydroxy-2-(2-pyridyl)-8-t4-(3-hydroxy-1-
methyl)piperidinyl]-4H-1-benzopyran-4-one hydrochloride.
Example 6
(+)-cis-5,7-Dihydroxy-2-(2-pyridyl)-8-t4-(3-hydroxy-1-
methyl)piperidinyl]-4H-1-benzopyran-4-one hydrochloride.
Example 7
( ~ ) -Ci8-5, 7-Dihydroxy-2-(3-pyridyl)-8-t4-(3-hydroxy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one hydrochloride.
E~ample 8
(+)-cis-5,7-Dihydroxy-2-(4-pyridyl)-8-t4-(3-hydroxy-1-
methyl)piperidinyl]-4H-1-benzopyran-4-one hydrochloride.
Example 9
(-)-cis-5,7-Dihydroxy-5-(2-chlorophenyl)-8-[4-(3-hydroxy-
1-methyl)piperidinyl]-4H-1-benzopyran-4-one
- 22 - 1336715
hydrochloride.
Example 10
(+)-cis-5~7-Dihydroxy-2-(2-chlorophenyl)-8-[4-(3-hy~loxy-
l-methyl)piperidinyl]-4H-1-benzopyran-4-one
S hydrochloride.
~xample 11
(~)-cis-5,7-Dihydroxy-2-(2-chlorophenyl)-8-[4-(3-hydroxy-
l-methyl)piperidinyl]-4H-1-benzopyran-4-one
hydrochloride.
E~ample 12
(+)-cis-5,7-Dihydroxy-2-(2,5-dichlorophenyl)-8-t4-(3-
hydroxy-l-methyl)piperidinyl]-4H-l-benzopyran-4-one
hydrochloride.
~xample 13
(+)-cis-5,7-Dihydroxy-2-(2,4-dichlorophenyl)-8-t4-(3-
hydroxy-l-methyl)piperidinyl]-4H-1-benzopyran-4-one
hydrochloride.
~ample 14
(~)-cis-5,7-Dihydroxy-2-(4-methylphenyl)-8-[4-(3-hydroxy-
1-methyl)piperidinyl]-4H-l-benzopyran-4-one
hydrochloride.
~xæmple lS
(+)-cis-5,7-Dihydroxy-2-(4-methylphenyl)-8-[4-(3-hydroxy-
l-methyl)piperidinyl]-4H-1-benzopyran-4-one
hydrochloride.
- 23 - 1336715
E~cample 16
( + ) -cis-5, 7-Dihydroxy-2- ( 4-aminophenyl ) -8- [ 4- ( 3-hydroxy-
1 -methyl ) piperidinyl ] -4H- l-benzopyran-4 -one
hydrochloride .
Example 17
( ~ ) -cis-5, 7-Dihydroxy-2- ( 3-bromophenyl ) -8- [ 4- ( 3-hydroxy-
1-methyl ) piperidinyl ] -4H-1-benzopyran-4-one
hydrochloride .
Example 18
1 0 ( ~ ) -c is - 5, 7 -Dihydroxy-2 - ( 2 -naphthyl ) -8 - [ 4 - ( 3 -hydroxy- 1-
methyl ) piperidinyl ] -4H-1-benzopyran-4-one hydrochloride .
E~ample 19
( + ) -cis-5, 7-Dihydroxy-2- ( 4-hydroxyphenyl ) -8- [ 4- ( 3-
hydroxy- 1-methyl ) piperidinyl ] -4H- l-benzopyran-4 -one .
l~xample 20
( + ) -cis-5, 7-Dihydroxy-2- ( 2-hydroxyphenyl ) -8- [ 4- ( 3-
hydroxy- l-methyl ) piperidinyl ] -4H- l-benzopyran-4 -one .
Esample 2 1
Active substance solutions which are suitable for
in~ection contain the constituents mentioned below and
can be prepared in a manner known per se by mixing the
substances with each other and filling sterile ampoules
with the solutions. The solutions for in~ections are used
for the treatment of tumors in a dose of 1-2 in~ection
units ( 1 in~ection unit = 1 ampoule) per day.
- 24 - 1336715
Constituents (per ampoule) Weight
(+)-cis-5,7-Dimethoxy-2-(2-thienyl)-8-[4-(3-acetoxy-1-
methyl)piperidinyl]-4H-l-benzopyran-4-one
Sodium chloride 50 mg
Methylparaben 5 mg
Sterile water 5 mg