Note: Descriptions are shown in the official language in which they were submitted.
1 337322
DESCRIPTION
HOT-DIP ZINC-ALUMINUM ALLOY COATED STEEL SHEET
FOR PREPAINTED STEEL SHEET, PROCESS FOR
PRODUCING THE SAME AND PREPAINTED STEEL SHEET
Technical Field:
The present invention relates to prepainted
steel sheets having excellent properties suitable for
use as construction materials such as roof materials
and wall materials as well as household electric
appliances, hot-dip zinc-aluminum alloy coated steel
sheets having properties superior to those of conventional
ones and suitable for use as the sheets of the prepainted
sheets and a process for producing them.
Background Art:
Steel sheets coated with zinc (Zn) or prepainted
steel sheets produced therefrom have been used in
construction materials or household electric appliances.
Further steel sheets coated with zinc-aluminum (Zn-Al)
alloy attract attention as materials taking the place
of Zn coated steel sheets, since they have corrosion
resistance, etc. superior to those of the Zn coated ones.
Various processes have been proposed heretofore
for producing the Zn-Al alloy coated steel sheets. These
processes include one comprising the use of a coating
bath comprising 5 to 25 wt.% of Al, 0.1 wt.% or less
I
2 1 337322
of Pb and the balance of Zn (see Japanese Patent
Publication No. 25220/1976); one comprising the use of
a coating bath comprising more than 3.5 wt.% but not
more than 10 wt.% of Al, and Mg, Be, Ti and Cu each in
a specified range of a small amount relative to the
concentration of Pb, Sn or both of them in the coating
bath (see Japanese Patent Publication No. 47055/1978);
one comprising the use of a coating bath comprising 3
to lS wt.% of Al, about 85 to 97 wt.% of Zn and small
amounts of rare earth elements (see Japanese Patent
Publication No. 500475/1982); and one comprising the
use of a coating bath comprising 0.05 to 2.0 wt.% of
Al, 0.01 to 0.1 wt.% of Mn and the balance of Zn and
unavoidable impurities (see Japanese Patent Publication
No. 32700/1985).
A principal object of these processes is to
reduce the weight loss of the coated steel sheets due
to corrosion by the addition of Al.
The loss due to corrosion of the coated steel
sheets in exposure tests in outdoor is usually reduced
as the Al concentration in the coating bath is increased
and, therefore, the corrosion resistance of the sheets
is improved. However, an alloy layer formed on the
interface with iron becomes thicker and the adhesion
and the workability of the coating layer are seriously
reduced as the Al concentration is increased.
Methods have been proposed to solve these
problems by adding small amounts of various elements.
3 1 337322
However, they have problems that the use of the limited
kinds of elements in limited amounts is troublesome,
that the coating pot must be exchanged in order to switch
over the coating bath and that the appearance of the
coated steel sheet surface is impaired. Under these
circumstances, the development of a process for producing
coated steel sheets which can satisfy the required
qualities with as little as possible elements added is
demanded.
The following properties are required of Zn-Al
alloy coated steel sheets, particularly those to be used
as sheets for prepainted steel sheets from the viewpoint
of the use of them:
~1) the corrosion resistance of the steel sheet surface
lS improved,
(2) a self-sacrificing rust-preventive power of Zn for
that part of iron which is exposed when the steel sheet
is cut is retained,
(3) no crack is formed in a bent part of the steel sheet
in the working step,
(4) the coating layer has an excellent adhesion which
is not reduced with time, and
(5) the surface smoothness is excellent.
The present applicant proposed a coated steel
sheet for prepainted galvanized steel sheets which is
produced by coating a steel sheet with hot dipping bath
comprising 0.3 to 3.5 wt.% of Al and the balance of Zn
and unavoidable impurities (see Japanese Patent
4 1 337322
Application No. 159469/1983).
It is apparent from the phase diagram of Zn-Al
alloy that the eutectic point is realized when the alloy
comprises 5 wt.% of Al (9S wt.% of Zn). When the Al
content is deviated to some extent from 5 wt.%, the
texture of the solidified alloy is quite different from
that of the 5 wt.% Al alloy unless it is quenched at
a very high speed. The Al-Zn alloy having 5 wt.% Al
content is eutectic and, therefore, its melting point
is low and Al and Zn are dispersed homogeneously
irrespective of the cooling rate. However, when the
Al content is less than 5 wt.%, for example, 1 wt.%,
a primary crystal of Zn containing only very small amount
of the Al component is formed in the coating layer and
the majority of the Al component remains in the finally
solidified grain boundary. Thus no coating layer having
homogeneous composition is formed. It has been believed
that an Al-Zn alloy having an Al content of 5 wt.% is
advantageous for homogeneously dispersing Al and Zn and
for giving a stable texture.
Disclosure of Invention:
After investigations of an Al-Zn alloy having
an Al content of 0.3 to 3.5 wt.% which is less than the
above-described value, 5 wt.%, the inventors have
completed the present invention. According to the present
invention, the qualities and properties required of Zn-Al
alloy coated steel sheets are satisfied or further
1 337322
improved.
Brief Description of Drawings:
Figs. l(a) to (c) are microphotographs of the
metal textures on the surfaces of the coating layers
formed in Example 5 of the present invention according
to Claim 4 and Comparative Example. They are X-ray images
of Al on the surface obtained with EPMA. Figs. l(a),
l(b) and l(c) are microphotographs of the metal textures
of the coating layer surfaces obtained when (steel sheet
temperature at dipping time) - (bath temperature) was
20C, -20C and -80C, respectively.
Figs. 2(a) and (b) show the concentration
distribution of Fe, Zn and Al in the thickness direction
of the hot-dip zinc-aluminum alloy coated steel sheets
produced in Example 5 of the present invention according
to Claim 4 and Comparative Example. They show the
concentration distributions obtained when (steel sheet
temperature at dipping time) - (bath temperature) was
20C and -80C, respectively.
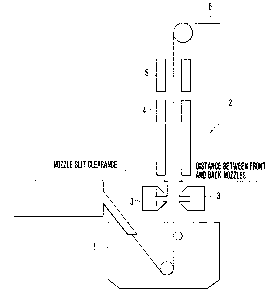
Fig. 3 is a schematic drawing of the hot dipping
equipment used in Example 6 of the present invention.
Figs. 4(a) to (c) are microphotographs of the
appearances of the metal textures of the coating layer
surface formed in Example 7 of the present invention
according to Claim 6 and Comparative Example. Fig. 4(a)
is a microphotograph of the metal texture of the coating
layer surface formed under conditions comprising (steel
6 1 337322
sheet temperature at dipping time) - (bath temperature)
of -60C, a nozzle slit clearance of 0.8 mm, an ejecting
pressure of gas of 1.0 kg/cm2 and a distance between
front and back nozzles of 50 mm. Fig. 4(b) is one formed
under conditions comprising (steel sheet temperature
at dipping time) - (bath temperature) of -20C, a nozzle
slit clearance of 0.6 mm, an ejecting pressure of gas
of 1.5 kg/cm and a distance between front and back
nozzles of 20 mm. Fig. 4(c) is one formed under
conditions comprising (temperature of steel sheet at
dipping time) - (bath temperature) of -80C, a nozzle
slit clearance of 1.2 mm, an ejecting pressure of gas
of 0.1 kg/cm and a distance between front and back
nozzles of 20 mm.
Figs. 5(a) and (b) show the thickness
distributions of the coating layers, wherein Fig. 5(a)
shows that of the coating layer having a surface not
reheated (Comparative Example) and Fig. 5(b) shows that
of the deposit coating layer having a surface reheated
at 460C (Example 8 of the present invention according
to Claim 7).
Figs. 6(a) to (c) are microphotographs of the
metal textures of the coating layer surfaces obtained
in Example 9 according to Claim 8 of the present invention
and Comparative Example. They are X-ray images of Al
on the surface obtained with EPMA. Figs. 6(a), 6(b)
and 6(c) are microphotographs of the metal textures of
the coating layer surfaces obtained when the cooling
7 1 337322
speed was 2C/sec, 17C/sec and 47C/sec, respectively.
Best Mode for Carrying Out the Invention:
The present invention described in Claims 9
and 10 relates to a prepainted steel sheet produced from
the above-described Zn-Al alloy coated steel sheet.
The prepainted steel sheets are those previously
painted with a paint. They are produced continuously
on a large scale by painting galvanized steel sheets
or zinc alloy coated steel sheets with a roll coater
usually after chemical conversion treatment. The demand
for them as starting materials for construction materials,
household electric appliances, business machines, etc.
is now increasing, since they have excellent, uniform
qualities, they can be supplied in large amounts and
no painting is necessary after application.
The properties required of the prepainted steel
sheets are mainly adhesion, corrosion resistance,
workability, weather resistance and scratch resistance.
However, it is quite difficult to satisfy all o the
required properties with only one paint.
Thus it was a usual practice to classify paints
into two groups in the prior art, i.e. those for
under-painting which could impart adhesion and corrosion
resistance and paints for toppainting which could impart
workability, weather resistance and scratch resistance
to further improve the qualities and properties of the
prepainted steel sheets. These paints were applied by
8 1 337322
the so-called two-coat two-bake process wherein the sheets
were baked after application of the under-painting paint
and baked again after application of the toppainting
paint in order to improve the qualities of the prepainted
steel sheets.
However, recently, durability over a period
of as long as, for example, 10 or 20 years is demanded
of the construction materials such as roof and wall
materials in various cases.
The term "durability" as used herein means
weather resistance and corrosion resistance. Namely,
an excellent durability means that the color tone and
gloss are substantially unchanged and no rust is formed
in 10 or 20 years.
The excellent weather resistance can be given
by using a paint having excellent properties against
chalking and fading.
However, it is difficult to inhibit the rust
formation over a long period of time.
The formation of red rust is often observed
in a portion of roof or wall material worked by roll
forming machine only several years after the construction,
though the timing of the rust formation varies depending
on the environmental conditions.
Such rust formation occurs even when the
material is exposed to the outdoor for only a short period
of time, since cracks are formed in the worked part
already prior to the use and in an extreme case, the
- ~ 337322
steel texture is exposed through the cracks formed in
the coating layer.
To solve this problem, it is necessary to
cover the coating layer with a painting film having
such an excellent elongation that it can withstand
the deformation in the course of working and to use a
steel sheet having a coating layer having such an
excellent workability that it is not cracked in the
course of working.
The former requirement was satisfied by
producing prepainted steel sheets or metal sheets
having excellent corrosion resistance after intensive
investigations. The inventors made investigations for
the purpose of satisfying the latter requirement.
After intensive investigations made for the
purpose of satisfying the latter requirement, the
inventors have succeeded in the production of Zn-Al
alloy coated steel sheets having excellent proper-
ties. After further investigations of prepainted
steel sheets, the inventors have found that two-coat
steel sheets having a performance equivalent to that
of three-coat steel sheets can be produced with the
Zn-Al alloy coated steel sheets of the invention are
used.
According to one aspect of the invention,
there is thus provided a process for the preparation
of a hot-dip zinc-aluminum alloy coated steel sheet
suitable for a prepainted steel sheet. The process of
the invention comprises the steps o~ coating a steel
sheet in a hot dipping bath comprising 0.3 to
3.5 wt.% of Al, 100 ppm or less of Pb and the balance
of Zn and unavoidable impurities, wherein the
temperature of the steel sheet dipped in the hot
B
... .
1 337322
dipping bath is lower than that of the hot dipping
bath, taking the steel sheet out of the hot dipping
bath and reheating the coated steel sheet to a
temperature above the melting temperature of the
coating layer.
The present invention also provides, in
another aspect thereof, a hot-dip zinc-aluminum alloy
coated steel sheet produced by a process as defined
above.
Thus, according to the invention, a hot-dip
zinc-aluminum alloy coated steel sheet for a
prepainted steel sheet is produced by coating a steel
sheet in a hot dipping bath comprising 0.3 to 3.5
wt.~ of Al, 100 ppm or less of Pb and the balance of
Zn and unavoidable impurities.
The Al concentration is thus limited
because when it exceeds 3.5 wt.%, the self-sacri-
ficing anticorrosive effect of Zn on iron is reduced
while when it is insufficient, the effect of
improving the corrosion resistance at the surface of
the coating layer is unsatisfactory. With at least
0.3 wt.% Al concentration, the workability is also
improved and this effect is remarkable when the Al
concentration is 0.5 wt.% or higher.
The Pb concentration is limited because
when it exceeds 100 ppm, the adhesion is reduced with
time due to intercrystalline corrosion and con-
sequently the corrosion resistance which is
particularly important for the prepainted steel sheet
is damaged. With 100 ppm or less of Pb, stable
adhesion which is not reduced with time can be
obtained. With an Al concentration of 0.3 to 3.5 wt.%
and a Pb concentration of 100 ppm or less, a zinc-
1 337322
aluminum alloy coated steel sheet which does notcause reduction in adhesion with time, has an
excellent workability and is quite suitable for use
as the sheet for a prepainted steel sheet can be
obtained.
The temperature of the hot dipping bath may
be one at which Zn and Al are molten to form a
homogeneous melt, such as about 430 to 480C.
Although a hot-dip zinc-aluminum alloy
coated steel sheet having excellent workability and
adhesion after aging can be obtained when the Al
concentration is in the range of 0.3 to 3.5 wt.% and
the Pb concentration is 100 ppm or less, it is
preferred to add 1/100 to 1 part, per part of Al and
Si. The addition of Si serves to inhibit the
formation of the alloy layer at the interface between
the steel sheet and the coating layer to thereby make
the formation of a thin alloy layer possible. Thus, a
zinc-aluminum alloy coated steel sheet having further
improved workability and adhesion after aging can be
obtained.
The Si concentration is limited because
when it is as low as about 1/200 of the Al
concentration, no improvement in the workability or
adhesion can be obtained. Another reason is that the
control of the addition of Si in an amount of as
small as 1/200 of Al is difficult, since the Al
concentration is as low as 0.3 wt.% or less. The
lower limit of the Si concentration is thus 1/100 of
the Al concentration.
Preferably, the hot dipping bath contains
0.01 to 1.5 wt.% of Mg, Mn or Cu. When metallic
elements effective in improving the corrosion
.,
B
1 337322
resistance of the galvanized steel sheet, such as Mg,
Mn or Cu, are added to the bath, the effects of the
present invention, i.e. satisfactory workability,
corrosion resistance and adhesion after aging are
further improved. With 0.01 wt.% or more of the
metallic elements, the effects can be obtained. The
addition of the metallic elements in an amount larger
than 1.5 wt.% is not preferred from the viewpoint of
the cost or efficiency of working. Mg, Mn and Cu may
be used alone or in combination. By adjusting the
amount of Pb to 100 ppm or less, a stable adhesion
after aging can be attained.
The temperature of the steel sheet dipped
in the hot dipping bath at the dipping time is
adjusted to a temperature lower than that of the
bath. Preferably, the temperature of the steel sheet
to be dipped in the hot-dip Zn-Al alloy coating bath
is adjusted to a temperature lower than the bath
temperature by at most 80C, particularly by 10 to
60C. By this process, a hot-dip zinc-aluminum alloy
coated steel sheet having an excellent surface
corrosion resistance and a high power of protecting
the edge of the steel from rust is obtained. Also,
the corrosion resistance in a bent part is improved.
Usually in the production of a hot-dip
coated steel sheet in hot dipping equipment provided
with a continuous annealing furnace, the temperature
of the steel sheet to be dipped in a hot dipping bath
is kept higher than a temperature of the bath from
the viewpoints of the adhesion and the heating effect
of the bath. Since the steel sheet is thicker than
the coating layer and the temperature of the steel
sheet is high, the cooling of the coating layer
B
1 337322
starts with its surface and the interface of the
coating layer with the steel sheet is solidified
layer. Consequently, the Al concentration is high in
the interface and the thickness of the alloy layer is
increased to thereby reduce the workability and the
self-sacrificing anticorrosive effect on the steel,
while the Al concentration on the coating layer
surface is low to reduce the corrosion resistance.
The temperature of the steel sheet dipped
in the hot dipping bath is kept below the bath
temperature contrary to the conventional processes in
order to initiate the cooling of the coating metal on
the side facing the steel sheet. By this process, the
inventors have succeeded in reducing the Al concen-
tration on the interface side and reducing the amount
of the alloy layer formed. In this process, the self-
sacrificing anticorrosive power (resistance to the
initial red rust formation) of Zn is retained.
Further, since the alloy layer formed is thin, no
crack is formed in the bent part.
On the other hand, the Al concentration in
the surface region of the coating layer is increased
and, therefore, the corrosion resistance of the
surface is improved (namely, the weight loss due to
corrosion is reduced).
The feature of the present invention thus
resides in that the temperature of the steel sheet to
be dipped in the hot-dip Zn-Al alloy coating bath is
kept below the temperature of the bath and the
temperature of the steel sheet to be pulled out of
the bath is kept below the bath temperature as far as
possible in order that the solidification of the
coating layer be started with the part in contact
~l
14
1 337322
with the steel sheet and is completed as soon as
possible. Such a process has never been known as yet.
The hot dipping bath temperature is such
that Zn and Al are molten to form a homogeneous melt,
for example, about 430 to 480C.
The temperature of the sheel sheet to be
dipped in the hot dipping bath is preferably kept
below the bath temperature by 10 to 80C. For
example, when the bath temperature is 480C, the
temperature of the sheel sheet to be dipped in the
hot dipping bath is kept in the range of 400 to
470C. This is because when the temperature of the
steel sheet to be dipped in the hot dipping bath is
equal to or lower than the bath temperature and also
that of the steel sheet to be pulled out of the bath
is lower than the bath temperature, the steel sheet
will have a thickness larger than that of the coating
layer and, since the temperature of the sheet pulled
out is lower than the bath temperature, the cooling
of the coating layer is started with the inner face
and the Al concentration is high in the surface
layer, since Al tends to concentrate in a part which
is solidified later. However, when the temperature of
the steel sheet is lower by more than 80C, the
adhesion of the coating layer is reduced and the
lowering in the bath temperature is serious to
thereby increase the operation cost.
The Al concentration in the grain
boundaries where solidification occurs later is
higher than that in the grain centers where
crystallization occurs in an initial stage. In
practice, the Al concentration in the surface layer
is uneven and it forms a honey-comb pattern in which
B
1 337322
parts of a relatively low Al concentration are
surrounded by parts of a high Al concentration.
However, the area of the parts of the high Al
concentration is large enough for improving the
corrosion resistance of the whole surface.
Although the Al-Zn crystals are also formed
in the surface layer upon cooling of the surface
layer, the homogeneous dispersion of Al in the
surface layer is also accelerated, since the cooling
velocity of the whole coating layer is increased by
dipping the steel sheet kept at a low temperature.
Further, the bath is preferably a low temperature
bath.
According to a preferred embodiment, the
amount of the molten zinc-aluminum alloy deposited on
the steel sheet is controlled with a gas wiping type
of equipment for controlling the amount of molten
zinc-aluminum alloy deposit under conditions
comprising a slit clearance of 0.6 to 2.4 mm, a
distance between front and back nozzles of 10 to 40
mm and an ejecting pressure of gas of 0.1 to 2.0
kg/cm2.
As described above, the Pb concentration in
the hot dipping bath must be controlled in 100 ppm or
less in order to obtain an excellent adhesion after
aging. However, when the Pb concentration is 500 ppm
or less, the surface of the coating layer has a
rippling pattern to impair the appearance thereof
unfavourably. After intensive investigations made for
the purpose of eliminating such a pattern from the
surface, the inventors have succeeded in obtaining an
excellent appearance by controlling the amount of the
molten zinc-aluminum alloy deposit with a gas wiping
16
1 337322
type of equipment under conditions comprising a
nozzle slit clearance of 0.6 to 2.4 mm, a distance
between front and back nozzles of 10 to 40 mm and an
ejecting pressure of gas of 0.1 to 2.0 kg/cm2.
These values are limited for the following reasons:
1. Nozzle slit clearance:
When the lower limit is less than 0.6 mm,
the secondary pressure variation of the gas is too
much to obtain a consistent appearance.
When the upper limit exceeds 2.4 mm, the
amount of the gas is too much and the energy loss is
serious.
2. Distance between front and back nozzles:
The lower limit is 10 mm, because when it
is less than 10 mm, the vibrating strip is apt to be
brought into contact with the nozzle to cause
trouble.
The upper limit is 40 mm, because a shorter
distance gives a better result and the appearance is
impaired when it exceeds 40 mm.
3. Ejecting pressure of gas:
The lower limit is 0.1 kg/cm2 because when
it is below 0.1 kg/cm2, the amount of the deposition
cannot be controlled.
The upper limit is 2.0 kg/cm2 because when
it exceeds 2.0 kg/cm2, the energy loss is large and a
more consistent appearance can be obtained with a
lower pressure.
The control of the amount of the deposition
is necessary in order to conform to Z 27 specified in
JIS G 3302 or G 90 specified in ASTM A 525.
After the steel sheet has been taken out of
the hot dipping bath, the coated steel sheet is
B
- I 337322
reheated to a temperature above the melting
temperature of the coating layer. The reheating
temperature is preferably 420C or higher, because
the appearance of the sheet can be improved at such a
high temperature. Although the reheating temperature
is preferably 420C or above, a high temperature is
not preferred from the viewpoints of both energy and
equipment cost. Thus, a temperature in the range of
420C to 560C is more preferred.
The effect obtained by maintaining the
temperature of the steel sheet dipped in the hot
dipping bath below the bath temperature can be
obtained even when the temperature of the steel sheet
dipped in the hot dipping bath is higher than that of
the hot dipping bath, because the temperature of the
steel sheet is lower than that of the coating layer
in the step of melting the coating layer surface
again and, therefore, the recrystallization of the
molten coating metal starts with the side in contact
with the steel sheet. The alloy layer formed between
the steel sheet and the coating layer is not molten
again, because it has a high melting point and,
therefore, the Al distribution in the alloy layer is
kept unchanged and the adhesion of the coating layer
is kept high.
In a preferred embodiment of the invention,
the hot-dip zinc-aluminum alloy coated steel sheet is
cooled to the solidifying point thereof at a rate of
at least 10C/sec. The lower limit of the cooling
rate is 10C/sec, because stable corrosion resistance
can be obtained at a cooling rate of at least
10C/sec. Although the higher limit thereof is not
limited, a cooling rate of 150C/sec or less is
B
18
1 337322
desirable from the viewpoint of the energy cost and
equipment.
According to a further aspect of the
invention, there is provided a process for producing
a prepainted steel sheet having an excellent
workability and corrosion resistance, comprising
forming a layer by chemical conversion treatment on a
hot-dip zinc-aluminum alloy coated steel sheet
produced by a process as defined above, and
thereafter forming a surface painting film layer
thereon.
According to yet another aspect of the
invention, there is provided a prepainted hot-dip
zinc-aluminum alloy coated steel sheet produced by a
process as defined above.
The surface painting film layer comprises
those of one-coat, two-coat, three-coat, four-coat
type, etc. Usually, a two-coat layer is used.
The present invention will be described in
detail with reference to a two-coat surface painting
film layer.
First, an under-painting paint is applied
to the sheet and baked.
The sheet used is a hot-dip zinc-aluminum
alloy coated steel sheet produced by a process as
defined above. The sheet may have a layer formed by
chemical conversion treatment having a thickness of
about 0.1 to 5 ~.
The chemical conversion treatment is
conducted in order to improve the corrosion resis-
tance of the sheet and the adhesion of the paint to
the steel sheet. The chemical conversion treatment
includes, for example, a treatment with a phosphate
B
19
1 337322
such as zinc phosphate, iron phosphate, manganese
phosphate or cobalt phosphate, and a treatment with a
chromate such as electrolytic chromate treatment and
applied chromate treatment.
As the under-painting paints, those
ordinarily used for the production of prepainted
steel sheets can be used. They include, for example,
paints prepared by mixing coloring pigment, rustproof
pigment, body, etc. in a resin solution mainly
comprising a resin such as epoxy, oil-free polyester,
acrylic or urethane resin. Among them, an under-
painting paint mainly comprising the epoxy resin
which has excellent adhesion and corrosion resistance
or the oil-free polyester resin which has also a good
workability is preferred. The thickness of the under-
paint is 1 to 15 ~, preferably 2 to 12 ~, because the
corrosion resistance and scratch resistance are
further improved with a thickness of at least 2 ~ and
the workability is further improved with that of 12
or less. When the thickness exceeds 12 ~, the cost is
increased.
The rustproof pigment may contain 5 to 35%
of at least one of strontium chromate, zinc chromate,
red lead, zinc plumbate, calcium plumbate, lead
cyanamide, basic lead chromate, basic lead
silicochromate, basic zinc molybdate and calcium zinc
molybdate depending on the use and environment. With
at least 5% of this pigment, the rust formation in an
early stage can be completely inhibited and no
blister is formed with 35% or less thereof.
After formation of the under-paint, a top-
paint of paint is applied thereto and baked to form
B
- I 337322
a topcoat. The top-painting paint comprises
preferably acrylic resin, oil-free polyester resin,
silicone polyester resin, silicone acrylic resin,
alkyd resin, polyurethane resin, polyimide resin,
polyamide resin, fluororesin or the like. The
thickness of the top-paint is 8 to 50 ~, preferably
10 to 45 ~, because the scratch resistance,
workability and weather resist-ance are improved with
the thickness of at least 10 ~ but the cost is
increased when the thickness is beyond 45 ~.
When the Pb concentration exceeds 100 ppm,
the adhesion after aging is reduced due to inter-
crystalline corrosion and, as a result, the corrosion
resistance of a worked part which is important
particularly in the prepainted steel sheet is also
reduced. The concentrations of Al and Pb must be thus
limited in order to produce the prepainted steel
sheet having excellent properties.
According to a preferred e~bodiment, the
chemical conversion layer is formed by treating the
sheet with a chromic acid solution containing silica
having an average particle diameter of 50 m~ and
specific surface area of 200 m2/g in such a manner
that the amount of the coating film after drying will
be 50 to 250 mg/m2 to impart an excellent scratch
resistance.
A chromic acid solution containing silica
having an average particle diameter of 50 m~ is used.
The smaller the average particle diameter of silica,
the better the workability and adhesion, though the
scratch resistance is not improved when the average
particle diameter is too small. When the average
B
-- I 337322
particle diameter is too large, the particles are
liable to be precipitated disadvantageously.
The specific surface area of silica in the
chromic acid solution is 200 m2/g. Although it varies
depending on the average particle diameter, no
scratch resistance can be obtained when the specific
surface area is excessively small.
The chemically converted layer is formed
wth the above-described chromic acid solution in such
a manner that the amount of the coating film after
drying will be 50 to 250 mg/m2. With at least
50 mg/m2 of the film after drying, both the scratch
and corrosion resistances are improved and with 250
mg/m2 or less thereof, the workability, adhesion and
scratch resistance are improved.
The following non-limiting Examples further
illustrate the present invention.
The materials to be coated were low-carbon
aluminum killed steel sheets (0.8 mm x 914 mm x coil)
in all the cases. The sheets were hot-dip coated with
Zn-Al alloy with Sendzimir continuous zinc coating
equipment.
Example 1
Hot-dip zinc-aluminum allow coated steel
sheets were produced with hot dipping baths having
various Al concentrations and the workability thereof
was examined in order to confirm the effects obtained
by the addition of Al according to the present
invention.
B ~ 1 337322
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 120 to 260 g/m2
Pb concentration: 50 ppm
(1) Test method:
The OT and 2T bending test methods according
to JIS G 3312 were employed. The term "OT bend" and
"2T bend" refer to the bends realized when a steel sheet
having a thickness of the base metal of T is bend with
a hand vise or other suitable means to give an inner
diameter of the bend of OT and 2T, respectively. The
cracks of the coating layer in the bend part were examined
and the results were classified into five groups. The
standard is shown in Table 1 and the results are shown
in Table 2.
Table 1 (Standard of evaluating degree
of cracking)
Standard
No crack formed.
4 Extremely slight crack formation observed.
3 Slight crack formation observed.
2 Crack formation distinct.
1 Crack formation remarkable.
B - 1 337322
Table 2
Workability
AQ wt%
O T 2 T
0
Comparative 0.1 1 2
0.2 2 3
0.3 3 4
O.S 4 5
1.0 4.5 5
1.5 4.5 5
Present
invention
2.0 4,5 5
2.5 S 5
3.0 S 5
3.5 5 5
4.0 5 5
5.0 5 S
Comparative
0.0 3 4
15.0 2 3
The numerals in -Table 2 are the averages of the results.
B 1~ 1 337322
Example 2
Hot-dip zinc-aluminum alloy coated steel sheets
were produced with hot dipping baths having various Pb
concentrations and the prolonged adhesion thereof was
examined in order to confirm the effects of Pb added
according to the present invention.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 120 to 260 g/m2
(2) Test method (adhesion after aging or adhesion after
working:
A paint was applied to the test pieces in a
thickness of about 5 ~ and then baked in order to prevent
formation of white rust. The test pieces were immersed
in hot water kept at 80C for 3 days and taken out.
The painting film was removed with a stripping agent.
A semi-spherical steel mass weighing 5 kg and having
a radius of 3/4 inch was dropped from a height of 500 mm.
An adhesive tape was applied to the projecting coated
surface to forcedly strip it to thus determine the
adhesion of the coating layer. The standard is shown
in Table 3 and the results are shown in Table 4.
~s 1 337322
Table 3 (Standard of adhesion after aging or
adhesion after~working)
Standard
No peeling observed.
4 Extremely slight peeling observed.
3 Slight peeling observed.
2 Peeling distinct.
1 Peeling remarkable.
B 1 337322
Table 4
Adhesion after aging
Pb (ppm)
AQ
wt~ 0 50 100 200 300 500 1000
0 5 5 5 5 5 5 5
0.1 5 5 5 4 4 4 4
0.2 5 5 5 3 2
0 3 5 5 5 3 2
0.5 5 5 5 2
1.0 5 5 5
1.5 5 5 5
2.0 5 5 5
2.5 5 5 5
3.0 5 5 5
3.5 5 5 5
4.0 5 5 5
5.0 5 5 5
*.The hot dipping bath comprised Al, Pb and the
hAlAnC~ of Zn.
The numerals in Table 4 are the averages of the results.
B ~ 1 337322
The following Example 3 will illustrate the
invention stated in Claim 2 of the present application.
Example 3
Hot-dip zinc-aluminum alloy coated steel sheets
were produced with hot dipping baths containing various
amounts of Si and the workability and adhesion thereof
were ex~mined in order to confirm the effects obtained
by the addition of Si according to the present invention.
(Test method)
A semi-spherical steel mass weighing 5 kg and
having a radius of 3/4 inch was dropped from a height
of 500 mm and an adhesive tape was applied to the
projecting coated surface to forcedly strip it to thus
determine the adhesion of the coating layer. The results
are shown in Table 5. The standard is the same as that
shown in Table 3.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 120 to 260 g/m2
Pb concentration: 50 ppm
In Table 5, the results of OT crack formation
according to the bending test are shown in the upper
row and those of OT tape tests on adhesion after working
are shown in the lower row.
When the Al concentration is in the range of
0.3 to 3.5 wt.~ and the Pb concentration is not higher
B ~ 1 33~3~2
than 100 ppm, hot-dip zinc-aluminum alloy coated steel
sheets having excellent workability and adhesion after
aging can be obtained. It is preferred, however, to
add 1/100 to 1 part of Si per part of Al to control the
formation of the alloy layer and, therefore, to form
only a thin alloy layer. By this process, a hot-dip
zinc-aluminum coated steel sheet further improved in
adhesion after aging can be obtained. When only
1/200 part of Si is added per part of Al, no improvement
is observed.
It was thus confirmed that when the Al
concentration was 0.3 to 3.5 wt.%, Pb concentration was
100 ppm or less and Si concentration was 1/100 to 1 part
per part of Al, an intended hot-dip zinc-aluminum alloy
coated steel sheet which has excellent wor~ability and
adhesion can be obtained.
Table 5
Workabillty ~upper row) and adheslon (lower low)
Ratlo of Sl to Al
3ath - ,3sltlon
~wt.~) 0 1/200 I/lO0 l/50 I/30 I/20 1/10 l/5 1/2 1/1 2/l
I /- / / / ~ / / / / /~ /
0 ~Q / I / / / _ / _ / _ / _ / _ / _ / _ / _
1 / - / - / - / 2 / 2 / 2 / 2 / 3 / 3 / 3 /
Comparatlve Q / 4 / _ / _ / _ / 5 / 5 / 5 / 5 / 5 / 5 / 5
0.2 AQ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
3 / - / 4 / 4 / 4 / 4 / 4 / 4 / i / 4 / 4 /
0'3 ~Q / 4 / - / 5 / 5 ~ 5 / 5 / 5 / 5 / S / 5 / 5
0.5 ~Q ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ 5
Present .
lnventlon
4.5 / ~.5 / 5 / 5. / 5 / 5 ~ 5 / 5 / 5 / 5 / 5 ~
1'0 ~Q / ~.5 / 4.5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5
~.5 / ~.5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 /
2.0 ~Q / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5
5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / -`I
a AQ / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5
5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / 5 / .
Comparatlve 5-0 ~Q / 5 / 5 / 5 / 5 / 5 / 5 / S / 5 / 5 / 5 / 5
The numerals of the ejvaluation results ln Table 5 are the averages of the results.
The following Example 4 will illustrate the
invention stated in Claim 3 of the present application.
Example 4
Hot-dip zinc-aluminum alloy coated steel sheets
were produced with hot dipping baths containing various
amounts of Mg, Mn or Cu and the corrosion resistance
and adhesion after aging thereof were examined in order
to confirm the effects obtained by the addition of it.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 120 to 260 g/m2
Pb concentration: 50 ppm
(Test method)
Test pieces prepared under the conditions shown
in Table 7 were subjected to the chromate treatment.
An epoxy resin under-paint having a thickness of 5 ~ and
then a silicone polyester resin top-paint having a
thickness of 15 ~ were formed. After baking, they were
subjected to a salt spray test according to JIS Z 2371
and the corrosion resistance in the OT part of each of
the prepainted steel sheets was examined. The results
are shown in Table 7. The standard is shown in Table 6.
- 3l- 1 337322
Table 6 (Standard of evaluation of
corrosion resistance)
Standard
No white rust formed.
4 Extremely slight white rust formation observed.
3 Slight white rust formation observed.
2 White rust formation distinct.
1 White rust formation remarkable.
The adhesion after aging was tested in the
same manner as that of Example 2 and the results were
evaluated according to the standard shown in Table 3.
When a metallic element such as Mg, Mn or Cu
capable of improving the corrosion resistance of the
galvanized steel sheet was added to the hot dipping bath,
the effect of the present invention could be further
improved. The effective concentration of the metallic
element was 0.01 wt.% or higher. A combination of Si
with Mg, Mn or Cu is also usable. By controlling the
Pb concentration below 100 ppm, a stable àdhesion after
aging could be obtained.
B 3~ t 337322
a~ ~D C
S JJ~ ~
~" .C
~:0 0 ~
.. , o
.t
,, -
~ n
U J- --~
~D ~ O cn
S~ 00
Q,o
~1 ~
E -- S
. O o ~
cc c~ ~cc ccc cc ccc .a
N N C C N N C C N N N C N N N C N N C C: N N N C 0
N N N N N N N N N E~
U O ~D ~ 0 0 0 0 0 ~ U O C
, ~ ~ 0 ~C C C ~ d ,, _, _I C
C N. . I d J3. 111 - . . . C N,q,, d ., 0. . _ ~ _
S~ ~ N ~ C .
~ ~ ~ ~) C~ X :E; ~ ~
_~ooouloo~u~ooo-n , ooo~oo_lu~ooou~ d r
-------......... , -.-.-..... --... -- p,
- , oo_l_~oo_~_~ooo_l ~ , ooo_100_1_1000_~ --
J I I I I I I I I I I I I _ I I I I I I I I I I I I
~ ooooooooooooo ~ ooooooooooooo
r O O O O O O O O O O O O O O O O O O O O O O O O O O O O Id ' ~
O 0000000000000 0 0000000000000
I I I I I I I I I I I I I I I I I I I I I I I I I I I I --I
O 0000000000000 o Ooooooooooooo ~tt
O
r ~ r
.
t~
The following Example 5 will illustrate the
invention stated in Claim 4 of the present application.
Example 5
Hot-dip zinc-aluminum alloy coated steel sheets
were produced at various steel sheet temperatures and
hot dipping bath temperatures as shown in Table 10 and
the workability and corrosion resistance thereof were
examined in order to confirm the effects of the present
invention.
The results are shown in Table 10.
In this example, the temperature difference
between the steel sheet and the bath was controlled to
be 0 to 80C to reduce the energy required to maintain
the bath temperature.
(Test methods)
Surface corrosion resistance test
The four edges of each of the test pieces having
a size of 60 mm x 60 mm were sealed by coating. Further
the whole surface of the test piece other than the surface
to be tested was also sealed by painting and then dried.
The painting was conducted in such a manner that the
area of the exposed surface of the test piece would be
50 mm x 50 mm. Then the test piece was thrown into a
salt spray testing instrument and tested according to
JIS Z 2371. After the completion of the test conducted
for 100 Hr., the test piece was taken out, corrosion
products were removed from the exposed surface thereof
and the test piece was weighed. The loss due to corrosion
- 3~ 1 3:~732;~
(g/m2) was determined by dividing the difference in weight
between that before the test and that after the test
with the area of the test surface. The standard is shown
in Table 8.
Table 8 ~Standa-rd of evaluation of
surface corrosion resistance)
Standard
Loss due to corrosion S0 g/m or less
4 Loss due to corrosion 50 ~ 75 g/m
3 Loss due to corrosion 76 ~ 100 g/m2
2 Loss due to corrosion 101 ~ 12S g/m2
1 Loss due to corrosion 126 g/m or above
Initial red rust test on edge
The test piece was placed in a salt spray
testing instrument for 160 Hr. and red rust formed on
the edges thereof was examined. The salt spray test
was conducted according to JIS Z 2371. The standard
of the examination of the red rust is shown in Table 9.
B 1 337322
Table 9 (Standard of examination of
initial red rust formed-on edge)
Standard
No red rust formed.
4 Extremely slight red rust formation observed.
3 Slight red rust formation observed.
2 Red rust formation distinct.
1 Red rust formation remarkable.
The lower is the temperature of the steel sheet
at the time of dipping into the bath, as compared with
the bath temperature, the better is the corrosion
resistance. The temperature difference between the sheet
and the bath is preferably within 80C to reduce the
energy required to maintain the bath temperature.
The Al distribution on the coating layer surface
at various steel sheet temperatus at dipping time is
shown in Figs. 1. The samples used for the determination
of this distribution were prepared under the following
conditions:
bath composition: 1 wt.% of Al, 0.005 wt.
of Pb, 0.02 wt.% of Si
and the balance of Zn
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
Figs. l(a), l(b) and l(c) are microphotographs
B ~ t 337322
of the metal textures obtained when (steel plate
temperature at dipping time) - (bath temperature) was
20C, -20C and -80C, respectively.
The Al distribution was determined with EPMA
(EMX-SM 7; a product of Shimadzu Seisakusho Ltd.).
Fig. 2(b) shows the distributions of Fe, Zn
and Al in the cross section of the hot-dip Zn-Al alloy
coated steel sheet. This figure substantiates the fact
that Al in the coating layer was distributed on the
surface layer thereof to improve the corrosion resistance.
Fig. 2(a) shows a cross section of the coating layer
of a hot-dip Zn-Al alloy coated steel sheet produced
by a conventional process. It is apparent that Al is
distributed densely in the alloy layer.
It is thus apparent from the above figures
that Al is distributed mostly in the surface layer of
the coating layer in the products of the present invention
unlike the products produced by a conventional process.
As described above, the present invention
provides a hot-dip Zn-Al alloy coated steel sheet which
has an excellent corrosion resistance of the coating
layer, which is prevented from the initial red rust
formation on the edges of the sheet and which has an
excellent workability of the coating layer.
B 1 337322
f ~ ~ ~ ..
a o n In U~
: c tn ~ O~
~ ~u
E~
~ O ~ e~ aO~ _ _, O _ _ _ O O _ ~,
o'a ~ ~ -- o o o o o o o o ~ ~ o ~i
00 0 Li. O ~ a
_~ v <~ ~ E a E '
a--n v;
_~ ~, ' o o o o o o o o ~ ~ o,
~ O 0. ~ ~ e~ ~ o ~
a <~ E . E ~ '~ '' 0
~.c o ~
u~ a-~ a~ v_
o O o o o O O o o o o
0
r ~d
c ~ ~ -- -- -- -- ---- _ _ _ L
~ ooooooo ooo o
,q ~ O O O O O O o O O
~_. . .. .. . . . . .
o o o oooo oo o o
'
~ o o oooo ~~ o o 0
O co _ ~ -, o _ .0
_- -- _
.
', , . ' <I~
B - 1 337322
The following Example 6 will illustrate the
invention stated in Claim 5 of the present application.
Example 6
The steel sheet was pulled out of the hot
dipping bath and the surface smoothness of the sheet
was improved by means of a gas wiping type of equipment
for controlling the amount of zinc deposit under
conditions comprising a slit clearance of 0.6 to 2.4 mm,
a distance between front and back nozzles of 10 to 40 mm
and an ejecting of 0.1 to 2.0 kg/cm . The appearance
(smoothness of the coating layer surface) of the produced
hot-dip zinc-aluminum alloy coated steel sheet was
ex~mined,
The conditions were as follows:
line speed: 100 m/min
sheet thickness: 0.8 mm
bath composition: 1 wt.% of Al, 0.005 wt.%
of Pb, 0.02 wt.% of Si
and the balance of Zn
bath temperature: 460C
dipping time in the bath: 4 sec .
Fig. 3 is a schematic drawing of the hot dipping
equipment having a gas wiping type of means for
controlling the amount of zinc deposit used in this
Example.
(Test method)
The appearance (smoothness) of the coating
layer surface was evaluated on the basis of the standard
B 1 337322
shown in Table 11. The results are shown in Tables 12(1)
and 12(2).
Table 11 (Standard of evaluation of appearance
smoothness of coating layer surface)
Standard
No ripple observed.
4 Extremely slight ripples observed.
3 Slight ripples observed.
2 Ripples distinct.
1 Ripples remarkable.
Although the Pb concentration in the bath must
be controlled to be 100 ppm or less to obtain an excellent
adhesion after aging as described above, a Pb
concentration of 500 ppm or less is not preferred for
obtaining a good appearance, because a rough ripply
pattern is formed on the surface with a Pb concentration
of 500 ppm or less.
It is apparent from Tables 12(1) and 12(2)
that a good appearance (smoothness) can be obtained by
the invention stated in Claim 5 of the present
application.
The control of the amount of the deposition
is necessary in order to conform to Z 27 of JIS G 3302
or G90 of ASTM A525. The control thereof in the range
of 50 to 400 g/m2 is easy in Example 6.
~io
B 1 337322
Table 12(1)
Upper row: appearance (smoothness of the coating layer surface)
Lower row: Possibility of control of the amount of deposition
Distance between front
Slit Gas and back nozzles (mm~
clearance ejecting
(mm) pressure
( kg/cm2 )
10 40 ~0
0.1 ~,~
sc1h~ sc1hl~ 5~ihle
Compara~ive 0 4 1.0
/ E~$c1hl~ / pOSCihl~ ~ ccihl~
2 :0 ~ 1 / ~
Ep~,5c1hl~ ~ po$c1hl~ cihl~
0.1 4 / 4 ~ 3 ~
~ 5c1hl~ / POSe1hl~ ~ po5c1hl~
0.6 1 0 4 / 4 /
/ E~c~1hl~ / ~ cc~hl ~ ~ 5cih~l~
2 0 3 / 3 /
Present ~ cc~hl~ 5c-hl~ / poss~hle
i~veu~ion O 1 5 /
- / po5Clhl~ 5c~hl~ 5~1hl~
5 / 4.5 / 3
.2 1.0
- / EK~5e~hl~ / pos~1hl~ / EOS~ihl~
2 0 4 ~ 4 ~ 3 /
/ IK~5c1hl~ / poss1hl~ ~ 55ihl~
The numerals of-the evaluation results in Table 12(1)
are the averages of the results.
B 1 337322
Table 12(2)
- Distance between front
Slit Gas and back nozzles (mm)
clearance ejecting
(mm) pressure
( kg/cm2 )
10 40 50
0.1 ,~//~/~/"
ssihle 5sihle ssihl~
5 / 4.5 / 4
2.0 1.0
- / p~ccihl~ / po5sihl~ / p~scihle
2 0 / ~
Present / p~sc~hl~ Ssihlf~ ccihle
invention ~ ~ ~
55ihl~ sslhle p~scihl~
5 / 4.5 / 4
2.4 1.0
/po5s~hle~poss~thl~ /po5Cihle
- 2 0 5 / 4' ~ 4 /
/ possihl~ / p~s5ih~e / poss~hle
0 1 ~ ~ 4 ~
csihl~ Ssihl ~ po55ihl~
Cbmparative 3 0 1.O / ~ ~
/pOscih]P~p~ccihl~possihle
4.5 / 4.5 / 4.5 /
2.0
/p~Sc j h~ e / poccihl~ / pOssihl~
The n~ ~r~lc of the-evaluation results in Table 12(2)
are the averages-of the results.
B ~- t ~73~
The following Example 7 will illustrate the
invention stated in Claim 6 of the present application.
Example 7
The steel sheet was pulled out of the hot
dipping bath and treated with a gas wiping type of
equipment for controlling the amount of zinc deposit
under conditions comprising a slit clearance of 0.6 to
2.4 mm, a distance between front and back nozzles of
10 to 40 mm and a gas ejecting pressure of 0.1 to
2.0 kg/cm2 to give a hot-dip zinc-aluminum alloy coated
steel sheet. The appearance (surface smoothness) of
the sheet was examined.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 250 g/m2
bath composition: 0.5 wt.% of Al, 0.005 wt.%
of Pb, 0.01 wt.% of Si
and the balance of Zn
(Test method)
The appearance (smoothness) of the coating
layer surface was evaluated on the basis of the standard
shown in Table 11. The results are shown in Table 13.
- B ~3 1337322
Table 13
(Bath
temp ) - Distance
(SteeI Slit Gas between Surface. Control of
sheet clear- e~ecting fron~ smooth- amount of
temp. at ance pressure and back ness deposition
dipping (mm) (kg/cm2) nozzle
time)('C) (mm)
Comparative -20 0.4 1 0 20 2 possible
-20 0.6 - 1.5 20 3 possible
-20 1.2 2.0 40 4.5possible
-45 2.0 1.5 10 4.5possible
Present
iuvæ~Lion -60 2.4 0.1 30 5 possible
-80 1.2 0.1 20 5 possible
-80 2.4 2.0 lQ 5possible
Comparative -60 0.8 1.0 50 1impossible
The numerals of ~he smco~hn~ss Ln Table 13 are
the a~erages of the results.
B ~ 1 337322
Figs. 4(a) to (c) show the appearances of the
sheets produced in this Example and Comparative Example.
Fig. 4(a) is microphotograph of a sheet produced
under conditions comprising (steel sheet temperature
at dipping time) - (bath temperature) of -60C, a nozzle
slit clearance of 0.8 mm, a gas ejecting pressure of
1.0 kg/cm and a distance between front and back nozzles
of 50 mm (the result of the evaluation of the
appearance: 1). Fig. 4(b) is that produced under
conditions comprising (steel sheet temperature at dipping
time) - (bath temperature) of -20C, a nozzle slit
clearance of 0.6 mm, a gas ejecting pressure of 1.5 kg/cm2
and distance between front and back nozzles of 20 mm
(the result of the evaluation of the appearance: 3).
Fig. 4(c) is that produced under conditions comprising
(steel sheet temperature at dipping time) - (bath
temperature) of -80C, a nozzle slit clearance of 1.2 mm,
a gas ejecting pressure of 0.1 kg/cm2 and a distance
between front and back nozzles of 20 mm (the result of
the evaluation of the appearance: 5).
By the process stated in Claim 6, a hot-dip
zinc-aluminum alloy coated steel sheet having excellent
appearance and corrosion resistance and being suitable
for use as a material for prepainted steel sheet could
be obtained.
The following Example 8 will illustrate the
invention stated in Claim 7 of the present application.
B 1 337322
Example 8
The hot-dip zinc-aluminum alloy coated steel
sheet produced by the process of Claim 5 or 6 was reheated
to various temperatures shown in Table 14 which were
above the melting temperature of the coating layer in
order to make its surface smooth. The appearance
(smoothness), thickness of the coating layer and Al
distribution in the obtained hot-dip zinc-aluminum alloy
coated steel sheet suitable for use as a material for
a prepainted steel sheet were ex~m;ned.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 250 g/m2
bath composition: O.S wt.% of Al, 0.005 wt.%
of Pb, 0.01 wt.% of Si
and the balance of Zn
The standard of the evaluation of the appearance
(smoothness) of the coating layer surface is shown in
Table 11 and the results thereof are shown in Table 14.
B 1 337322
Table 14
Reheating temp. Smoothness of
(C) coating layer surface
not reheated 3
Comparative 380 3
400 3
420 4
Present 460 4.5
invention 500 4 5
560 4.5
The numerals of the evaluation results are the averages
of the results.
The surface smoothness was remarkably improved
at a reheating temperature of 420C or above.
The results of the determination of the
thicknesses of the coating layers obtained without any
reheating and with the reheating at 460C are shown in
Figs. 5.
It is apparent from Figs. 5 that the scattering
in the thickness of the coating layer was narrow when
the reheating was conducted according to the present
invention. The scattering of the thickness of the coating
layer was closely related to the appearance. Namely,
the narrower the scattering, the less the formation of
the ripples.
The thickness of the coating layer was
determined with a micro-fluorescence X-ray device
B ' I 337322
(SPT-157 SLS; a product of Seiko Denshi Co., Ltd.) with
a beam diameter of 0.1 mm.
The distribution of Al atoms, etc. was examined
with ESCA. The distribution of Fe, Zn and Al in the
cross section of the coating layer on the hot-dip An-Al
alloy coated steel sheet is shown in Table 15. The
results support the fact that Al is distributed in the
surface layer of the coating layer and the coated steel
sheet of the present invention has an excellent corrosion
resistance.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 250 9/m2
bath composition: 1 wt.% of Al, 0.003 wt.%
of Pb, 0.01 wt.% of Si
and the balance of Zn
The ESCA instrument used was JPS-90 SX of JEOL,
Ltd. The acceleration voltage (V) was 500 and the etching
rate was 250 A/min (in terms of SiO2) (the etching rate
of Zn is about 4 times as high as that of SiO2).
B 1 337322
Table 15 (Atomic distribution change
with depth from surface layer)
Depth (Atomic ,6) (Atomic ~)(Atomic %)(Atomic
0 3.21 40.58 38.95 17.25
33 A 5.62 57.94 8.17 28.25
- 200A 13.62 53.27 7.76 25.33
Surface
layer 1200A 95.11 1.97 0.86 2.04
6200 A 98.18 1.13 0.30 0.37
11200A 97.85 0.70 0.99 0.44
The following Example 9 will illustrate the
invention stated in Claim 8 of the present application.
Example 9
Hot-dip zinc-aluminum alloy coated steel sheets
were produced at various cooling speeds as shown in
Table 16 in order to obtain a stable corrosion resistance
The workability and corrosion resistance of the sheets
were examined.
The conditions were as follows:
sheet thickness: 0.8 mm
bath temperature: 460C
dipping time in the bath: 4 sec
amount of deposition: 120 to 260 g/m2
'1~
B 1 337322
bath composition: 1 wt.% of Al, 0.005 wt.
of Pb, 0.01 wt.~ of Si
and the balance of Zn
The workability test was conducted in the same
manner as that of Example 1 and the results were evaluated
on the basis of the standard shown in Table 1.
In the corrosion resistance test, the initial
red rust formation in the edges was examined in the same
manner as that of Example 5 and the results were evaluated
on the basis of the standard shown in Table 9.
Table 16
Corrosion
Cooling speed Workability re(Sedtran5t
SST 160 Hr
Comparative 5 5 3
Present 20 5 4
invention 30 5 5
The numerals of the evaluation results are the averages
of the results.
B 1 337322
It is apparent that the corrosion resistance
was improved when the cooling was conducted at a speed
of at least 10C/sec after the hot dipping.
Figs. 6(a) to (c) are microphotographs showing
the metal textures of the coating layer surfaces. They
are X-ray images of Al on the surfaces formed with EPMA.
Figs. 6(a), 6(b) and 6(c) are microphotographs of the
metal textures of the coating layer surfaces obtained
when the cooling speed was 2C/sec, 17C/sec and 47C/sec,
respectively.
It is apparent from Figs. 6(a) to (c) that
as the cooling speed was increased, the Al distribution
on the coating layer surface became homogeneous and Al
concentrated in the surface layer.
The following Examples 11 to 19 will illustrate
the invention stated in Claim 9 of the present
application.
Example 11
Hot-dip zinc-aluminum alloy coated steel sheets
were produced with baths containing various amounts of
Al and Pb in order to confirm the effects of Al and Pb
added to the bath according to the present invention
(Example 1). Each of them was used as the material sheet.
It was treated with a chromate. An epoxy resin paint
containing 15% of a rustproof pigment was applied thereto.
After baking at 210C for 35 sec, an under-paint having
a dry thickness of 3 ~ was given. Then an oil-free
polyester resin paint as the top-painting paint was
B I ~ 337322
applied thereto and baked at 220C for 45 sec to form
a top-paint having a dry thickness of I1 ~.
The properties of the prepainted steel sheets
thus prepared were examined. The results are shown in
Table 17.
The workability was determined by the same
2T bending test method as that of Example 1 and the
results were evaluated on the basis of the standard shown
in Table 1.
The adhesion was determined also in the same
manner as that of Example 1. Namely, after the 2T bending
test, an adhesive tape was applied to the 2T part of
the prepainted steel sheet and the forced stripping test
was conducted. The results were evaluated on the basis
of the standard shown in Table 3.
The corrosion resistance test was conducted
according to JIS Z 2371 in the same manner as that of
Example 4. The flat part and 2T part were sub~ected
to the salt spray test (SST, 1000 Hr.). The results
were evaluated on the basis of the standard shown in
Table 6.
The workability, adhesion and corrosion
resistance were determined immediately after the
preparation of the prepainted steel sheets and after
six months.
When the Al concentration was in the range
of 0.3 to 3.5 wt.%, the prepainted steel sheets having
excellent properties could be obtained.
B S~ 1337322
When the Pb concentration exceeded 100 ppm,
the workability, adhesion and corrosion resistance were
reduced with the elapse of time.
Table 17
Corroslon reslstance
Bath composltlon Workablllty Adheslon SST lO00 Hr
(wt.%) 2T 2T
flat part 2T part
lmmedl- lmmedl-
ately after ately after ll f.~l after ~ after
~Q rb z~ produc- mo th8 produc 6 p~ c~- 6 p:~uc~- 6
tlon n tion months ._0-, months -_on months
Comparatlve O.l O.Ol balance 3 3 5 5 5 5 3 3
0,3 0,005 balance 4,5 4,5 5 5 5 5 4,5 4,5
Present l.0 0.005 balance 5 5 5. 5 5 5
3.5 O.Ol balance 5 5 5 5 5 5 5 5
W
5.0 0.005 balance 5 5 5 5 5 5 5 S
0.1 0.02 balance 3 2.5 5 4 5 4 3 2.5
0.3 0.03 balance 4.5 2 5 a 5 2 4.5 2
Comparative
1.O O,03 balance 5 1 5 1 5 1 5 1 ~,~
3.5 0.02 balance 5 1 5 1 5 1 5 r~
5.0 0 02 hA 1 An~e 5 1 5 1 5 1 5
The numerals of the evaluatlon results are the averages of the results.
B I 337322
Example 12
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared with hot dipping baths containing various
amounts of Si in order to confirm the effect of Si added
to the bath according to the present invention
(Example 3~. Each of them was used as the material sheet.
It was treated with a chromate in the same manner as
that of Example 11. An under-paint and then a top-paint
were formed thereon to prepare a prepainted steel sheet
in the same manner as that of Example 11. The
workability, adhesion and corrosion resistance of the
prepainted steel sheet were determined in the same manner
as that of Example 11. The conditions and the results
are shown in Table 18.
B ss 1 337322
Table 18
Corrosion
Bath composition (wt.~) resistance
SST 1000 Hr
Work- Adhe-
ability sion
Al Si/Al Pb Zn 2T 2T part 2T part
o 1.5 5 5 1.5
c- ~rative 0.1 1 / 10 2.5 5 5 2.5
1 / 1 3.5 5 5 3.5
Present 1 / 100 4.5 5 5 4 5
invention o 31 / 5 4 . 5 5 5 4 5
c ,~rative 2 / 1 0.005 hAlAn~e 4.5 5 5 4.5
1/ 200 5 5 5 5
Present 1.01 / 5 o 5 5 5 5
invention 1 / 2 5 5 5 5
c ,~rative o 5 5 5 5
Present 3 . 5 1 / 10 5 5 5 5
invention 1 / 1 5 5 5 5
1/ 100 5 5 5 5
Comparative 5 o 1 / 5 5 5 5 5
2 / 1 5 5 5 5
The numerals of the evaluation results are the
averages of the results.
B 1 337322
Example 13
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared with hot dipping baths containing various
amounts of Mg, Mn or Cu in order to confirm the effect
of them added to the bath according to the present
invention (Example 4). Each of them was used as the
material sheet. It was treated with a chromate. An
epoxy resin paint containing 15% of a rustproof pigment
was applied thereto. After baking at 210C for 35 sec,
an under-paint having a dry thickness of 3 ~ was given.
Then an oil-free polyester resin paint as the top-painting
paint was applied thereto and taked at 220C for 45 sec
to form a top-paint having a dry thickness of 11 ~.
The workability, adhesion and corrosion
resistance of the prepainted steel sheets were determined.
The conditions and the results are shown in Table 19.
The workability was determined by the 2T bending
test in the same manner as that of Example 1 and the
results were evaluated on the basis of the standard shown
in Table 1.
The adhesion in the 2T part of the prepainted
steel sheet was determined also in the same manner as
that of Example 11 and the results were evaluated on
the basis of the standard shown in Table 3.
The corrosion resistance test was conducted
according to JIS Z 2371 in the same manner as that of
Example 4. The flat part and 2T part were subjected
to the salt spray test (SST, 1000 Hr.). The results
- B ~31- 133 7322
were evaluated on the basis of the standard shown in
Table 6.
It is apparent from Table 19 that when 0.01
to 1.5 wt.% of one or more of Mg, Mn and Cu was(were)
added to the bath, the workability, adhesion and corrosion
resistance of the prepainted steel sheet were further
improved.
- B s~ 1 337322
Table l9
Corroslon
Bath e ,~slt~on ~wt.~) Work- Adhe- SST lOOO ~r
abll~ty slon
2T 2T
~Q PbSi and Cu Zn part 2~ part
c, ~a~-ve 0 ~;55 5 ~.5
0.011~ 5 - 5 5 5
- 0.03U~ S S S S
1.0 U~ S S. S S
1.5 ~ S S S S
O.OlUII 5 5 5 5
Prrs~nt 0 3 0,30.02Un h~lAr~ 5 5 5
1.2 l~n 5 5 5 5
1.5 Ull 5 5 5 5
O.OlCu 5 5 S. S
-O~Cu 5 5 5
0.08CU S S 5 5
1.5 Cu S S S S
~e 0 ~.5S 5 ~.5
o.olU8 5 5 5 5
0.02l~ 5 5 5 5
O.Ogl4~ 5 5 5 5
1.5 U8 5 5 5 5
O.OlUn 5 5 5 5
~vltlon -S O.OOS O.S 0.03Un ~ n~-e 5 5 5 5
l.l Un 5 5 5 5
1.5 Un 5 5 5 5
O.OlCu S S 5 5
0.03Cu 5 5 5 5
O.O9Cu 5 S 5 5
1.5 Cu . 5 5 5 5
The ~ 1~ of tha eY~ results are the ~ Y ,of the rr~
B 1 337322
Example 14
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared by the process stated in Claim 5 in which
the slit clearance and the distance between front and
back nozzles of the gas wiping type of deposit control
equipment were varied. The sheets were painted and baked
in the same manner as that of Example 12 to form
prepainted steel sheets. The surface smoothness of these
sheets was determined on the basis of the standard shown
in Table 11 in the same manner as that of Example 6.
The conditions and the results are shown in Table 20.
The conditions were as follows:
bath composition:
Al concentration 1.0 wt.%
Pb concentration 0.005 wt.%
Si concentration 0.02 wt.%
Zn concentration the balance
According to the present invention, the
prepainted steel sheet having a good surface smoothness
could be obtained.
B 1 337322
Table 20
Smoo~hnesc after coatLng
Slit Gas
clear- ejecting
ance pressure
(mm) (kg/cm2) Distance between front and
back nozzles (mm)
Comparative 0. 4 2 2
0.6 4 4
1.2 5 4 5 3
Present 1 0
invention
2.0 5 4.5 4
2.4 5 4.5 4
Comparative 3 o 5 4 5 4 5
The numerals of t-hle smoo~hness test results are
the averages.
B 1 337322
Example 15
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared by varying the bath temperature and the
temperature of the steel sheet in order to confirm the
effect of the temperature of the steel sheets to be dipped
in the hot dipping bath according to the present invention
(Example 5). Each of the sheets was used as the material
sheet. It was treated with a chromate. An epoxy resin
paint containing 15% of a rustproof paint was applied
thereto. After baking at 210C for 35 sec, an under-paint
having a dry thickness of 3 ~ was given. Then an oil-free
polyester resin paint as the top-painting paint was
applied thereto and baked at 220C for 45 sec to form
a top-paint having a dry thickness of 11 ~.
The workability, adhesion and corrosion
resistance of the prepainted steel sheets were determined.
The conditions and the results are shown in Table 21.
The workability was determined by the 2T bending
test in the same manner as that of Example 1 and the
results were evaluated on the basis of the standard shown
in Table 1.
The adhesion in the 2T part of the prepainted
steel sheet was determined also in the same manner as
that of Example 11 and the results were evaluated on
the basis of the standard shown in Table 3.
The corrosion resistance test was conducted
according to JIS Z 2371 in the same manner as that of
Example 4. The flat part and 2T part were subjected
- B 1 337322
to the salt spray test (SST, 1000 Hr.). The results
were evaluated on the basis of the standard shown in
Table 6.
It is apparent from Table 21 that when the
temperature of the steel sheet to be dipped in the hot
dipping bath is lower than the temperature of the bath,
well-balanced, quite excellent workability, adhesion
and corrosion resistance were obtained.
-- - B 1 337322
o C o E~
~ ~ o ~
~q _ O .
O ~_~
I ~
0~ S~
;3: ~
~ - 0
,~,~ . ~ _ o o o oo o o o ~ ~ o
~ J O ~ TI T
<q u ~ ~a o. J
s - oo o o ol o o o o o o ~
~ oq
dP <~ J~
3 o
o
O ~ C
.,
, ~Is
O _ _
~, ~ o . o o o o . o o o o
h cn o . Q
C)
S
O OO ~ U~ o o S~
o _ _ _ C~ C~ C~ O O --
' O
~n
1: _I h
O ~ ~
r <D
c ~
~ ~ O S
p,-,l C~ E~
B 1 337322
Example 16
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared by the process stated in Claim 6 in which
the temperatures of the bath and the sheet to be dipped
in the bath, and the slit clearance, gas ejecting pressure
and the distance between front and back nozzles of the
gas wiping type of deposit control equipment were varied.
The sheets were treated with a chromate and then painted
and baked by the two-coat/two-bake process in the same
manner as that of Example 11 to form prepainted steel
sheets. The surface smoothness of each of the prepainted
steel sheets was determined on the basis of the standard
shown in Table 11 in the same manner as that of Example 6.
The conditions and the results are shown in Table 22.
The bath composition was as follows:
Al concentration 0.5 wt.%
Pb concentration O.OOS wt.%
Si concentration 0.01 wt.%
Zn concentration the balance
It is apparent from Table 22 that the surface
smoothness of the prepainted steel sheets could be
remarkably improved according to the present invention.
B 1 337322
Table 22
.(Bath
temp.) - Gas Distance Smooth-
(Steel Slit eject- between ness
sheet clear- ing front after
temp~ at . ance pres- and back paint--
dipping (mm) sure nozzles ing
time)(C) (kg/c~) (mm)
Comparative -20 0.4 1.0 20 2
-20 0.6 1.5 20 3
-20 1.2 2.0 40 4.5
-45 2.0 1.5 10 4.5
Present
invention -80 2.4 0.1 30 5
-80 1.2 0.1 20 5
-80 2.4 2.0 10 5
ComparatiYe -80 0.8 1.0 50
The numerals of the smoothness test results are
the averages of the results.
- B 1 337322
Example 17
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared by the process stated in Claim 7 in which
the reheating temperature was varied. The sheets were
treated with a chromate and then painted and baked by
the two-coat/two-bake process in the same manner as that
of Example 11 to form prepainted steel sheets. The
surface smoothness of the prepainted steel sheets was
determined on the basis of the standard shown in Table 11
in the same manner as that of Example 6.
The smoothness test results and the reheating
temperatures are shown in Table 23.
The bath composition was as follows:
Al concentration 0.5 wt.%
Pb concentration 0.005 wt.%
Si concentration 0.01 wt.%
Zn concentration the balance
The prepainted steel sheets having excellent
smoothness could be thus prepared by the present
invention.
- B ~ 1 337322
Table 23
Reheating temp. Smoothness
(C) after painting
not reheated 3
Comparative 380 3
400 3
420 4
460 4.5
Present
invention 500 4 5
560 4.5
The numerals of the smoothness test results are the averages
of the results.
Example 18
The hot-dip zinc-aluminum alloy coated steel
sheets prepared at various cooling speeds by the process
stated in Claim 8 were used. Each of the sheets was
treated with a chromate and then painted and baked by
the two-coat/two-bake process in the same manner as that
of Example 11 to form prepainted steel sheets. The
workability, adhesion and corrosion resistance of the
prepainted steel sheets were determined. The results
are shown in Table 24 together with the cooling speeds.
The workability was determined by the 2T bending
test in the same manner as that of Example 1 and the
results were evaluated on the basis of the standard shown
in Table 1.
The adhesion in the 2T part of the prepainted
- B 1 337322
steel sheet was determined also in the same manner as
that of Example 11 and the results were evaluated on
the basis of the standard shown in Table 3.
The corrosion resistance test was conducted
according to JIS Z 2371 in the same manner as that of
Example 4. The flat part and 2T part were subjected
to the salt spray test (SST, 1000 h). The results were
evaluated on the basis of the standard shown in Table 6.
The bath composition was as follows:
Al concentration 1.0 wt.%
Pb concentration 0.005 wt.%
Si concentration 0.01 wt.%
Zn concentration the balance
The prepainted steel plates having excellent
workability, adhesion and corrosion resistance could
be prepared by the present invention.
Table 24
Corrosion
Cooling resistance
hi 1 i tvA~lh~s~ n
(C/sec) 2T 2T SST 1000 ~r -~
. flat part 2T p~t
Comparative 5 5 5 5 S
p ~se~--
invention 30 5 5 5 5
The numerals of the evaluation results are the averages
of the results.
GC~
B 1 337322
The following Example 20 will illustrates the
invention stated in Claim 10 of the present application.
Example 20
Prepainted steel sheets of the present invention
were prepared and the properties of them were determined
as follows to confirm the effect of the present invention.
Hot-dip zinc-aluminum alloy coated steel sheets
were prepared in a hot dipping bath having the following
composition (Example 1) and they were used as the
materials:
The bath composition:
Al concentration 1.0 wt.%
Pb concentration 0.005 wt.%
Si concentration 0.01 wt.%
Zn concentration the balance
Then the sheets were subjected to chemical
conversion treatment to form a coating film in various
amounts on the materials (hot-dip zinc-aluminum alloy
coated steel sheets) with a chromate solution (type A)
having a Cr to Si ratio of 55:45 which comprised a mixture
of a solution containing silica having an average particle
diameter of 10 m~ (specific surface area: 200 m2/g) and
a solution containing silica having an average particle
diameter of 50 m~ (specific surface area: 50 m2/g) in
a ratio of 1:1; a chromate solution (type B) having a
Cr to Si ratio of 55:45 comprising only the solution
containing silica having an average particle diameter
of 50 m~ (specific surface area: 200 m /g) or a phosphate
-- B ~ i 337322
solution.
Then an epoxy resin paint containing 15% of
a rustproof pigment was applied to each of the treated
zinc-aluminum alloy coated steel sheet. After baking
at 210C for 35 sec, an under-paint having a dry thickness
of 3 ~ was given. Then an oil-free polyester resin paint
as the top-painting paint was applied thereto and baked
at 220C for 45 sec to form a top-paint having a dry
thickness of 11 ~.
The scratch resistance, workability, adhesion
and corrosion resistance of the prepared prepainted steel
sheets were determined. The results are shown in Table 27
together with the conditions.
The scratch resistance was determined by
applying a copper coin to the painted surface at an angle
of 45 and moved under a load of 3 kg. The results were
evaluated on the basis of the standard shown in Table 26.
The workability was determined by the 2T bend
test in the same manner as that of Example 1 and the
results were evaluated on the basis of the standard shown
in Table 1.
The adhesion in the 2T part of the prepainted
steel sheet was determined also in the same manner as
that of Example 11 and the results were evaluated on
the basis of the standard shown in Table 3.
The corrosion resistance test was conducted
according to JIS Z 2371 in the same manner as that of
Example 4. The flat part and 2T part of the prepainted
B 1 337322
steel sheet were subjected to the salt spray test
(SST, 1000 Hr.). The results were evaluated on the basis
of the standard shown in Table 6.
The prepainted steel sheets having not only
excellent workability, adhesion and corrosion resistance
but also an excellent scratch resistance could be prepared
by the present invention.
Table 26 (Standard of evaluation of scratch resistance)
Standard
S The area of the exposea under-paint was less
than 10% and no sheet was exposed.
4 The area of the exposed under-paint was 10 to
70% and no starting sheet was eYrose~.
3 me area of the ~Yros~ under-paint was more
than 70% and that of the starting sheet was
less than 30%.
2 The area of the ~Yrose~ under-paint was 30 to
70%.
1 The area of the e~rO5e~ under-paint was more
than 70%.
- B 1 337322
Table 27
Corrosion
resistance
Pretreatment Amount SST lOOO Hr
: (ch~ 'cAl of Scratch Work- Adhe-
conv~rSi~ coatlng resist- ability sion
trea ~Pntfilm ance 2T 2T
solution) (mg¦m2) flat 2T part
2 5 5 5 5
100 3 5 5 5
C ;-r~tive C~ e 150 3 5 5 ' 5
200 3 5 5 5 5
300 2 5 5 5 5
3 5 5 5 5
- 100 4 5 5 5 5
~.~s~
i~lvPn1-ign- ~~ B~ 150 4.5 5 5 5 5
200 4.5 5 5 5 5
300 2 5 4 5 5
C ~ ative
P~ e1000 5 4 3,5 4 4
The chromate solution of type A had a Cr to
Si ratio of 55:45 and comprised a mixture of a solution
containing silica having an average particle diameter
of 10 m~ (specific surface area: 200 m2/g) and that having
an average particle diameter of 50 m~ (specific surface
area: 50 m /g) in a ratio of 1:1.
The chromate solution of type B had a Cr to
Si ratio of 55:45 and comprised only a solution containing
silica having an average particle diameter of
50 m~ (specific surface area: 200 m2/g).
The numerals of the evaluation results in the
above Table are the averages of the results.