Note: Descriptions are shown in the official language in which they were submitted.
~lSC-6624
~ 337054
-- 1 --
This application îs a division of Canadian
patent application serial no. 562,q71 filed March 30,
lq88 .
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to a corrosion
resistant plated steel strip. More particularly, the
present invention relates to a high corrosion and r~st
resistant plated steel strip having a zinc-based alloy
base plating layer and thus usef~l for transportation
vehicles, for example, cars and trucks, building mate-
rials, and electric appliance.
2. Description of Related Arts
It is known that a steel strip plated with
zinc and a zinc-based alloy exhibits an enhanced resis-
tance to corrosion and rust. This corrosion resistance
of the plating layer consisting of zinc or a zinc-based
alloy is mainly derived from a self-sacrificing anti-
corrosional action of zinc.
However, it is also known that, if a steel
strip plated with zinc or a zinc-based alloy is used in
a corrosional circumstance, partic~larly in the presence
of salt, zinc is dissoived at a relatively high rate,
and th~s the corrosion resistance of the plated steel
strip cannot be maintained at a high level.
The reasons for the above-mentioned phenomenon
are as follows.
First, zinc has a higher ionization tendency
and lower electric potential than those of iron.
Therefore, an excessively large Zn-Fe coupling current
flows, in a zinc-plated steel strip and th~s zinc is
dissolved at a high rate.
3~ Second, the corrosion product of zinc has a
high cond~ctivity of the corrosion electric current, and
th~s the membrane of corrosion prod~ct is easily dis-
solved.
- 2 - l 337054
To avoid the above-mentioned disadvantages,
attempts have been made to plate a steel strip
substrate surface with a zinc-based alloy containing
iron and/or nickel. The resultant plating alloy layer
has a high electric potential than pure zinc and a
smaller potential difference between iron and the zinc
alloy than that between iron and pure zinc. This
feature restricts the flow of corrosion current through
the plated steel strip, and thus the plating layer can
protect the steel strip substrate over a longer period.
Japanese Examined Patent Publication (Kokoku)
No. 58-15,554 dated March 26, 1983 discloses a plated
steel strip having a plating layer comprising a zinc-
iron alloy or a zinc-nickel alloy. This plating layer
lS is disadvantageous in that an iron component in the
zinc-iron alloy-plating layer is corroded so as to form
red rust. In the zinc-nickel alloy-plating layer, the
corrosion rate of nickel is very low. This feature
results in a remaining of nickel in the state of metal
in the corroded plating layer, and the metallic nickel
on the steel strip substrate undesirably promotes
perforation corrosion of the steel strip substrate.
Japanese Unexamined Patent Publicatikon
(Kokai) Nos. 61-127,900 (June 16, 1986), 61-270,398
(November 29, 1986), 61-235,600 (October 20, 1986) and
61-266,598 (November 26, 1986) discloses a corrosion-
resistant plated steel strip having a zinc-based
plating layer containing alumina or silica colloidal
particles dispersed therein.
However, the corrosion-preventing effect of
the alumina and silica colloidal particles is
unsatisfactory. Also, the alumina or silica colloidal
particle-containing plating layer exhibits a poor
appearance.
Japanese Examined Patent Publication No. 49-
3610 dated January 28, 1974 and Japanese Unexamined
Patent Publication No. 61-270,398 dated November 29,
_ 3 - l 337054
1986 discloses a plated steel strip having a zinc-iron
alloy-plating layer. This plated steel strip exhibits
an enhanced corrosion resistance after being coated
with an organic paint, and thus is useful for
industrial purposes. However, a further enhancement of
the corrosion resistance is strongly desired.
Japanese Examined Patent Publication (Kokoku)
Nos. 61-36078 (August 16, 1986) and 58-56039 (December
13, 1983) and Japanese Unexamined Patent Publication
(Kokai) No. 61-270,398 (November 29, 1986) discloses a
plated steel strip having a plating layer comprising
co-deposited zinc and chromium, thus exhibiting an
enhanced resistance to corrosion. However, the content
of chromium in the plating layer is very small, and
thus the corrosion resistance of the resultant plated
steel strip is unsatisfactory.
In conventional co-deposition method of zinc
and chromium from an electric plating liquid containing
zinc ions and trivalent chromium ions, chromium can be
co-deposited in a very small amount of 0.005 to 5%
based on the total weight of the co-deposited zinc and
chromium. An increase in the concentration of the
trivalent chromium ions in the plating liquid does not
increase the content of chromium in the resultant co-
deposited zinc-chromium alloy plating layer, and
results in a decreased adhesion of the resultant zinc-
chromium alloy plating layer to the steel strip
substrate and in a remarkably decreased electric
current efficiency.
Accordingly, the conventional zinc-chromium
alloy plating method can not be industrially utilized.
Japanese Examined Patent Publication (Kokoku)
No. 58-56039 (December 13, 1983) discloses that, when a
zinc-chromium alloy containing 10 to 100 ppm of
chromium is plated from an acid zinc plating liquid,
the resultant plating layer surface has a pearl-like
gloss.
- 3a- 1 337~54
Also, an increase in the content of chromium
should result in an increase in the corrosion
resistance of the resultant plated steel strip.
However, it has been found that when the content of
S chromium in the zinc-chromium alloy plating layer is
increased to a level of more than 1~ by weight, the
resultant plating
/
/
` _ 4 _ 1 3 3 7 ~ 4
layer becomes dark grey in color and exhibits ~neven
stripe-shaped patterns, d~e to the increase in the
content of chromium. Therefore, the plated steel strip
having a zinc-chromi~m alloy-plating layer containing 1%
by weight of chromi~m is useless as a commercial prod~ct.
The prod~ction of a zinc-chromium alloy plating layer
having both a pearl-like gloss and an enhanced corrosion
resistance is very difficult.
Further, it has been fo~nd that the increase
in the content of chromium in the zinc-chromi~m alloy
plating layer res~lts in a decrease in the phosphate
coating layer-forming property of the plating layer.
That is, when a phosphate chemical conversion treatment
is applied to the zinc-chromi~m alloy plating layer, a
large content of chromi~m in the res~ltant plating
layers, ca~ses the resultant plating layer to exhibit a
significantly decreased adhesion property to phosphate
membrane. Accordingly, even if a painting layer is
formed on the zinc-chromium alloy plating layer, the
increase in the corrosion resistance of the res~ltant
plated steel strip is unsatisfactory.
Japanese Unexamined Patent Publication
(Kokai) Nos. 60-50179 (March 19, 1985) and 58-98172
(June 10, 1983) discloses a plated steel strip having a
zinc, zinc-nickel alloy or zinc-iron alloy plating
layer. The conventional plated steel strip is usually
coated with an organic paint layer having a thickness
of 0.5 to 2.5 ~m. The organic paint layer is effective
for enhancing the corrosion resistance of the plated
3~ steel strip, but when the organic paint layer is
cracked, the corrosion resistance of the plated steel
strip is borne only by the plating layer. Therefore,
the duration of the corrosion resisting activity of
conventional plating layer is unsatisfactory.
~5 Japanese Unexamined Patent P~blication (Kokai)
No. 61-270398 discloses an iron-zinc alloy s~rface
plating layer formed on a zinc-based base plating layer.
_ 5 _ 1 3 3 7 0 5 4
This iron-zinc alloy s~rface plating layer effectively
increases the corrosion resistance of a paint-coated
steel strip. However, when the iron-zinc alloy plating
layer is formed on a zinc-chromi~m alloy base plating
layer, the corrosion potential of the zinc-chromi~m
alloy base plating layer is lower than that of the
iron-zinc alloy plating layer, and thus the resultant
plated steel strip sometimes exhibits an unsatisfactory
corrosion resistance under a certain corrosion circum-
stance.
To produce a zinc-chromium alloy plating layer
containing more than 5% by weight of chromium, it is
important to maintain the contents of zinc ions (Zn2+)
and chromium ions (Cr3 ~ in a plating liquid at a
necessary high level.
When chromium ions (Cr3 ) are fed in the form
of chromium sulfate or chromium chloride into the
plating liquid, the content of sulfate ions (S042 ) or
chlorine ions (Cl ) in the plating liquid is increased,
2Q and this large content of sulfate ions or chlorine ions
disturbs the smoothness of the plating procedure.
Chromium ions (Cr ) cannot be fed in the form of
chromium oxide (Cr203) or metallic chromi~m, because
they are not sol~ble in an acid plating liquid even when
the liquid has a pH of 1.0 or less.
Chromium ions (Cr3 ) may be fed into the
plating layer in the form of chromium hydroxide
(Cr(OH)3) or chromium carbonate (Cr2(C03)2), but they
are only partly dissolved in the plating liq~id and the
non-dissolved portion thereof deposits from the plating
liquid, because the hydroxide and carbonate of chromium
are easily oxidized with air into chromi~m oxide which
is insoluble in the plating liquid. Prevention of the
oxidation of the chromium hydroxide and carbonate is
possible but is very expensive, and thus is not ind~s-
trially practical.
It is also possible to use a sol~ble anode
-
- 6 - 1 ~ 3~
consisting of metallic chromi~m to feed chromi~m ions
(Cr3 ) from the anode. However, in this method, metal-
lic chromi~m anode is electrically dissolved in a m~ch
larger amo~nt than a necessary amo~nt for plating a
cathode and, therefore, the content of the chromi~m ions
(Cr3 1 in the plating liq~id cannot be maintained at a
constant level.
Accordingly, the provision of a method effec-
tive for contin~o~sly feeding chromium ions (Cr3 ) and
for maintaining the content of the chromium ions (Cr3 )
in the plating liq~id at a req~ired constant level is
strongly desired.
SUMMARY OF TH~ INVENTION
An object of the present invention is to provide a
corrosion resistant plated steel strip having an excel-
lent resistance to r~st and a method for prod~cing the
same.
Another object of the present invention is to
provide a corrosion resistant plated steel strip pro-
vided with a zinc-chromi~m alloy plating layer contain-
ing more than 5% by weight of chromi~m and having a good
gloss and appearance, and a method for prod~cing the
same.
Still another object of the present invention is to
provide a corrosion resistant plated steel strip pro-
vided with a zinc-chromium alloy plating layer firmly
bonded to a steel strip s~bstrate and a method for
prod~cing the same in a high efficiency.
F~rther object of the present invention is to
provide a corrosion resistant plated steel strip pro-
vided with a zinc-chromi~m alloy plating layer having an
enhanced bonding property to a phosphate chemical
conversion membrane layer and to a paint coating layer,
and a method for prod~cing the same.
A still f~rther object of the present invention is
to provide a corrosion resistant plated steel strip
~sef~l as a paint coated steel strip having an excellent
1 337054
- 7 -
resistance to corrosion and rust, and a method for
producing the same.
The above-mentioned objects can be attained by
the corrosion resistant plated steel strip of the
present invention which comprises a substrate con-
sisting of a steel strip and a principal plating layer
formed on one surface side or each of two surface
sides of the steel strip substrate and comprising a
co-deposited zinc-chromium based alloy comprising
chromium in an amount of more than 5% by weight but
not more than 20% by weight and the balance consisting
of zinc.
The above-mentioned corrosion resistant plated
steel strip can be produced by the method of the
present invention which comprises forming a principal
plating layer on one surface side or each of two
surface sides of a substrate consisting of a steel
strip, a principal plating layer comprising a zinc-
chromium based alloy by a co-deposition electroplating
procedure using an acid plating liquid containing zinc
ions and trivalent chromium ions in an adequate
amount.
The steel strip substrate is directly coated with
the principal plating layer. Alternatively, the steel
strip substrate is directly coated with an additional
plating metal layer and then with the principal
plating
/ /
/
/ /
~ - 8 - 1 337054
layer. Otherwise, the principal plating layer is coated
with an additional plating metal layer.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows an X-ray diffraction pattern of an
embodiment of the zinc-chromium alloy-plating layer of
the plated steel strip of the present invention, which
embodiment contains the n phase;
Figs. 2 to 5 respectively show an X-ray diffraction
pattern of another embodiment of the zinc-chromium
alloy-plating layer of the plated steel strip of the
present invention, which embodiment does not contain the
n phase;
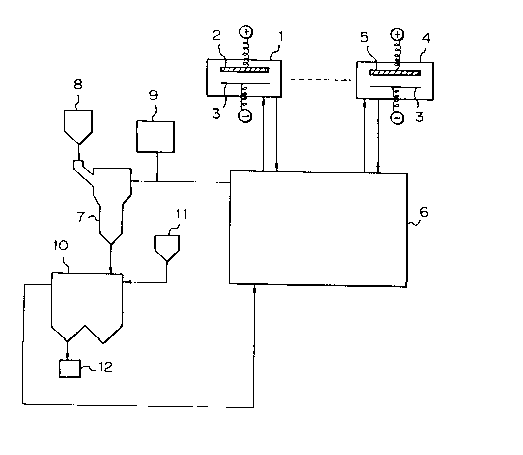
Fig. 6 shows an embodiment of apparatus for contin-
uously carrying out the method of the present invention;
Fig. 7 is a cross-sectional view of an embodiment
of the dissolving vessel usable for the apparatus as
shown in Fig. 6; and,
Fig. 8 shows an another embodiment of the apparatus
for continuously carrying out the method of the present
invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the plated steel strip of the present invention,
at least one surface of a substrate consisting of a
steel strip is coated with a specific zinc-based alloy-
principal plating layer. The specific zinc-based alloy
can be selected from (1) co-deposited zinc-chromium
alloys comprising more than 5% by weight but not exceed-
ing 40% by weight, preferably 7% to 40% by weight, of
chromium and the balance consisting of zinc, and (2) co-
deposited zinc-chromium-iron family metal alloys com-
prising more than 5% by weight of chromium, 5% by weight
or more of at least one member selected from iron family
metals, namely, iron nickel and cobalt, the total amount
of the chromium and the iron family metal being 40~ by
weight or less, and the balance consisting of zinc.
It is known that chromium is in the passive state
in the presence of oxygen, and thus exhibits an excellent
9 1 33 ~
resistance to corrosion in a diluted acid aqueo~s
solution. However, when chromium is bro~ght into
contact with zinc, the chromium exhibits a low electro-
chemical potential close to that of zinc and, therefore,
the zinc-chromium alloy plating layer exhibits a self-
sacrificing corrosion resistance. When the zinc-chromium
alloy-plating layer is corroded in a wet condition, the
resultant corrosion prod~ct is assumed to be a basic
chloride of trivalent chromium which is a water insoluble
multin~cle~s complex. This corrosion prod~ct can serve
as a corrosion resistance material for the steel strip
substrate.
Accordingly, the chromi~m-containing zinc-based
alloy principal plating layer of the present invention
can exhibit a superior corrosion and rust resistance
which cannot be attained by a conventional plating layer
comprising a zinc-iron alloy or zinc-nickel alloy.
In the zinc-based alloy principal plating layer of
the present invention, the content of chromium must be
more than 5% by weight but not exceeds 40% by weight.
If the content of chromi~m is 5% by weight or less, the
resultant plated steel strip exhibits an unsatisfactory
corrosion resistant and rust resistance. When the
content of chromi~m is more than 40%, the resultant
plated steel strip is disadvantageous in that the
resultant plating layer exhibits an unsatisfactory
bonding strength to the steel strip substrate, i.e., the
res~ltant plated steel strip exhibits an ~nsatisfactory
anti-powdering property.
In the zinc-chromium-iron family metal alloy-
plating layer of the present invention, the iron family
metal in a content of 5% by weight or more an uniform
microstr~ct~re is formed in the resultant plating layer.
When the plated steel strip is s~bjected to a phosphate
chemical conversion treatment, the zinc-chromi~m-iron
family metal alloy plating layer having the ~niform
microstr~ct~re forms a dense, even phosphate crystal
- lo - 1 3 3 7 0 5 4
layer thereon. This plated steel strip having a dense,
even phosphate crystal layer exhibits an excellent
paint-coating property. For the above-mentioned effects,
the content of the iron family metal in the plating
S layer must be 5% by weight or more.
- In the method of the present invention, at least
one surface side of a steel strip s~bstrate is plated
with an acid plating liquid containing zinc ions and
trivalent chromium ions (Cr3 ) or a mixture of trivalent
chromi~m ions with ions of at least one iron family
metal to provide a co-deposited zinc-chromium alloy
principal plating layer or a co-deposited zinc-chromi~m-
iron family metal alloy plating layer.
In the acid plating liquid, us~ally, the zinc ions
are in an amount of lO to lS0 g/l, the trivalent chromi~m
ions are in an amo~nt of lO to lO0 g/l and the ion
family metal ions are in an amount of lO to lO0 g/l.
Usually, the zinc ions and the chromi~m ions in the
acid plating liquid are in the total amount of 0.2 to
3.0 mole/l.
In the formation of a zinc-chromium alloy plating
layer of the present invention, the acid plating liquid
contains, for example, zinc ions (Zn ) and chromium
ions (Cr3~) in a total amount of 0.2 to l.2 mole/l, at
least one type of anions selected from sulfate ions and
chlorine ions, complex ion-forming agent for the
trivalent chromium ions, and 0.2 to 5.0 mole/l of an
antioxidant consisting of at least one member selected
from, for example, formic acid, formates, amino radical-
containing organic compounds, for example, amino acidss~ch as glycine, urea, amines and amides.
The acid plating liquid may further contain 4 mole/l
or less of an electric conductivity-increasing agent
consisting of at least one member selected from ammonium
sulfate, ammonium chloride, ammoni~m bromide and other
ammonium halides, alkali metal halides and alkali metal
sulfates. The acid plating liquid may still further
1 337054
-- 11 --
contain a pH-b~ffer consisting of at least one member
selected from boric acid, phosphoric acid, alkali metal
salts and ammonium salts of the above-mentioned acids.
In the acid plating liq~id, when the total amo~nt
of the zinc ions and chromi~m ions is less than
0.2 mole/l, the plating efficiency is sometimes unsatis-
factory and when the total amo~nt is more than
1.2 moles/l, the plating liq~id is sat~rated, and th~s
sometimes cannot be applied to plating operation.
When the amo~nt of the antioxidant is less than
0.2 mole/l, the complex ion formation from the trivalent
chromium ions and the oxidation-preventing effect are
sometimes ~nsatisfactory. When the amo~nt of the
antioxidant is more than 5.0 mole/l, the plating liq~id
is sometimes sat~rated, and th~s cannot be ~sed for a
plating operation. Also, when the amo~nt of the electric
cond~ctivity-increasing agent is more than 4 moles/l,
the plating liq~id is sometimes sat~rated and becomes
~nstable.
2n The plating operation is preferably carried o~t at
a c~rrent density of 10 to 300 A/dm2. When the c~rrent
density is less than 10 A/dm2, the ind~strial efficiency
of the plating operation is sometimes ~nsatisfactory.
Also, when the c~rrent density is more than 300 A/dm2,
the chromi~m ions cannot diff~se into the plating
interface of the steel strip s~bstrate at a satisfactory
diff~sing rate, and therefore, discharge of hydrogen
ions on the plating interface of the steel strip
s~bstrate occ~rs at a high rate and causes a rapid
increase in pH of the plating liq~id to an extent s~ch
that the pH cannot be controlled by the pH b~ffer. D~e
to the above-mentioned phenomena, the plating operation
cannot be carried o~t ~nder ordinary conditions.
The plating liq~id may flow at a flow speed of 0 to
200 m/min. The increase in the flow speed of the
plating liq~id decreases the thickness of interface
layer formed between the steel strip s~bstrate s~rface
- 12 - 133rO~
and the plating liq~id. This decrease ca~ses electro-
deposition intermediates, for example, Cr2 or Zn2+
dissociated from the ligant thereof to flow away from
the interface layer, and th~s decrease the plating
S efficiency. These phenomena can be prevented by con-
trolling the contents of the above-mentioned additives
to an adeq~ate level to prepare a satisfactory plating
layer.
The plating operation is preferably carried out at
a temperat~re of 20C to 70C. A plating temperat~re of
lower than 20C sometimes ca~ses an ~ndesirably in-
creased viscosity of the plating li~id and thus,
diffusion of ions in the plating liquid is restricted
and the plating efficiency is decreased. A plating
temperature of higher than 70C sometimes ca~ses unde-
sirable dissociation of ligants from chromium complex
ions, and th~s normal plating proced~res cannot be
carried o~t.
~n the formation of the zinc-chromium-iron family
metal alloy-plating layer, preferably the content of the
iron family metal in the plating layer is not more than
0.5 moles/l. If the content of the iron family metal is
more than 0.5 moles/l, the chromium complex ion-forming
agent and the antioxidant are consumed for forming iron
family metal complex ions to an extent such that the
chromium complex ion formation is restricted and,
therefore, the electrolytic deposition of chromium is
hindered.
The zinc-based alloy-plating layer of he present
invention preferably further comprises 0.2% to 20% by
weight of fine particles of at least one metal oxide
dispersed therein. The metal oxide is preferably
selected from oxides of silicon, al~min~m, zirconium,
titanium, antimony, tin, chromi~m, molybdenum and
ceri~m. The metal oxide fine particles dispersed in the
plating layer enhance the corrosion resistance of the
plated steel material. The mechanism of enhancement of
13 1 337~54
the corrosion resistance d~e to the presence of the
metal oxide fine particles is not completely clear, b~t
it is ass~med that the corrosion prod~ct of chromium
formed in the plating layer is fixed on the s~rface of
the metal oxide fine particles, to enhance the corrosion
and rust resistance of the plating layer.
Also, the presence of the metal oxide fine parti-
cles in the acid plating layer promotes the co-deposi-
tion of chromi~m in an amo~nt of more than 5~ by weight
lQ with zinc and the fine particles.
When the content of the metal oxide fine particles
is less than 0.2% by weight, the corrosion resistance-
enhancing effect becomes unsatisfactory.
A content of the metal oxide fine particles exceed-
ing 20% by weight is no longer effective for increasing
the corrosion resistance of the resultant plated steel
strip. Also, an excessively large content of the metal
oxide fine particles sometimes res~lts in a decrease in
the bonding strength of the plating layer to the steel
strip s~bstrate s~rface.
The metal oxide fine particles preferably have a
size of 1 ~m or less and use in the form of colloidal
particles.
The zinc-based alloy plating layer containing the
metal oxide fine particles of the present invention can
be prod~ced by ~sing an acid plating liq~id containing
20 to 80 g/l of zinc ions, 10 to 70 g/l of chromium ions
(Cr3 ), 2 to 200 g/l, preferably 10 to 100 g/l of at
least one type of metal oxide fine particles and, if
necessary, 10 to 70 g/l of at least one type of iron
family metal ions, at a current density of 50 to
250 A/dm2, preferably 70 to 250 A/dm2, more preferably
120 to 250 A/dm2. The acid plating liquid preferably
has a pH of 1.0 to 3Ø
In the plated steel strip of the present invention,
the base plating layer is preferably in an amo~nt of 5
to 50 g/m .
- 14 -
1 337054
In the plated steel strip of the present invention,
the principal plating layer is directly formed on the
surface of the steel strip substrate. Alternatively,
the surface of the steel strip s~bstrate is coated with
an additional plating metal layer and then with the
principal plating layer. The principal plating layer
may be coated with an additional plating metal layer
(s~rface layer).
Where the plated steel strip of the present inven-
tion is coated with a paint or lacquer, especially a
cationic electrodeposition paint, the zinc-based alloy
principal plating layer is preferably coated with an
additional plating metal layer comprising a zinc or a
zinc alloy.
Where an iron-zinc alloy comprising 60% by weight
or more of iron and the balance consisting of zinc is
plated on the principal plating layer, the res~ltant
additional plating surface layer has an enhanced bonding
property to a phosphate chemical conversion membrane and
to a cationic electrodeposition paint coating layer, and
thus the resultant paint-coated steel strip has a smooth
surface without crater-like defects.
The zinc-chromium-iron family metal alloy base
plating layer usually has a corrosion potential of -0.9
to -0.8 volt determined in accordance with a calomel
electrode standard in a 5~ NaCl solution. Also, an
additional plating s~rface layer comprising 60% by
weight of iron and the balance consisting of zinc has a
corrosion potential of about -0.8 volt determined in the
same manner as mentioned above. The corrosion poten-
tials of the above-mentioned base and s~rface plating
layers are close to each other, and th~s the combination
of the above-mentioned base plating layer and the
s~rface plating layer is very effective for enhancing
the corrosion and r~st resistances of the plated steel
strip.
The additional plating metal layer may be arranged
-
- 15 - 1~370~4
between the substrate and the principal plating layer to
firmly bond the s~bstrate to the principal plating layer
therewith and to increase the corrosion resistance of
` the resultant plated steel strip.
The additional coating layer preferably has an
amo~nt of l to lO glm2.
The additional coating layer of the present inven-
tion may contain, as an additional component, a small
amo~nt of at least one member selected from Ni, Cr, Al,
P, Cu, Co, Mn, Sn and Cd.
The surface of the principal plating layer of the
present invention preferably has a glossiness of 80 or
more, determined in accordance with JIS Z 8~41, 60/60.
Generally, an acid plating liquid containing zinc
ions and trivalent chromium ions exhibits a special
electrodepositing property. That is, an increase in the
concentration of zinc ions in the plating liq~id accel-
erates the deposition of zinc but sometimes restricts
the deposition of chromi~m. Also, an increase in the
proportion of chromi~m ions (Cr3 ) in the plating liquid
sometimes causes the deposition of zinc to be restricted
and hinders the deposition of chromium.
Also, the principal plating layer of the present
invention sometimes exhibits an undesirable white grey
or black grey color, and has a n~mber of stripe-patterned
blocks.
The above-mentioned disadvantages can be removed by
adding a polyoxyalkylene compound to the plating liq~id.
That is, in the plating liquid containing the polyoxy-
alkylene compound, zinc and chromium can be co-deposited
at a high current efficiency. Also, the resultant
principal plating layer has an improved glossiness of 80
or more and a good appearance.
Namely, the surface of the principal plating layer
has an uniform stainless steel-like silver white color
which is different from the milk ~hite color of a
zinc-plating layer surface. When a r~st-preventing oil
` - 16 - 1 337054
or press oil is applied onto the principal plating layer
of the present invention, the oil coating layer is
glossy and it is easy to detect cracks or scratches
formed thereon. However, when the r~st-preventing oil
or press oil is applied to a conventional zinc-plating
layer, the oil layer has no gloss and it is diffic~lt to
detect cracks and scratches on the zinc-plating layer.
The polyoxyalkylene compound usable for the present
invention is of the formulae:
2 ( 1 )n
and
2 ( 1 )n
wherein Rl represents an alkylene radical, R2 represents
a member selected from a hydrogen atom, alkyl radicals,
a phenyl radical, a naphthyl radical and derivatives of
the above-mentioned radicals, and n represents an
integer of 1 to 2000.
For example, the polyoxyalkylene compo~nds ~sable
for the present invention incl~de the following com-
po~nds.
Polyoxyethylene (polyethylene glycol)
HO - (CH2-CH2-O)n-H
n = 1 to 2000
Alkyl-polyoxyethylene ether
R-O-(CH2-CH2-O)n-H
n = 1 to 2000
R = an alkyl radical of the form~la:
m 2m+1
wherein m = 0 to 20
Alkylphenyl-polyoxyethylene ether
~ O-(CH2-CH2-0)n~H
R
wherein:
n = 6 to 2000
R is as defined above
_ is as defined above
Alkylnaphthyl-polyoxyethylene ether
- 17 - 1 337054
O- (CH2-CH2-0) n~H
R ~ ~b
n = 4 to 2000
R and m are as defined above.
Polyoxypropylene (polypropyleneglycol)
HO ~ CH-CH2-Ot H
\CH3 I n
n = 3 to 2000
Alkyl-polyoxypropylene ether
R-O tCH-CH2-O~ H
CH3 n
n = 1 to 2000
R and m are as defined above.
Alkylphenyl-polyoxypropylene ether
O ~ CH-CH2-0)--H
,~ CH3 n
R ~
n = 6 to 2000
R and m are as defined above.
Alkylnaphthyl-polyoxypropylene ether
O tCH-CH2-O~H
R ~ CH3 n
n = 4 to 2000
R and m are as defined above.
3n Polyoxymethylene compo~lnd
R ' 1-- (CH2-O) n~H
n = 3 to 5000
R' 1 represents a hydrogen atom, alkyl
radical or aryl radical
-ethoxylated naphthol (EN)
O- (CH2CH2-0) nH
- 18 - 1 337054
n = 1 to 20
and
Ethoxylated--naphthol s~lfonic acid (ENSA)
O- (CH2-CH2-0) nH
n = 1 to 20
Preferably, the polyoxyalkylene compo~nd is added in an
amo~nt of 0.01 to 20 g/l of the plating liq~id.
When the polyoxyalkylene compo~nd is ~sed as an
additive, the plating proced~re is preferably carried
o~t by ~sing an acid plating liq~id containing 10 to
150 g/l of zinc ions, 10 to 150 g/l of chromi~m ions
(Cr3 ), 0.01 to 20 g/l of the polyoxyalkylene compo~nd
at a pH of 3 to 0.5 at a c~rrent density of 50 A/dm or
more, more preferably 50 to 250 A/dm at a temperat~re
of 40C to 70C. Also, the plating liquid preferably is
circ~lated at a flow speed of 30 to 200 m/min.
In an embodiment of the present invention, the
principal plating layer comprising a zinc-chromi~m alloy
comprising more than 5% by weight b~t not exceeding 40%
by weight of chromi~m and the balance consisting of zinc
is prepared by an electroplating operation in an acid
plating liq~id containing 10 to 150 g/l of zinc ions and
10 to 100 g/l of trivalent chromi~m ions (Cr3 ), the
total concentration of the zinc ions and the trivalent
chromi~m ions being in the range of from 0.5 to
3.0 mole/l, at a c~rrent density of 150 A/dm2 to
300 A/dm .
The acid plating liq~id contains acid ions s~ch as
s~lfate ions and/or chlorine ions and preferably has a
pH of 0.5 to 3Ø Also, the acid plating liq~id may
contain an electrocond~ctivity-increasing agent consist-
ing of at least one selected from, for example, Na , K ,
NH4 and Mg2 ions which does not co-deposit with zinc
and chromi~m on the s~bstrate s~rface. F~rther, the
-
- 19 - 1 337054
plating liquid may contain a small amount of at least
one type of additional metal ions, for example, Cr 6,
Ni, Co, Fe, Mn, Cu, Sn, Cd and Pb ions, which are
co-deposited with zinc and chromi~m.
The plating liquid preferably has a temperature of
40 to 70C and is circulated at a flow speed of 30 to
200 m/min.
In an embodiment of the present invention, the base
plating layer of the plated steel strip is coated with a
chromate layer. The chromate coating layer is pref-
erably coated with a resin layer.
The chromate coating layer can be formed on the
base plating layer by any conventional chromate treat-
ment method, for example, coating type chromate treat-
ment, reaction type chromate treatment, and electrolysistype chromate treatment. In the coating type and reaction type chromate
treatment methods, the chromate treating liquid contains
Cr 6 ions and/or Cr 3 and an additive consisting of at
least one member selected from inorganic colloids,
acids, for example, phosphoric acid, fluorides, and
aqueous solutions or em~lsion of organic resinous
materials.
For example, a typical phosphoric acid and
fluoride-containing chromate treating liq~id comprises
30 g/l of chromic acid, 10 g/l of phosphoric acid, 4 g/l
of titanium potassium fluoride and 0.5 g/l of sodium
fluoride. A typical silica-containing chromate treating
liq~id comprises 50 g/l of chromic acid containing 40%
of trivalent chromi~m and 100 g/l of silica colloid.
The inorganic colloid may be selected from silica,
alumina, titania, and zirconia colloids. The acid can
be selected from oxygen acids, for example, molybdic
acid, t~ngstic acid, and vanadic acid.
The chromate treating liq~id preferably contains a
substance capable of reacting with zinc to form a
water-insoluble s~bstance, for example, phosphoric acid,
-
- 20 - 1 3 3 7 ~ 5 4
polyphosphoric acid, and/or another substance which can
be converted to a water-insoluble substance by hydro-
lysis, for example, silicofluorides, titanofluorides,
` and phosphates.
The inorganic colloids are effective for fixing a
small amount of hexavalent chromium in the resultant
chromate coating layer, and the phosphoric acid com-
po~nds and fluoride compounds are effective for promot-
ing reactions of chromate with base plating layer. The
phosphoric acid compound and the silica colloid are used
in a concentration of 1 to 200 g/l and 1 to 800 g/l,
respectively.
The chromate treating liquids may be mixed with a
resinous material which is not reactive with the
chromate treating liq~id, for example, an acrylic
resinous material.
The electrolysis type chromate treatment is carried
out by using a treating li~uid-comprising sulfuric acid,
phosphoric acid, and/or halogen ions, and optionally, an
inorganic colloid, for example, SiO2 colloid and/or
A12O3 colloid, and cations, for example, Co and/or Mg
ions, in addition to chromic acid.
The electrolytic chromate treatment is usually
carried out by a cathodic electrolysis and can be ~sed
-5 in conjunction with an anodic electrolysis and/or an
alternating current electrolysis.
Generally, the chromate coating layer is in an
amount of S to 100 mg/m . A chromate coating layer in
an amount of less than 5 mg/m2 sometimes exhibits an
3n unsatisfactory bonding property to a paint coating
layer. Also, a chromate coating layer in an amount of
more than 100 mg/m sometimes causes the resultant
chromate coated plated steel strip to exhibit a de-
creased welding property.
The chromate coating layer is preferably coated
with an organic resin coating layer having a thickness
of 0.5 to 2.5 ~m. The resin is preferably selected from
-
- 21 - ~ 3~0~
epoxy resins, acrylic polymer resins, polyester resins,
polyurethane resins, and olefin-acrylic polymer resins.
The organic resin coating layer may contain an additive
consisting of at least one member selected from anti-
rusting agents, for example, SiO2 , a surface tensionand viscosity-controlling agent, for example, amino-base
surfactant, and lubricants, for example, wax.
A resin coating layer having a thickness of less
than 0.5 ~m sometimes exhibits an unsatisfactory corro-
sion resistance-enhancing effect. A resin coating layer
having a thickness of more than 2.5 ~m sometimes ca~ses
the res~ltant resin coated plated steel strip to exhibit
a poor welding property, a reduced cationic electro-
deposition paint-coating property, and a poor pressing
workability.
In an embodiment of the plated steel strip of the
present invention, the principal plating layer compris-
ing a zinc-chromi~m alloy is coated with an additional
plating layer comprising zinc or a zinc-bast alloy, for
2~ example, 60% or more of zinc and t~e balance consisting
of at least one member of iron, nickel, manganese and
cobalt. This type of additional plating layer exhibits
a good phosphate layer-forming property in an immersion
type phosphate chemical conversion treatment. The
additional coating layer may contain a small amount (for
example, 1% or less) of at least one additional metal
selected from Sn, Cd, Al, Pb, Cu, Ag, P, C, O, Sb, B,
and Ti.
In an embodiment of the plated steel strip of the
present invention, the principal plating layer compris-
ing a zinc-chromium alloy preferably does not contain
the n phase.
Stable intermetallic compounds are not known in
many types of zinc-chromium alloys, but in view of the
X-ray diffraction patterns of the zinc-chromium alloys
in the base plating layer, it has been found that the
X-ray diffraction patterns have a pl~rality of unknown
-
- 22 1 3 3 7 0 5 4
-
peaks spaced from each other with face intervals d
val~es which cannot be identified as a zinc phase ~n
phase) or a chromi~m phase. These peaks are ass~med to
denote a certain type of zinc-chromi~m alloy phase.
In Figs. 1 to 5, the axis of the abscissas repre-
sents a value (degree) of 2~ at the C~ target and the
axis of the ordinates represents the intensity of the
X-ray.
Figure 1 shows an X-ray diffraction pattern of a
zinc-chromi~m alloy plating layer which contains 9% ~y
weight of chromium, and has an n phase.
In Fig. 1, peak A (d = 2.10 A) and peak B (d
O O
= 2.47 A) correspond to the n phase, peak C (d = 2.21 A)
is assumed to correspond to a zinc-chromi~m alloy phase,
and the peak at d = 2.023 A corresponds to the a-Fe
derived from the steel strip substrate.
Fig~re 2 shows an X-ray diffraction pattern of a
zinc-chromi~m alloy-plating layer containing 7% by
weight of chromium. This pattern has no peak at d
= 2.10 A and d = 2.47 A, which correspond to the n
phase. The peak C (d = 2.276 A) is ass~med to corre-
spond to a type of zinc-chromi~m alloy phase, and
therefore, this zinc-chromium alloy-plating layer does
not have the n phase.
Referring to Fig. 3 in which an X-ray diffraction
pattern of a zinc-chromi~m alloy-plating layer contain-
ing 12% by weight of chromi~m is shown, no peak was
fo~nd at d = 2.10 A and d = 2.47 A. The peak C (d
= 2.212 A) and peak D (d = 2.138 A) are ass~med to
3n correspond to certain types of zinc-chromi~m alloy
phases and, therefore, this zinc-chromi~m alloy-plating
layer does not have the n phase.
Referring to Fig. 4, in which an X-ray diffraction
pattern of a zinc-chromi~m alloy-plating layer contain-
ing 15% by weight of chromi~m is shown, no peak appearedat d = 2.10 A and d = 2.47 A. The peak D (d = 2.129 A)
and peak E (d = 2.348 A) are ass~med to correspond to
- 23 - 1337054
certain types of zinc-chromium alloy phase. In view of
Fig. 4, it is clear that this zinc-chromium alloy-
plating layer does not have the n phase.
In Fig. 5, in which the X-ray diffraction pattern
of a zinc-chromium alloy-plating layer containing 27% by
weight of chromi~m is shown, no peak appears at d
= 2.10 A and at d = 2.47 A. The peak D (d = 2.123) is
ass~med to correspond to a certain type of zinc-chromi~m
alloy. From Fig. 5, it is clear that the zinc-chromium
alloy-plating layer does not contain the n phase.
The zinc-chromium alloy-plating layer not contain-
ing the n phase, as shown in Figs. 2 to 5, causes the
resultant plated steel strip, especially, after paint-
coating, to exhibit a higher corrosion and rust resis-
tance than that of the zinc-chromi~m alloy plating layer
containing the n phase. Us~ally, when the zinc-chromi~m
alloy plating layer is exposed to corrosive conditions,
the corrosion product of chromium forms a corrosion
resistant membrane on the steel strip substrate s~rface.
The corrosion prod~ct prod~ced in the n-phase free
zinc-chromium alloy plating layer is effective for
restricting an excessive local cell action in the
plating layer and for preventing a separation of the
paint from the base plating layer. However, the
zinc-chromi~m alloy-base plating layer containing the n
phase exhibits lower effect of the above-mentioned
restriction and prevention.
The n phase-free zinc-chromi~m alloy-base plating
layer can be prod~ced by electroplating a steel strip
s~bstrate with acid plating liq~id containing 0.01 to
20 g/l of a polyoxyalkylene derivative as described
hereinbefore, at a c~rrent density of 50 A/dm2 or more.
When an additional coating layer comprising 60% by
weight or more of iron and 40% by weight or less of zinc
is formed on the n phase-free zinc-chromi~m alloy-
principal plating layer, the res~ltant two-layer-plated
steel strip exhibits an improved phosphate chemical
-
- 24 - 1 3 3 7 0 5 4
conversion coating layer-forming property and an enhanced
cationic electrodeposition paint coating property
layer-forming p,operty, and th~s the cation electro-
deposition paint-coated steel strip has a smooth coating
s~rface witho~t crater-like coating defect.
In the method of the present invention for produc-
ing a zinc-based alloy principal plating layer on a
s~rface of a steel strip s~bstrate, the electroplating
proced~re can be contin~o~sly carried out by contin~-
lQ o~sly feeding zinc ions (Zn ) and trivalent chromi~m
ions (Cr3 ) to an acid plating liq~id in s~ch a manner
that a metallic zinc and an aq~eo~s sol~tion containing
hexavalent chromi~m ions (Cr ) are brought into contact
with the acid plating liq~id containing zinc ions and
trivalent chromi~m ions.
The metallic zinc is dissolved in the acid plating
liquid while generating hydrogen gas and is converted to
zinc ions. The hexavalent chromi~m sol~tion, for
example, a chromic acid sol~tion, is mixed with the acid
plating liq~id; the hexavalent chromium promotes the
dissol~tion of the metallic zinc and is converted to
trivalent chromi~m ions.
When the metallic zinc is bro~ght into complete
contact with the hexavalent chromi~m sol~tion, the
entire amo~nt of the hexavalent chromi~m is converted to
trivalent chromi~m ions and no non-converted hexavalent
chromi~m remains.
The metallic zinc can be dissolved in the acid
plating liq~id by a competitive reaction with H ions
and with the hexavalent chromi~m. Therefore, when a
base plating layer comprising a zinc-chromium alloy
having a high content of chromi~m is formed, it is
necessary to increase the contrib~tion of the reaction
with the hexavalent chromi~m. The reaction rate of the
hexavalent chromi~m is controlled by a rate of diff~sion
of the hexavalent chromium to the s~rface of the metallic
zinc. Accordingly, it is preferable to ~se a dissolving
~ - 25 - 1 337054
vessel which can carry o~t the contact of the metallic
zinc with the hexavalent chromi~m at a high contact
efficiency.
Th~s type of dissolving vessel is preferably
provided with a hopper for feeding the metallic zinc, a
vessel for containing the metallic zinc, means for
feeding an aq~eo~s solution of hexavalent chromi~m into
the vessel, and means for circ~lating an acid plating
liq~id thro~gh the vessel.
When a batch type dissolving vessel is ~sed, the
vessel is preferably provided with shaking, stirring or
gas-blowing means to increase the contact efficiency.
The contin~o~s dissolving vessel can be one of a
fl~idizing vessel, filling vessel, and tower mill.
In the dissolving vessel for the metallic zinc and
hexavalent chromium, preferably the metallic zinc is
fixed in the vessel so that the metallic zinc cannot
move by the flows of the hexavalent chromi~m sol~tion
and the acid plating liq~id or by hydrogen gas b~bbles
2Q generated on the metallic zinc particle or plate s~r-
faces. For this p~rpose, a perforated plate is pref-
erably arranged at an ~pper portion and a bottom portion
of the dissolving vessel. The perforated plate allows
the acid plating liq~id to flow therethro~gh at a
desired flow speed. This flow of the acid plating
liq~id is effective for enhancing the contact efficiency
of the metallic zinc with the hexavalent chromi~m. The
acid plating liq~id preferably flows at a space velocity
of 0.5 cm/sec or more in the dissolving vessel. In a
dissolving vessel in which the metallic zinc is fixed
and th~s cannot move with the flow of the acid plating
liq~id, the relative velocity of the acid plating liq~id
to the metallic zinc is preferably 5 cm/sec or more.
The metallic zinc may be in any shape, for exam-
ple, plate, grains, or fine particles. In order to
allow the acid plating liq~id to flow at a satisfactory
relative flow speed to the metallic zinc and to have a
- 26 - 1337054
relatively large s~rface area thereof, preferably the
metallic zinc is in the form of grains or particles
having a size of 10 mm to 0.1 mm.
After the reaction in the dissolving vessel has
been completed, the residual content of hexachromium
ions (Cr ) in the acid plating liq~id is preferably
less than 10 g/l. Also, the acid plating liquid is
preferably introd~ced into the dissolving vessel at room
temperature or more, but not more than 80C, more
0 preferably 30C to 70C, which is the same as the
plating temperature.
The hexavalent chromi~m-feeding liq~id contains
chromic acid, dichromic acid and/or chromi~m chromate,
and preferably, does not contain anions and cations
other than those mentioned above, to maintain the
composition of the acid plating liq~id at a constant
value.
The chromium chromate is prepared by reacting
anhydro~s chromic acid with a red~cing s~bstance, for
example, a lower alcohol compo~nd, for example, ethyl
alcohol and propyl alcohol, a polyhydric alcohol, for
example, glycerol, and ethylene glycol, an organic acid,
for example, formic acid or oxalic acid, or starch or
saccharose so that a portion of the hexavalent chromium
(Cr6 ) is red~ced to trivalent chromi~m (Cr3 ). In the
preparation of the chromi~m chromate solution, the
reducing organic s~bstance is used in an amount such
that substantially the entire amo~nt of the red~cing
organic s~bstance added to the chromic acid solution is
consumed and s~bstantially no non-reacted substance
remains in the res~ltant chromium chromate sol~tion.
The hexavalent chromi~m feeding liq~id may contain a
chromate, for example, sodi~m chromate, in a small
amount which does not substantially affect the composi-
tion of the acid plating liquid.
In the method of the present invention, preferablya lead-based electrode is used as an insol~ble anode,
- 27 - 1 3 3 7 0 5 4
stronti~m carbonate and/or bari~m carbonate is fed into
the acid plating liq~id, and a portion of chromi~m to be
fed into the acid plating liq~id consists of chromi~m
s~lfate.
The ~se of an insol~ble anode is advantageo~s in
that the shape and dimensions of the anode can be
maintained constant even when contin~o~sly ~sed for a
long period, a distance between a cathode consisting of
a steel strip s~bstrate to be plated and the anode can
be maintained at a constant val~e, and therefore, the
plating proced~re can be continuo~sly carried o~t ~nder
constant conditions.
AlSo, the distance between the anode and cathode
can be shortened so as to red~ce a voltage loss gen-
erated d~e to the resistance of the plating liq~id.
F~rther, the plating proced~re can be contin~ed over a
long period witho~t replacement of the anode, and th~s
provides a high productivity and high economical effi-
ciency.
However, when the insol~ble anode is ~sed, the
electric c~rrent is transmitted by a generation of
oxygen gas (2 d~e to an electrolysis of water or
electrolytic oxidation reaction of components in the
plating liq~id. In a plating liquid containing zinc
ions and trivalent chromi~m ions, the trivalent chromium
ions are oxidized to form hexavalent chromi~m, and the
res~ltant hexavalent chromi~m is acc~m~lated in the
plating system, and therefore, it is necessary to red~ce
the hexavalent chromium to prod~ce trivalent chromi~m
ions.
In the above-mentioned method of the present
invention, the hexavalent chromi~m generated d~e to the
insol~ble anode is red~ced by the metallic zinc fed into
the plating liq~id, and the concentration of the hexa-
~5 valent chromi~m in the plating liq~id is maintained at avery low level.
The plating proced~re in accordance with the
`~ -
- 28 - 1337~54
present invention is preferably carried o~t in a number
of plating cells each having an insoluble anode.
However, some of the plating cells may have a soluble
anode, for example, a chromium anode. The type of anode
to be placed in the plating cells can be desired by
taking into consideration the contribution of the
metallic zinc to the red~ction of hexavalent chromium
and the consumption of electric current for the oxida-
tion of trivalent chromium on the insoluble anode, so
that an undesirable accumulation of hexavalent chromium
in the plating liq~id is avoided.
The insoluble anode preferably comprises lead, a
lead tPb) based alloys containing at least one member
selected from Sn, Ag, In, Te, Tl, Sr, As, Sb and C~,
PbO2 , Pt, Pt-based alloys containing at least one
member selected from Ir, Pd, Ru and Ph, oxides of Rh and
Ru, or a Ta-based amorphous alloy containing at least
one member selected from Ru, Rh, Pd, Ir, Pt and Ni.
The most economical insol~ble anode is one formed
of a Pb or a Pb-based alloy.
The insoluble anode is used mainly in a s~lfate-
containing plating liquid in which a small amount of Pb
is dissolved. The concentration of Pb dissolved in the
plating liquid is preferably restricted to a level of
3 ppm or less, to prevent an undesirable decrease in the
bonding property of the resultant zinc-chromi~m alloy
plating layer to the steel strip substrate. The in-
crease in the concentration of Pb in the plating liquid
can be prevented by adding Sr carbonate and/or Ba
carbonate to the plating liq~id. When Sr or Ba carbonate
is converted to Sr or Ba sulfate, which is insol~ble in
water, in the plating liq~id, the deposition of the
resultant sulfate ca~ses Pb dissolved in the plating
liq~id to be co-deposited therewith. Also, the Sr or Ba
carbonate is effective for eliminating an excessive
amount of sulfate ions from the plating liq~id. This
allows chromium to be fed in the form of s~lfate, for
- 29 - 1 3 3 7 0 5 4
example, Cr2(SO4)3 or Cr(OH)(SO4) to the plating liq~id
and the amo~nt of metallic zinc to be added to the
plating liq~id to be red~ced.
The method of the present invention will be f~rther
S explained below.
Referring to Fig. 6, a plating apparat~s comprises
at least one plating cell 1 having an insoluble anode 2
and at least one other plating cell 4 having a soluble
anode 5. In each of the cells 1 and 4, a steel strip
s~bstrate 3, which serves as a cathode, is plated with a
plating liq~id. The plating liq~id is circ~lated
thro~gh a tank 6 and the cell 1 or 4. Metallic zinc is
fed from a hopper 8 into a dissolving vessel 7, a
portion of the plating liquid is fed from the tank 6
into the dissolving vessel, and hexavalent chromi~m is
fed from a tank 9 into the dissolving vessel 7 to be
mixed with the plating liquid. In the dissolving
vessel 7, the hexavalent chromium comes into contact
with the metallic zinc and is converted to trivalent
chromi~m ions, and a portion of the metallic zinc is
converted to zinc ions dissolved in the plating liq~id.
The res~ltant plating liq~id is fed from the dissolving
vessel 7 to a deposition vessel 10, and Sr or Ba carbo-
nate is fed from a hopper 11 to the deposition vessel 10
to eliminate excessive amo~nts of Pb and s~lfate ions.
The res~ltant deposits are removed thro~gh a filter 12
to the o~tside of the plating system. The filtered
plating liq~id is fed from the deposition vessel 10 to
the plating liq~id tank 6, and then into the plating
cells 1 and 4.
Additional amo~nts of zinc and chromi~m corre-
sponding to the cons~mption thereof in the plating cells
are prepared in the dissolving vessel 7 and are fed into
the tank 6 so that the concentrations of zinc and
chromi~m are maintained at a constant val~e.
Fig~re 7 shows a cross-sectional view of a
dissolving vessel ~sef~l for the method of the present
1 337054
invention, in which metallic zinc is fixed so that the
metallic zinc is not moved by a flow of a liquid con-
taining hexavalent chromi~m.
Referring to Fig. 7, grains of metallic zinc are
charged from a hopper 8 into a dissolving vessel 7
thro~gh a d~ct 16 so that a layer 13 consisting of the
metallic zinc grains is formed on a perforated bottom
plate 14 while a perforated ~pper plate lS is elevated
by a plate-moving device comprising a motor 18, guide
bar 19, rod 20a and rod 20b. When the metallic zinc
grain layer 13 is formed, the ~pper plate 15 is placed
on the layer 13 and is rotated by a motor 21 so that the
upper face of the layer 13 becomes smooth and horizon-
tal. Then the upper plate lS is fixed on the metallic
zinc grain layer 13 so that the metallic zinc grains are
fixed between the upper and bottom plates 15 and 14.
A mixture of the plating liquid with a solution of
hexavalent chromium is fed to the dissolving vessel 7
through the cond~it 16. The mixture is passed thro~gh
the metallic zinc grain layer 13 between the perforated
bottom and upper plates 14 and 15 while the hexavalent
chromi~m is converted to trivalent chromium ions and the
metallic zinc is converted to zinc ions.
The resultant fresh plating liquid is discharged
from the dissolving vessel 17 thro~gh a discharging
cond~it 17 and is fed to the deposition vessel (not
shown in Fig. 7).
The above-mentioned method of the present invention
can be carried out in the presence of the organic
reducing substance mentioned above, added to the plating
liquid. The organic reducing substance is preferably
selected from lower monohydric alcohols, for example,
ethyl alcohol and propyl alcohol, polyhydric alcohols,
for example, glycerol and ethyleneglycol, reducing lower
aliphatic acids, for example, formic acid and oxalic
acid, and starch and saccharose.
The reducing organic substance is preferably
- 31 - ~ 3~70~4
contained in a concentration of 50 g/l or less pref-
erably, 0.1 to 30 g/l in the plating liq~id. If the
concentration of the red~cing organic s~bstance is more
than 50 g/l, the resultant zinc-based alloy plating
layer sometimes exhibits an unsatisfactory bonding
strength to the steel strip substrate.
The plating liq~id containing the red~cing organic
substance preferably further contains bromine ions
(Br ). The bromine ions (Br ) in the plating liquid are
preferentially oxidized before the trivalent chromi~m
ions (Cr3 ) on the insol~ble anode and are converted to
Br2. The res~ltant Br2 reacts with the reducing organic
substance and is returned to Br . D~ring the above-
mentioned activity, the bromine ions (Br ) in the
red~cing organic substance-containing plating liquid
serves as a catalyst for preventing an ~ndesirable
generation of hexavalent chromium on the insol~ble
anode. The bromine ions may be added in the form of a
alkali or ammonium salt, NaBr, KBr, or NH4Br.
Generally, the concentration of bromine ions in the
plating liq~id is 40 g/l or less.
The plating liquid containing the red~cing organic
substance and Bromine ions can be prepared by using, for
example, an apparatus as shown in Fig. 8.
Referring to Fig. 8, a portion of a plating liquid
contained in a tank 6 is fed into a reaction vessel 31,
and a hexavalent chromium sol~tion in a tank 32, a
red~cing organic substance in a tank 33 and, if neces-
sary, a s~lf~ric acid solution in a tank 34 are fed into
the reaction vessel 31. In this reaction vessel 31, the
hexavalent chromium is red~ced to trivalent chromium
ions, the res~ltant plating liquid is controlled to a
desired temperature in a heat exchanger 35, and, if
necessary, is ret~rned to the tank 6. The heat-exchanged
plating layer is fed to a dissolving vessel 37 and is
brought into contact with metallic zinc supplied from a
hopper 36 to the dissolving vessel 37. Also, a portion
- 32 - 1 3 3 ~ O ~ ~
of the plating liquid in the tank 6 is fed to the
dissolving vessel 37. The metallic zinc is converted to
zinc ions and is dissolved in the plating liquid. Also,
non-reacted hexavalent chromium in the plating liquid is
reduced with the metallic zinc and is converted to
trivalent chromium ions.
The plating liquid is fed to a deposition vessel 38
and, if necessary, is mixed with a bromine ion solution
fed from a tank 39. The plating liquid is then sepa-
rated from the deposition and returned to the tank 6.
EXAMPLES
The present invention will be further explained by
way of specific examples, which are representative and
do not in any way restrict the scope of the present
invention.
In the examples, the resistance of a specimen to
corrosion was determined as follows.
(1) Preparation of paint-coated specimen
A specimen consisted of a plated steel strip
was subjected to a dipping type chemical conversion
treatment with zinc phosphate, and the treated specimen
was then coated with a cathodic ED coating layer having
a thickness of 20 ~m.
(2) Cyclic corrosion test
A specimen was subjected to a cyclic corrosion
test (CCT) in which a salt spray test was combined with
a drying-wetting-cooling test.
In one cycle test, the specimen was wetted at
a temperature of 50C and a relative humidity of 85% for
15.5 hours, was dried at a 70C for 3 hours, was sub-
jected to a salt spray test at a temperature of 50C for
2 hours, was left at room temperature for 2 hours, and
then was salt spray-tested at 50C for 1.5 hours. the
test was repeated 30 times. After the test was com-
pleted, a decrease in weight of the specimen due to
corrosion and the number of perforations per dm2 formed
in the specimen, were measured.
_ 33 - I 3 3 7 ~ 5 4
(3) Salt spray test
This test was carried out in accordance with
Japanese Industrial Standard (JIS) Z 2371, and the
.percentage of the area in which red rust was generated,
based on the total surface area of specimen was measured.
Examples 1 to 16
In each of Examples 1 to 16, a cold rolled steel
strip consisting of a continuously cast and box-annealed
aluminum-killed steel and having a thickness of 0.8 mm
and a width of 15 cm was degreased and pickled in a
usual manner and then electroplated with an acid plating
liquid having the composition as shown in Table 1 at the
current density at the temperature shown in Table 1.
The resultant principal plating layer had the composi-
tion shown in Table 1.
-- 34 --
~ 33~0~
N
T 5 I` r` r` o r~ r ~
N _I O O O ~D O O O O O O O O O O O O
N t~ N
-
!r~ _
O O O O O U~ O O O O O O O O O O
c ~ ~ ~r o ~r ~ ~ ~r ~r ~ ~ ~ ~r ~ ~r ~r
_I
Q.
~ _
.,~ ~ _
C)O ~OOOOOOOOOOOOOOOO
U~ O O
-- N~SI N
u,~ N ~ --
~, O
N
U ~ o o o ~-- ~ o o o o o o o o o
I U~ --1 N N N
D _
~ ~ ~D a~ O N ~ ~ ~ N ~ O N N N ~ N
~ _
N
c~ o o ~ o o o o r~ r~ 1-- 1` 0 0 0 1`
C~ --
~ . ,
F: O _1 N ~ ~ 1~1 ~ r` ~ ~ O _1 N t") ~ In ~D
8 ~ ~ ~ ~ ~ ~ ~
- r
- 35 ~ 1 3 3 7 ~ 5 4
r d~
dP d~ O
o ~ o u~ o u~ ~ ~ ~ o o~ I`
~ ~ I~ o r~ o u~ o ~ _~ o o o u~ o o
,,~3 c cl c ~ c~ c c c c c ~ c~ c
o e~
o u^t ul o o o o o o o o o o o o o
c ~ ~ -
c~
~ c l l ~
oooooooooooooooo
U ~ ~ D CO O ~ X CO CO : CO O O O a~ ~r
a ~ ~ ~ ~ ~ ~
~ _ In ~
~D ~ O C~ ~ O ~ ~i O ~ O
aJ ~, ,' ~s
o~ o~ o~ o~ o~ o~
9~ ~ c-l c ~ ~ cl c~
~o ~ z z z --~ z z
L ~ C ~ ~ C'
-- Z 14 Z C~
O :~:
C'
., m
~ Z
~ ~ _
C~
~ Qz _I ~ r~ ~ In ~ t~ co a~ O
~_ - 36 - 1 337054
Examples 17 to 46 and Comparative Examples 1 to 7
In each of Examples 17, 19, 34 and Comparative
Examples 1 to 4, the same steel strip as that mentioned
.in Example 1 was plated with a principal plating layer
having the composition and the amount as shown in
Table 2.
In each of Examples 19, 20, 21, 26 to 33, 38 to 40,
and 42 to 46 and Comparative Examples 5, 6 and 7, the
same steel strip as that described in Example 1 was
plated with a base plating layer having the composition
and the amount as shown in Table 2, and then with a
surface plating layer having the composition and the
amount shown in Table 2.
In each of Examples 22 to 25, 35 to 37 and 42, the
same steel strip as that described in Example 1 was
plated with a base plating layer, then with an interme-
diate plating layer, and finally, with a surface coating
layer; each layer having the composition and the amount
shown in Table 2.
The resultant plated steel strips exhibited the
corrosion resistance as indicated in Table 2.
Table 2 clearly indicates that the plated steel
strips of the present invention have an enhanced corro-
sion resistance even if the thickness of the principal
plating layer is small, and therefore, are useful for
cars, trucks and electric devices.
- 37 - 1 3 3 7 ~ 5 ~
V
0 ~ ~ o o o o o o o -, o
B . ~,~ o ~1
o _~ o o o U~ o
o .. _
V
U -
E ~ "~ o ~ ~D,~ ,,~
0 ~ ~1 ~ o ~ o _~ ~ ~ ~ ~ o
0 _
~V
0 t,q
t. ~ o æ o ,~, ", O O O O
n o o
~n 0 B~
gg oO og
' !~ ' ' ' ~ ~ '
C~ C
I ~.
0 g, S
g g Og t~
m _I c ~ c C ~ 'ZC Z ~ ~;
E
" B ~ ' ' ' ' ' ' ' '
_,
~o ~ o,, o ,, ~ U~ o~ U~
~
u '; 3 ';
o
-- 38 --
1 337~4
tVn ~- ~ ~ o o o o o o o o o
1 0 t.` O _1
V~ ,~ ., AU
~a ~ ,~
, z _I o o o In O t.~ O O
o ~
V
V U t~
tn-,l-- v
C~` E r~ `D t~
tD ~ ~ t,'~ t.~t _i t.`~ O o
- tn
.,
~V
o~ 0
~ o o o o o o o o o
V ~V ra ~ .a - u~ ~ o -~
r, o o
U~ ~ V ~ ~o
gg gg gg gg gg gg gg gg ~
a ~ C ~ C 2~ C ~ C ~ C ~ C ~ C C q~ ~ C
N ~4 N N 1~ N ~4 N 1~ N 1-~ N N 1~ ~ N
~ ~ O ~ U~ U~ g O ~ U~
t~
t _I N 1~ N ~ N N
_l .
d~ t~ t~ tJP tJO t~ tJP tP t~ t~ tlP tJO t~ t~ t~ t~ ~0 t~
' ' 9~ Q~ 0 ~ g ~ ~ m In 1~ o~ _~
a ~
N LS N 1~ N N 1~ N 1~ N Z N Z N ~; N ~3 Z
o
I
E
I U~ o o I O
~C Vc' j~
E ~ --
~;
U~ U~ o o o o o t`
,
Z ~ :
-
-- 39 --
1 337054
V ........... . ~
0 ~ ~ o o o o o o
~} ~y o .,~ o ,~"
~U , ~ o o o o o o
. V
.,~
" U t~
~n
E ~n 1~
' 2i' '~ ~
JJ
0 0
o o o o o o
~- ~ v ~ ~ tt~
r-l h tn O O ~U
t~ _ rD ttl
rO tO r.10 rP rP d~ rP r.'~ rO r.~O rP rJ~
h t~ t~l '~ ~ t~ ~~ ~O~ n ~ ~-t
"" ~ r~l L~ t.`~ ~ t.`~ ~ t.`~ C~ t.`Cl t~ 'Z i~
tl
h t 1.~
h . ; ~,
C
tt~ ~ r~l
r-~ .
h g ~ ,D ,J~ J ,''P '~ r~ tn g ~ g ~ U dO ,J
~ V~ ~ ~ ~ ~ ~ ~ ~ 0
C t.~ 3 CJ U ~ ~ U vl ~ u a ~ &~
- ~
n
, ~
~ I c
C .~; ~, I I I I I
~.
o o o o ~ o
_I
~ o~ o ,~ ~ ~7
cJ
I
-- 40 --
1 3370~
V - ~
o o o o o o o
~8 V ~
~0
Cl. ` ~
~I r Z O O O O O O O
O
C ~ V
~ U ro~
U~ ~ V
~l U S ~ ~ ~
'~ ~ O O O O O O O
_ ~ ~ r
_ V
~9 ~ O O O O O O O
V 0 V ~ ~1 0
O O
U~ V ~ ~P ~
OO ~0gO ggO gg OO OO
~ 0 ~ C ~ ~ C U~ ~ ~g C C~ C~
- , ~ 2 U.
~ aJ
L~ . V ' ~.
~, C ~ C ~; C ~ C
' I ~ ~ ~ ~ ~ ~ ~ ~ ~ a~ ~ $ ~, ~ ~ o o o o
0 0
- ~
v~ o o
C
a~ ~
o o ~ o o o
~ 0 ~ ~ ~
I~ Q O~ O
13 ;
S
1 337~54
V - ~
o~ ~ ~ o o o o o o
~} ~ o ~,~ o ,"
- .8 ,,
c~, .. 5, ~,
o o o o o o
O . _
. V
U t~~
' ~ U ~ E
a~ ~. -,~ ~ o o o o o o
~ o o o o o o
.~ a
01 0 0 ~
o ~o o o o ~ o o
C C~ ~
~L g o
I .1. r~ ~ I I I I I
t~ o o u~ u r~ ~o
~ o
--~ , as~ ~
o
g~ I Q 01
~8
. N~
E
~;
~ ~ o o:1 o o o
Q--I
_1 N ~ ~ ~r
r
- 42 - 1 3 3 7 ~ 5 4
Examples 47 to 53 and Comparative Examples 8 to 10
In Example 47, a cold steel strip having a thick-
ness of 0.6 mm was plated in an acid plating liquid
containing 43 g/l of zinc ions (zn2 ) 15 g/l of tri-
valent chromium ions (Cr3 ), 18 g/l of sodium ions,sulfate ions in an amount corresponding to the metal
ions, and 19 g/l of silica colloid at a pH of 2.0, a
temperature of 50C, and a current density of 150 A/dm2,
while flowing the plating liquid at a flow speed of
60 m/min.
The resultant principal plating layer had the
composition and the amount shown in Table 3.
In each of Examples 48 to 53 and Comparative
Examples 8 to 10, the same procedures as those described
lS in Example 47, except that the composition of the
plating liquid was modified so that the resultant
plating layer had the composition and the amount shown
in Table 3.
In Example 52, the principal plating layer was
coated with a surface plating layer having the composi-
tion and the amount shown in Table 3.
The resultant plated steel strip was subjected to
corrosion tests.
In the salt spray test, the corrosion resistance
was represented by a ratio (%) of an area of the speci-
men surface which was covered by red rust after salt
spray testing for 720 hours, to the entire area of the
specimen surface.
Also, a specimen was chemical conversion treated
with zinc phosphate and then coated with a cathodic ED
paint at a thickness of 20 ~m. The paint coated speci-
men was subjected to a cross-cut salt-spray test for 600
hours. The corrosion resistance of the paint-coated
specimen was represented by the maximum width of blis-
ters formed on the surface of the specimen.
Furthermore, the appearance of the cathodic EDpaint-coated steel strip was evaluated by a naked eye
-
~ 43 ~ ~ 337:054
test and the resultant evaluation was represented as
follows.
Excellent --- no craters found on the paint coating
layer
Good --- 10 or less paint coating layer craters
found per dm2
Bad --- more than 10 craters found per dm2.
The results are shown in Table 3.
-
-- 44 --
1 3370:~4
o 8 n t~
C n _ rn rn u~ rn rn rn ~n rn rn rn
v $ ~
~ 8 ~
v ~v" O vn ,a o o o o o o o ~ O ,o
U~ rn ~ O~P Z rr~
rn
5.1, ~ rD
v r~
,a ~
r _ ~_ I I I I I I I
rD
~ - ~ l l l l l z
~ v
J rJ~ D O ~n _1 o I o
O ~ ~ O
~D rDr,~ ~ ~1 ~ r.~ r~ o r~
r.~ ~ rD ~ ~ ~ rD O a~
L~ ~n o r~ D r~ 1~ r7 0 rn
r.~ o o ~n o ~n ~ r~ In
I~ rD ~ O ~ ~ ~ rD r~ O
n ~n m ~n _r
r ~ r
_ 45 - 1 337054
Examples 54 to 61
In each of Examples 54 to 61, the same steel strip
as that described in Example 47 was plated in an acid
plating liquid having the composition as indicated in
Table 4 and under the conditions indicated in Table 4.
The resultant plating layer had the composition as
indicated in Table 4, and the resultant plated steel
strip had the corrosion resistance indicated in Table 4.
~ 46 ~ 1 3 3 7 0 5 4
r. c
., ~o
o o o o o o o o
Ul-- h
~ ul O a~
.~,
~ O O O ~ O ~ ~ O
o ~ Z ~ v~
- ~a
rC = = : = : :
a) ~
o o o o o o o o
'3
. . C
O-- ~ _l _, ,~, _~ U~
n.
,C '~
.
~ .~ ~ u~ ~ g g 8 g u~
C~'
+ + + +~r +"~ ~ +, +,~
rc~
r-~ C ~ ; ~
~ o ~ ~o
o ~ ~
- ~x r
o o o ot`l o ot~`l o~ o
~ r~ r1 r~ ~
+ ~ I
In , U
1 ~3~
- 47 -
Examples 62 to 71 and Comparative Examples 11
and 12
In Example 65, the same steel strip as that men-
` tioned in Example 47 was plated in a s~lfuric acid
S plating liquid containing 56 g/l of zinc ions, 44 g/l of
trivalent chromium ions, 15 g/l of sodium ions, and
1 g/l of a polyethylene glycol (n = 20 to 60) at a pH of
2.0, a temperature of 50C, a flow speed of the plating
liquid of 60 m/min, and a current density of 100 A/dm2.
The resultant principal (base) plating layer had
the composition and the amount as shown in Table 5.
In each of Examples 62 to 64 and 66 to 71 and
Comparative Examples 11 and 12, the same procedures as
those described in Example 65 were carried out except
that the composition of the plating liquid was modified
so that the resultant plating layer had the composition
and the amount as indicated in Table 5.
In Example 71, the resultant principal plating
layer was coated with an additional surface) plating
layer having the composition and the amount shown in
Table 5.
The resultant plated steel strip was subjected to
the same corrosion tests as described in Examples 47
to 53, and the glossiness of the plated surface was
measured in accordance with JIS Z 8741. The results are
shown in Table 5.
1 337054
-
-- 48 --
_,
. _ dP
~: , , , , , , , , , o
_1 ~ In ~ ~ ~ ~ O
o U~ o ~ U~ o ~ o
O r7 ô , O O
... , o g
o o o o ~ o
,~ n ~ 1l 'i u
C ~ ~ ~ ~ _ _ .
'i ' 'i 'i 'i 'i o; 'i 'i
~ : , : ' o
-- ~ ~. 2 ~ ~ ~ 2
.`~ i ,,
'~
Z ~ I I I I I I I L~ 'Z _ j
o ~ ~ ~ O
~ I
1 337~5~
-- 49 --
"
~ 0
o U~ U~ s
o
., o
U
U ~ ~ O U~ ~ ~ ~ ~ ~r
C ~
_ I o
~ "
o ~
U~ o
~ ~D ~ O O O O O O ~ O ~D O
. u~ o ~ 1-- ~ r,~
u~ a~ O ~
o ~ A
~ ~ U~ ~D
rn
l ~-~
~ 8 ~
~ u ~
U ~
~ - a ~ ~
U~ ~
o o o o o o o o o o o o
o a~
U~
~ O ~ ~ ~ ~ ~ ~ o ~ ,~ ~1 C~
3 ~ ~ ~ ~ D ~ ~ ~
_ 50 _ 1 337054
Examples 72 to 80 and Comparative Examples 13 to 16
In each of Examples 72 to 80 and Comparative
Examples 13 to 16, the same steel strip as that men-
tioned in Example 47 was plated in a plating liquid
having the composition as indicated in Table 6 and under
the plating conditions indicated in Table 6.
The resultant principal (base) plating layer had an
amount of 20 g/m and the composition as shown in
Table 6.
The plated steel strips in Examples 72 to 80
exhibited a good degree of glossiness of 80 or more and
had an even silver white appearance.
The comparative plated steel strips of Comparative
Examples 13 and 16 had a milky white appearance, which
is similar to that of a zinc-plated steel strip. The
comparative plated steel strips of Comparative Exam-
ples 14 and 15 had an ~neven grey or black grey appear-
ance.
The plated steel strip was subjected to the salt
spray test for 720 hours.
In the plated steel strips of Examples 72 to 80, no
red rust was found on the surface thereof, but in the
comparative plated steel strips of Comparative Exam-
ples 13 and 16, red rust was formed within 24 hours of
the salt spray test. In the comparative plated steel
strips of Comparative Examples 14 and 15, red rust was
formed within 48 ho~rs and 360 hours of the salt spray
test, respectively.
- 51 - 1 337054
, ~, U ~ ~ " ~ o~~ , ~
1~1
O ~ ~ a~ 0 ~ cr- a~ U~ o
~ _
OOOOOOOOO OOOO
~-OOOOOOOOO OOOO
S I ~
~ . ~~_
,~ ~ O U~ ,~~ ~r, o 1~ o ~ u~ ~ ~
z ~ z +x ~ ~ z z z ~ ~
o
q : s : s s : : q : s
~ ~ ul o o - ~ o - ~
qo _ `D i
~o : ~ ;i
Il 11 _~ 11 U
-- c c c
s
L. L. ~ L Z
r~ ~ ~ ou~ o ~ ~ ~ ~ r~ a~ a~ ~ er
C ~1 _1 r~
- 52 - ~ 337~5~
Examples 81 to 85 and Comparative Examples 17 to 19
In each of Examples 81 to 85 and Comparative
Examples 17 to 19, the same steel strip as that de-
scribed in Example 47 was plated in an acid plating
liq~id having the composition indicated in Table 7 and
~nder the conditions indicated in Table 7.
The res~ltant principal plating layer had an amount
of 20 g/m and the composition as indicated in Table 7.
When s~bjected to the salt spray test for 720 ho~rs,
the plated steel strips of Examples 81 to 85 did not
r~st, b~t in the comparative plated steel strips of
Comparative Examples 17 to 19, red r~st formed within
48 ho~rs of the salt spray test.
53 _ 1 3 3 7 0 5 4
a~
,o ~ C,) _ ,
0 ~ ~
o o
O
~ ~ CJ oo o o o o o o
O~ ~ o ~In InIn ~ n u~ ul
.,~ ~
.~ c
r ~, ,~
..~ O
C ~i' ~
~ ~ ~ OU~ ~ 0 .,.
r
0~ 0 ~ ~,~
' ~ ++ +Z ~ Z Z Z
a ^ ~ a
_ _ c ~ n
nS ~ U~ U,
O +
OC~ ~
+ ~ ~D o o '`
~C ~ ~ ~ ~ ~ o
C~ _
C ~ ~ ~ ~ Ln O c~,
- S4 - 1 3 3 7 0 5 4
Examples 86 to 92 and Comparative Examples 20 to 23
In Example 86, the same cold rolled steel strip as
that described in Example 47 was electroplated in a
sulfate type plating liquid containing 56 g/l of zinc
ions, 44 g/l of trivalent chromi~m ions, 15 g/l of
sodi~m ions, and 1 g/1 of polyethyleneglycol having a
molecular weight of 1500, at a pH of 2.0, a temperature
of 50C, a flow speed of the plating liquid of 60 mtmin,
and a current density of 100 A/dm .
The resultant plating layer had the amount and the
composition indicated in Table 8.
In Each of Examples 87 to 92 and Comparative
Examples 20 to 23, the same plating proced~res as those
described in Example 86 were carried out except that the
composition of the plating liquid and the plating
conditions were modified so that the resultant plating
layer had the composition as indicated in Table 8.
The plated steel strips were subjected to a chromate
treatment of the type indicated in Table 8.
(a) The coating type chromate treatment was
carried o~t in such a manner that a chromate treating
liquid containing 50 g/l of chromic acid, which contains
40% of trivalent chromium (Cr3 ), and 100 g/l of SiO2
colloid, was coated on the surface of the plated steel
strip by an air-wipe method, and then dried at a temper-
ature of 100C for one minute. The amount of the coated
treating liq~id layer was controlled by controlling the
concentration of the treating liquid and by the air-wipe
operation.
~b) The reaction type chromate treatment was
carried out by coating the surface of the plated steel
strip with a treating liq~id containing 50 g/l of
chromic acid, 10 g/l of phosphoric acid, 0.5 g/l of NaF,
and 4 g/l of K2TiF6 by a roll coater, and by drying the
coated treating liquid layer at a temperat~re of 60C.
The amount of the coated treating liquid layer was
controlled by controlling the concentration of the
- 55 - 1 33 7a~ 4
treating liq~id and the roll-coating operation.
(c) The electrolysis type chromate treatment was
carried out by s~bjecting the plated steel strip to a
cathodic electrolysis treatment with a treating liquid
containing 30 g/l of chromic acid and 0.2 g/l of sulfuric
acid at a current density of 3 A/dm2, by washing with
water, and by drying. The amount of the chromate was
controlled by controlling the quantity of electricity
(Co~lomb) applied to the treating liquid.
The chromate-coated steel strips were coated with
the resinous materials as shown in Table 8. The resino~s
materials contained a r~st-preventing agent, ~or example,
SiO2 , hardening-promoting agent, catalyst, lubricant,
and water-wetting promoting agent. The coating operation
with the resinous material was carried out by using a
roll coater and the coated resinous material was cured
at a temperat~re of 140C to 170C for 10 seconds to 30
seconds.
The resin-coated steel strips were subjected to the
salt spray test in which a time (hours) in which red
rust formed on 2% of the surface area of specimen was
measured.
Also, the resin-coated steel strips were drawn with
a 10~ strain, and then subjected to the same salt spray
test as that mentioned above.
The res~lts are shown in Table 8.
-
- 56 - 1 3 3 7 0 5 4
. ~ '$
c U~ ~ 8 8 8 8 8 8 8 8 $ o o
0 C ~ o CO o o o~ o o
U ~ ~ C) O ~
a ~ ~ s~ u ~ 0
r~ c 0 ~ 8 8 8 8 8 8 8 8 8 8 8
0 0 1 ~ L1 0 0 0 0 0 0 0 0 0 0 0
o ~
,..
~ o U~ ~_ ~ ~ o o o o o o
-rl C U
q~ U ~ '
O r1
JJ ~ _
~J O O O O O O O O O O O
O' ~r~
~ ~
r
~ -
~ 2 ~ 2
,~
L
I Co'~ ~ I I I I I I I
2 z ~ z
:
~_ ~CCD ¢~ ~ ~ ~ O
L1
U r~
V
~, ~D ~ ~ ~ o ,~ ~ , o ,~
- 57 - 1 33~5 4
Examples 93 to 103 and Comparative Examples 24
to 28
In Example 94, a cold rolled steel strip having a
thickness of 0.7 mm was plated in a s~lfate type plating
liq~id containing 76 g/l of zinc ions, 31 g/l of tri-
valent chromi~m ions, 25 g/l of iron ions, 12 g/l of
sodi~m ions, and 1 g/l of a polyethyleneglycol having a
molec~lar weight of 1500, at a pH of 1.5, a temperature
of 50C, a flow speed of the plating liquid, and a
current density of 100 A/dm2. The resultant plating
layer had the composition and the amount as indicated in
Table 9.
In each of Examples 93 and 95 to 103 and Compara-
tive Examples 24 to 28, the same proced~res as those
described above were carried o~t except that the compo-
sition of the plating liq~id was modified so that the
resultant plating layer had the composition as shown in
Table 9.
In Examples 102 and 103, the plated steel strip was
further plated with an additional ~surface) plating
layer having the composition and the amount as shown in
Table 9.
The resultant plated steel strips were s~bjected to
the following tests.
a) Salt spray test
This test was carried out in accordance with
JIS Z 2371 for 720 hours. A ratio (%) of the rusted
area to the entire area of the specimen was determined.
b) Phosphate chemical conversion treatment
After an ordinary phosphate chemical conver-
sion treatment was applied to a specimen, the density of
the resultant phosphate crystals was observed.
c) Water-proof, paint adhesion test
A specimen was subjected to an immersion type
phosphate chemical convertion treatment in a ~s~al
manner, and then to a cathodic electrodeposition paint-
coating treatment to form a paint-coating layer having a
- S8 -
1 337054
thickness of 20 ~m. The paint coated specimen was
intermediate coated, water-polished, and upper coated to
provide a final coat having a total thickness of 80 ~m.
` The specimen was immersed in water at a temperature of
40C for 10 days, and thereafter, was cross-cut to form
100 squares (2 mm x 2 mm). An adhesive tape was adhered
to the cross-cut s~rface of the specimen and was peeled
from the surface. The number of peeled squares of the
coating was counted.
1- d) Corrosion test or paint-coated specimen
The phosphate chemical conversion-treated and
paint-coated specimen having a thickness of paint-
coating layer of 22 ~m was cross-cut in the same manner
as mentioned above, and was subjected to the salt spray
test for 840 hours. The maximum width of blisters
formed in the specimen was meas~red.
e) Appearance of paint coated specimen
A specimen was subjected to an ordinary
phosphate chemical conversion treatment and then to a
cathodic electrodeposition paint coating proced~re under
a voltage of 300 V. The appearance of the resultant
paint-coated specimen was observed, and the number of
craters formed on the specimen surface was measured.
f) Powdering property test
This test was carried out in such a manner
that an adhesive tape was adhered on a surface of a
specimen, and the specimen was folded so that the
adhesive tape was on the inside of the folded specimen.
Then the specimen was opened and the adhesive tape was
3~ peeled from the specimen. The maxim~m width of a
portion of the specimen on which powder of the plating
layer was adhered was measured.
The results are shown in Table 9.
-
- s9 -
1 337054
.
oooooooooooo ooo
-
C
... ~C ,,,,,,,,,oo, , .~.~
0 .
~ o 8 E~ O
o
- ~ û
~ 1- o U~ u~ ,rl In U'l U'l U'l 1-') 0 0 0 0 0 0 0
~q ~ 0 .1
a, ~ o 8 3,
0 WS,,
, .I .~
OOOOOOOOOOOO OIIO
w
. ~ -., 0
0
~ 2
.- o
o.
~0 ~ ~ ~
a~ 0~ ~ g
J V ~ dP 'J ~ . I .1 .-~
. ~ ~ 0 0 a
0~ 0
, . _
~ 0~ ~
.-- , I .
.
~D -
IIIIIIIIIO~I
IIIIIIIIIOO
OOOOOOOOOOOO OOOO
- ,~ -
.~. 0 _ ~ .t Ir~ O 11- 0 0 ~ `I O
,, , ~ t4 ~ ~ ~ Z Z
'~_ V ,n
C~ O .~ V In ~c> ~ o r~ o ~o ~ ~ u~
c~ a~ ~ ~ o. ~ o~ o~ o~ o o
.o , . - .
.
1 337054
- 60 -
Examples 104 to 112 and Comparative Examples 29
and 30
In Example 111, a cold rolled steel strip ha~ing a
thickness of 0.7 mm was electroplated in a sulfate type
plating liq~id containing 56 g/l of zinc ions, 44 g/l of
trivalent chromi~m ions, 15 g/l of sodi~m ions, and
1 g/l of a polyethylene glycol having a molec~lar weight
of 1500 at a pH of 2.0, a temperat~re of 50C, a flow
speed of the plating liq~id of 60 m/min, and a c~rrent
density of 100 A/dm . The resultant base plating layer
was plated with a s~rface plating layer having the
composition as indicated in Table 10~
In each of Examples 104 to 110 and 112 and Compara-
tive Examples 29 and 30, the same plating procedures as
those described above were carried o~t except the base
plating layer-forming proced~res and the surface plating
layer-forming proced~res were modified so that the
res~ltant base plating layer and the s~rface plating
layer had the compositions indicated in Table 10,
-- respectively.
The plated steel strips were subjected to the same
salt spray test, phosphate chemical conversion treat-
ment, and corrosion test for the paint-coated steel
strip as described in Example 93, with the following
exception.
In the corrosion test for the paint-coated speci-
men, the cross-c~t specimen was exposed to the o~tside
atmosphere. D~ring the expos~re, a 5% saline sol~tion
was sprayed on the specimen once a week. The expos~re
3Q was contin~ed for 10 weeks. Thereafter, a maxim~m width
of blisters formed in the specimen was meas~red.
The res~lts are shown in Table 10.
- 61 - 1 3 3 7 0 5 4
oo o o o o o o o o U~
0~ r -- ~ ~ F ~ . . . . .
~: a~ o ~
U ~ ~ o U U~
a
r ~
t ~ 1J - a -
~ ~ o o o o o O o o o o o
tn ~ $ dP --' ~
h
O~J OU~ U~
tl5 0 Il~ ~ _I ~ t~
~ O
~ 8
o
O ~ O O U~ o o u~ o
o ~ ~ ~o o r o o ~o o
E o o o o o o ~n o o o o
,~ _
i~ r~
,~ -- ' b~ ' ' ' .
C~ I ~ U
a) u
u~ ~ I~ o ~ ~ O
m ~
~7 o ~o ,n ~ In ~r ~ o o a~
~, .
a~ '
B~ . ~ ~ u~ ~D ~ 00 al o ~ o
~ o ~ o o o o o o
3 ~ ~ ~
62 1 337054
Examples 113 to 119 and Comparative Examples 31
to 35
In Example 113, the same cold rolled steel strip as
that mentioned in Example 111 was plated in a s~lfate
type plating liq~id containing 56 g/l of zinc ions,
44 g/l of trivalent chromi~m ions, 15 g/l of sodium
ions, and 1 g/l of polyethyleneglycol having a molec~lar
weight of 1500, at a pH of 2.0, a temperat~re of 50C, a
flow speed of the plating liq~id of 60 m/min, and a
current density of 100 A/dm .
The plated steel strip was s~bjected to a reaction
type chromate treatment to form a chromate layer in an
amo~nt of 50 mg/m .
In each of Examples 114 to 119 and Comparative
Examples 31 to 35, the same proced~res as those men-
tioned above were carried o~t except that the composi-
tion of the plating liq~id and the plating conditions
were modified so that the resultant plating layer had
the composition as indicated in Table 11, and the
chromate treatment was carried o~t- as shown in Table 11.
a) Coating type chromate treatment
Same as that described in Examples 86 to 92.
b) Reaction type chromate treatment
Same as that described in Examples 86 to 92.
c) Electrolysis type chromate treatment
Same as that described in Examples 86 to 92,
except that the treating liq~id contained 50 g/l of
chromic acid, 0.4 g/l of s~lf~ric acid, 20 g/l of
phosphoric acid, and 11 g/l of zinc carbonate.
The res~ltant chromate-coated steel strips were
s~bjected to the following corrosion tests.
a) Salt spray test for chromate-coated specimen
The corrosion resistance was represented by a
time in which 2% of the s~rface area of the specimen was5 covered with red r~st.
b) Salt spray test for stretched specimen
The same test as mentioned above was applied
-
- 63 - 1 3 3 7 5 4
to a chromate-coated specimen, which was stretched at a
10% strain.
The res~lts are shown in Table 11.
-
- 54 - 1 3 3 7 5 4
C ~ _ o o o o o o o o o o o o
0 0 ~ ,~ O O r r~ O ~ O 0~ U~ U. ~l U~
-- ~ ~ ~ ~ ~ ~ A
0 - ~D
u~ ~ n ~
o o o o o o o o o o o o
0~ C ~ ~ ~ o o o o o o o o o o o o
u, o ~ o~ ~ 0~
~ A ~ A ~ A A A
r ~
O O O O O O O O O O O O
3 ~ ~ ~ ~ ~ ~ ~
~ ~ a~ ~ 0 ~ a 0
0
r ~ rc ~ r ~ r-- r
r-l ~ ~ ~\ t ~ t Y 5
~ c ~ r ~ r
g ~~ 2 .~ ~ 2 ~ ~ ~ ,~ ~ ~ ~
r
Z ~ ~Z
r~
~ ~ ~ ~ u~ o o
el C, t~ a~ tl~ tO a~ t~ t~ O O
r-l r--I
c~ r-l
al ~ r1
r~ r~ L~ ~ ~ o 1~ oo a~ d ~ ,-1 t,~l t~
r~l r-l r I rr~ r-l r 1 r-l ~ r t ~ t~
1 337054
- 65 -
Examples 120 to 128 and Comparative Examples 36
and 37
In each of Examples 120 to 128 and Comparative
Examples 36 and 37, the same cold rolled steel strip as
that described in Example 111 was plated in a s~lfate
tape plating liq~id having the composition, and ~nder
the conditions, indicated in Table 12. In Comparative
Example 27, a ~s~al zinc plating layer was formed on the
steel strip.
The res~ltant principal plating layers exhibited
the X-ray diffraction patterns shown in Figs. 1 to 5.
The X-ray diffraction patterns were determined by a
specimen-rotating method using a C~ target ~nder 45 kV
at 150 mA, and at scanning speed of 2 deg./min.
Also, the res~ltant principal plating layers had
the composition and the amo~nt shown in Table 13 and the
X-ray diffraction patterns had peaks at the locations as
indicated in Table 13.
In Examples 125 to 127, the principal plating
layers were coated with additional (s~rface) plating
layers having the compositions shown in Table 13.
The plated steel strip was s~bjected to the corro-
sion tests.
Referring to Table 13, the salt spray test was
carried o~t in accordance with JIS Z 2371 for 720 hours,
and the res~lt is represented by a ratio (%) of red
r~sted area to the entire area of the specimen s~rface.
The cyclic corrosion test was carried o~t by wetting a
specimen at a temperature of 50C and a relative h~midi-
ty of 85% for 16 ho~rs, ~y drying the specimen at 70C
for 3 ho~rs, by immersing the specimen in a 5% salt
sol~tion of 50C for 2 ho~rs, by leaving the specimen at
room temperat~re in the ambient atmosphere, and by salt
spraying at 50C in accordance JIS Z 2371 for one ho~r.
The above-mentioned operations more repeated for 672
ho~rs. The res~lt was represented by a maxim~m depth of
pits formed in the specimen.
- 66 - 1~70~4
The corrosion test for paint-coated specimen was
carried o~t in the following manner. A specimen was
s~bjected to an immersion type phosphate chemical
conversion treatment and then to a cathodic electro-
deposition paint coating to form a paint coating layerhaving a thickness of 20 ~m. The coated specimen was
cross-cut and the s~bjected to the same salt spray test
as mentioned above, and to a cyclic corrosion test in
which a cyclic treatment comprising salt spraying at
50C for 17 ho~rs in accordance with JIS 2371, drying at
70C for 3 ho~rs, salt spraying a 5% NaCl sol~tion at
50C for 2 ho~rs, and leaving in ambient atmosphere for
2 ho~rs, was repeated for 2016 ho~rs, and the res~lt is
represented by a maxim~m depth of pits formed in the
specimen.
The plated steel strips and the paint-coated steel
strip of Examples 120 to 127 in which the res~ltant
zinc-chromi~m alloy plating layers did not have the
n-phase exhibited a higher corrosion resistance than
that of Example 128 in which the res~ltant zinc-chromi~m
alloy plating layer had the n-phase.
~ 337054
-- 67 --
S~
~ o o o o o o
,C E~ ~
- t 1 ~ ~ r`~D el' n o
.,, .~ ~
~5 ~
_
~,a ,¢ ~ ~ o 0~
r~ ~ o o o
_i ~ ~ r~ r~
,~ -" ~ Q ~
, l o
~ - ~
z o~
U+ r~l
N O O N N U~Il') '
N ~0 0 Cl~ N N N N
Z;
O
_1 ~ _J ~ ~ ~r x
-- ~ 33~054
-- 68 --
.
E~0 ~ ~ ~o o ~D ~o o ~ o
o o o ' o o o o o o o o
o o o u~
~3 a)
o u~ o t~ o o u~
o o o o o o o o o o o
U~ o o o o o o o o o o o
CL JJ Ul ~ o
E I ~ ~
., ~ ~
- ~ o C ~ I I I I I o o U~ I I I
oP I I I I I o U~
o r~ _ _ _
o ~:
C C C
o ~
E ~ ~o o o o o o o o o o
O ~ --I ~ ~ ~ o
C _ ~ ~ U~ ~ ~ ~ U~ ~_I ~ o
z ~ ~ :
z : : : : : : ~
1 33705~
- 69 -
Examples 129 to 134 and Comparative Example 38
In each of Examples 129 and 134 and Comparative
Example 38, the same cold rolled steel strip was plated
in a sulfate or chlorine type plating liquid having the
composition, and under the plating conditions, indicated
in Table 14.
The resultant plating layers of Examples 129 to 133
did not have the n phase, but the resultant plating
layers of Example 134 and Comparative Example 38 did
have the n phase.
The plated steel strips were subjected to the same
cyclic corrosion test described in Examples 120 to 129.
The results are shown in Table 14.
1 337~54
-- 70 --
C ~ ~ . -~ o o o
N ~~ ,~ ~, ~ U $
o,~
o ~ ~ O O ,~, ~ O
o C
$ .~S ~ o
o ~ Z
'- O ~~ dP 0~ 1~ ~o ~ _I
C dO O ~
~ ~ CJ o o o o o o o
Z~ O o ~ O O O
~ ~~ _
O O O o O o
C _~ I ul o~~ I,n
~ I + ~ +~ +z Z
o ~
~ o ~ a
~ r ~ : : 5 ~
r~ O
O ~ ~ O O O U~
r~ r '~;~
+ -1 I ' ;~ Z Z
rl ~ GO a~~ U~ ~D m U7
n. ('~
~ O
-
- 71 - 1~37054
Example 135
The same cold rolled steel strip as that described
in Example 111 was contin~o~sly plated in a sulfate type
plating liq~id comprising 107 g/l of zinc ions, 40 g/l
of trivalent chromium ions, 14 g/l of sodium ions,
anions consisting of s~lfate ions, and 2 g/l of poly-
ethylene glycol having a molecular weight of 1500 at a
pH of 1.3, a c~rrent density of 150 A/dm , a flow rate
of the plating liquid of 60 m/min, and a temperature of
1~ 50C by using an anode consisting of an insoluble
Pb-4%Sn electrode, until the total q~antity of electric-
ity applied to the plating proced~re reached
10,000 Co~lomb/l. The res~ltant plating layer comprised
15% by weight of chromi~m and 85~ by weight of zinc.
After the 10,000 Co~lomb/l loading, it was found that
the concentration of hexavalent chromi~m ions (Cr6+) was
increased to 0.57 g/l.
The plating liq~id was mixed with 1.8 g of metallic
zinc powder per liter of the plating liquid and with an
aq~eous CrO3 solution corresponding to 0.3 g/l of Cr per
liter of the plating liq~id, and the mixt~re was stirred
at a temperature of 50C until a uniform plating liq~id
was obtained. The resultant refreshed plating liquid
contained zinc ions and trivalent chromium ions at a
similar content to that in the original plating liquid.
The content of Cr6 in the refreshed plating liquid was
0.1 g/l or less.
The refreshed plating liquid was ~sed for the same
continuous plating procedure as that mentioned above at
lO,O00 Coulomb/l.
The above-mentioned cyclic process consisting of
the continuo~s plating proced~re and the refreshing
proced~res for the ~sed plating liquid was repeated 6
times, until the load applied to the plating liq~id
reached 60,000 Coulombs/l.
After the above-mentioned contin~ous plating
procedures were completed, all the res~ltant plating
~ 72 _ 1 3~ ~054
layers were composed of about 15% by weight of chromi~m
and about 85% by weight of zinc, and had a good appear-
ance.
After each refreshing proced~re, the contents of
zn2 and Cr3 in the refreshed plating liquid were
substantially the same as those of the original plating
liquid and the content of Cr6 was 0.1 g/l or less.
Example 136
The same plating and refreshing procedures as those
described in Example 135 were carried o~t, with the
following exception.
The original sulfate type plating liquid comprised
84 g/l of zinc ions, 49 g/l of trivalent chromium ions,
14 g/l of sodium ions, 2 g/l of a polyethylene glycol
having a molecular weight of 1500 and anions consisting
of sulfate ions, and had a pH of 1.2. The current
density was 100 A/dm2. A Pt anode was used.
After the lO,000 Coulomb/l load plating procedure,
the resultant plating layer was composed of 15% by
weight of chromium and 85% by weight of zinc, and the
used plating liquid contained 0.1 g/l or less of Cr6 .
In the refreshing proced~re, an aqueo~s chromium
chromate solution in an amount corresponding to 0.3 g/l
of Cr was used in place of CrO3. The aqueous chromi~m
chromate solution was prepared by adding starch to an
aq~eous anhydrous chromic acid sol~tion to reduce a
portion of the anhydrous chromic acid and contained 30%
of Cr3 and 70% of Cr6 based on the total amount of
chromi~m.
Each of the res~ltant refreshed plating liquids
contained zinc ions and trivalent chromium ions in the
same contents as those of original plating liquid and
O.l g/l or less of Cr6+ ions.
Example 137
3S The same plating and refreshing procedures as those
described in Example 135 were carried o~t with the
following exception.
1 337~54
- 73 -
The original plating liq~id comprised 84 g/l of
zinc ions, 49 g/l of trivalent chromium ions, 14 g/l of
sodi~m ions, anions consisting of s~lfate ions, 2 g/l of
a polyethyleneglycol having a molec~lar weight of 1500
and had a pH of 1.2. The anode consisted of a Pb-1%Ag
electrode.
After 10,000 Co~lomb/l load plating procedure, the
used plating liq~id contained 0.76 gtl of Cr6 and
14 ppm of pb, and the res~ltant plating layer was
composed of 15~ by weight of chromi~m and 85% by weight
Of zinc.
The CrO3 sol~tion was replaced by an aq~eo~s
chromi~m s~lfate sol~tion in an amo~nt corresponding to
O.3 g/l of chromi~m. In the refreshing proced~res,
1.6 g of SrCO3 per Q of the plating liquid were f~rther
added to and dissolved in the plating liq~id.
Each refreshed plating liq~id contained zinc and
trivalent chromi~m ions in the same contents as those in
the original plating liq~id and 0.1 g/l or less Of c6
ions and 1 ppm or less of Pb.
Example 138
Referring to Fig. 7, the dissolving vessel 7 having
a diameter of 500 mm was charged with 330 kg of metallic
zinc grains having a size of 2 mm to form a metallic
zinc grain layer having a height of about 300 mm. The
metallic zinc grain layer was pressed between the bottom
and ~pper perforated plates 14 and 15.
A feed sol~tion comprising 80 g/l of zinc ions,
40 g/l of trivalent chromium ions, 14 g/l of sodi~m
30 ions, O. 2 g/l~ in terms of Cr6 , of chromic acid,
1.5 g/l of a polyethylene glycol having a molecular
weight of 1500 and anions consisting of s~lfate ions and
having a pH of 1.0, was fed from a plating vessel (not
shown in Fig. 7) to the dissolving vessel 7 thro~gh the
cond~it 16 and passed thro~gh the metallic zinc grain
layer. The res~ltant refreshed plating liq~id was
ret~rned to the plating vessel.
_ 74 - 1 3 3 7 0 5 4
The above-mentioned proced~res were continued for
one ho~r. It was found that 36 kg of metallic zinc were
dissolved in the plating liq~id to reduce Cr6 ions into
Cr3 ions. The content of Cr6 in the plating liquid at
the o~tlet 17 was 0.1 g/l or less. That is, abo~t 90%
of the dissolved metallic zinc contributed to the
reduction of the Cr6 ions.
Examples 139 to 142 and Comparative Example 39
In each of Examples 139 to 142 and Comparative
Example 39, a cold rolled steel strip was continuously
plated in a plating liquid having the composition, and
under the plating condition, as indicated in Table 15,
until the total load reached 10,000 Coulomb/l. After
completion of the contin~o~s plating proced~re, it was
found the used plating liquid in Examples 139 to 142
contained a small amo~nt of hexavalent chromium ions as
shown in Table 15, whereas the used plating liquid in
Comparative Example 19 contained a relatively large
amount lO.~5 g/l) of hexavalent chromium ions.
That is, the organic red~cing agent and bromine
ions contained in the plating liquid were effective for
restricting the generation of the hexavalent chromium
ions.
1 337054
-- 75 --
~ .t t --
:: g!,
C oo u ~ u~ CD
. . S~
O -
O t . . ~
O ~ '~ _
.t O
V~ ; ' t _ o ~ '~
t O O O O
~ ~_ o
0~
N
D _
r ~ ~ O ,~, ~ ~ ~ ~
~' O 'Ut~ r
~' ~ tl,.t~
.~ . ', _
. ~
" t O o O o
S . ''~
O
g . ~ : :
~ a~ a~
t ~_1
.~ ' _
~ ~ a.1 .t It~
~0 ~ CD ~r o
3 -~ a r~
~ ~ o ~ a~
+
~ o ~
r
z ~ r r r
- 76 - 1 3 3 7 0 5 4
Example 143
The ~sed plating liq~id in Example 140 was mixed
with a chromic acid aq~eo~s sol~tion in an amo~nt
` corresponding to 0.3 g/l of chromi~m and 0.9 g/l of
formic acid and the mixt~re was heated at a temperature
of 70C to red~ce the hexavalent chromium. The res~l-
tant plating solution contained 0.1 g/l or less of
hexavalent chromium.
The plating solution was f~rther mixed with zinc
carbonate (ZnCO3) in an amount corresponding to 1.8 g/l
of zinc and the amount of the plating solution was
controlled so that the resultant refreshed plating
liq~id contained zinc ions and trivalent chromi~m ions
in the same contents as those in the original plating
liq~id.
The above-mentioned plating and refreshing proce-
d~res were repeated 6 times until the total load applied
to the plating liq~id reached 60,000 Coulomb/l.
All of the plated steel strip had a zinc-chromium
alloy plating layer composed of 15% by weight of chromi~m
and 85% by weight of zinc. Also, all of the refreshing
plating liquid contained zinc ions and trivalent chromium
ions in the same contents as those in the original
plating liq~id and 0.1 g/l or less of hexavalent
chromium.