Note: Descriptions are shown in the official language in which they were submitted.
2 1 337842 73486-3D
This is a division of Application S.N. 604,058 filed
June 2, 1989 for "Artificial Spinal Fusion Implants".
The present invention relates to an artificial fusion
implant to be placed into the intervertebral space left after the
removal of a damaged spinal disc, and a driving member for
inserting the implant.
The purpose of the present invention is to provide an
implant to be placed within the intervertebral disc space and
provide for the permanent elimination of all motion at that
location. To do so, the device is space occupying within the disc
space, rigid, self-stabilizing to resist dislodgement, stabilizing
to the adjacent spinal vertebrae to eliminate local motion, and
able to intrinsically participate in a vertebra to vertebra bony
fusion so as to assure the permanency of the result.
At present, following the removal of a damaged disc,
either bone or nothing is placed into the space left. If nothing
is placed in the space the space may collapse which may result in
damage to the nerves; or the space may fill with scar tissue and
eventually lead to a reherniation. The use of bone is less than
optimal in that the bone obtained from the patient requires
additional surgery and is of limited availability in its most
useful form, and if obtained elsewhere, lacks living bone cells,
carries a significant risk of infection, and is also limited in
supply as it is usually obtained elsewhere, lacks living bone
cells, carries a significant risk of infection, and is also
limited in supply as it is usually obtained from young accident
victims. Furthermore, regardless of the source of the bone, it is
only marginal structurally and lacks a means to either stabilize
-
2a 1 337842 73486-3D
itself, against dislodgement, or to stabilize the adjacent
vertebrae.
A review of all possibly related prior art will
demonstrate the novelty of the present invention.
There have been an extensive number of attempts to
develop an acceptable disc prothesis (an artificial
1 337842
--3--
~ disc). Such devices by design would be used to replace a
damaged disc and seek to restore the height of the
interspace and to restore the normal motion of that spinal
joint. No such device has been found that i5 medically
acceptable. This group of prosthetic or artificial disc
replacements seeking to preserve spinal motion which are
different from the present invention include:
Patent No. 3,867,728 STUBSTAD - describing a
flexible disc implant.
Patent No. 4,349,921 KUNTZ - describing a
flexible disc replacement with rope or file like surface
projections to discourage device dislocation.
Patent No. 4,309,777 PATIL - describing motion
preserving implant with spike outer surfaces to resist
dislocation and containing a series of springs to urge the
vertebrae away from each other.
Patent No. 3,875,595 FRONING - describing a
motion preserving bladder like disc replacement with two
opposed stud like projections to resist dislocation.
Patent No. 2,372,622 FRENCH (FASSIO) - describing
a motion preserving implant comprising complimentary
opposed convex and concave surfaces.
In summary then, these and other similar devices
resemble the present invention only in that they are placed
within the intervertebral space following the removal of a
damaged disc. In that thy seek to preserve spinal motion,
they are diametrically different from the present invention
which seeks to permanently eliminate all motion at that
spinal segment.
A second related area of prior art includes those
devices utilized to replace essentially wholly removed
vertebrae. Such removal is generally necessitated by
extensive vertebral fractures, or tumors, and is not
associated with the treatment of disc disease, or therefore
related to the present invention. While the present
invention is to be placed within the disc space, these
prior devices cannot be placed within the disc space as at
least one vertebrae has already been removed and there no
longer remains a "disc space.~ Furthermore, all of these
devices are limited in that they seek to perform as
-4- 1 3 3 7 8 4 2
temporary structural members mechanically replacing the
removed vertebrae (not a removed disc), and do not
intrinsically participate in supplying osteogenic material
to achieve cross vertebrae bony fusion. Therefore, again
unlike the present invention which provides for a source of
osteogenesis, use of this group of devices must be
accompanied by a further surgery consisting of a bone
fusion procefure utilizing conventional technique. This
group consisting of vertebral struts rather than disc
replacements would include the following:
Patent No. 4,553,273 WU - describing a turnbuckle
like vertebral strut.
Patent No. 4,401,112 REZAIAN - describing a
turnbuckle like vertebral strut with the addition of a long
stabilizing staple that spans the missing vertebral body.
Patent No. 4,554,914 KAPP - describing a large
distractible spike that elongates with a screw mechanism to
span the gap left by the removal of a entire vertebrae and
to serve as an anchor for acrylic cement which is then used
to replace the missing bone (vertebrae).
Patent No. 4,636,217 OGILVIE - describing a
vertebral strut mechanism that can be implanted after at
least one vertebrae has been removed and which device
consists of a mechanism for causing the engagement of
screws into the vertebrae above the vertebrae below the one
removed.
In summary then, this group of devices differs
from the present invention in that they are vertebral
replacements struts, do not intrinsically participate in
the bony fusion, can only be inserted in the limited
circumstances where an entire vertebrae has been removed
from the anterior approach, and are not designed for, or
intended to be used for the treatment of disc disease.
A third area of prior art related to the present
invention includes all devices designed to be applied to
one of the surfaces of the spine. Such devices include all
types of plates, struts, and rods which are attached by
hooks, wires, and screws. These devices differ
significantly from the present invention in that they are
not inserted within the disc space, and furthermore do not
~5~ 1 337842
intrinsically participate in supplying osteogenic material
for the fusion.
Therefore, with these devices where permanent
spinal immobilization is desired an additional surgery
_ 5 consisting of a spinal fusion performed by conventional
means or the use of supplemental methylmethacrylate cement
is required. Such devices, applied to the spine but not
within the disc space, would include the following:
Patent No. 4,604,995 - STEPHENS - describing a
"U" shaped metal rod attached to the posterior elements of
the spine with wires to stabilize the spine over a large
number of segments.
Patent No. 2,677,369 - KNOWLES - describing a
metal column device to be placed posteriorly along the
lumbar spine to be held in position by its shape alone and
to block pressure across the posterior portions of the
spinal column by locking the spine in full flexion thereby
shifting the miximum weight back onto the patient's own
disc.
Other devices are simply variations on the use of -
rods (e.g. Harrington, Luque, Cotrel-Dubosset, Zielke),
wires or cables (Dwyer), plates and screws (Steffee), or
struts (Dunn, Knowles).
In summary, none of these devices are designed
for or can be used within the disc space, do not replace a
damaged disc, and do not intrinsically participate in the
generation of a bony fusion.
Other prior art possibly related to the present
invention and therefore, to be considered related to "Bony
Ingrowthn. Patents related to this feature describe either
methods of producing materials or devices to achieve the
same. Such patents would include:
Patents No. 4,636,526 (DORMAN), No. 4,634,720
(DORMAN), No. 4,542,539 (ROWEl, No. 4,405,319 (COSENTINO),
35 No. 4,439,152 (SMALL), No. 4,168,326 (BROEMER), No.
4,535,485 (ASHMAN), No. 3,987,499 (SCHARBACH), No.
3,605,123 (HAHN), No. 4,655,777 (DUNN), No. 4,645,503
(LIN), No. 4,547,390 (ASHMAN), No. 4,608,052 (VAN KAMPEN),
No. 4,698,375 (DORMAN), No. 4,661,536 (DORMAN), No.
40 3,952,334 (BOKROS), No. 3,905,047 (LONG), No. 4,693,721
- 1 337842
--6--
_ (DUCHEYNE), No. 4,070,514 (ENTHERLY).
However, while the present invention would
utilize bone ingrowth technology, it would do so with
conventional technology.
~- 5 The final area of related prior art to be
- considered is that of devices designed to be placed within
the vertebral interspace following the removal of a damaged
disc, and seeking to eliminate further motion at that
location.
Such a device is contained in Patent No.
4,501,269 BAGBY describing an implantable device, limited
instrumentation, and a method; whereby a hole is bored
transversely across the joint and then a hollow metal
basket of larger diameter is then pounded into the hole and
lS then filled with the bone debris generated by the drilling.
The present invention differs from the prior art devices in
the following ways:
1. UNIVERSAL APPLICABILITY WITHOUT CONTOURING OF
THE INTERSPACE. The present device will fit any patient,
anywhere throughout the spine, in any intervertebral disc
space, and without alteration of that interspace regardless
of its natural size or shape.
2. RESTORATION AND PRESERVATION OF THE
INTERSPACE. The present invention will restore the
intervertebral space to its premorbid dimensions, and do so
by having the implant fit the space rather than having to
modify the interspace, by bone removal from the vertebrae,
to accommodate the implant.
3. END PLATE PRESERVATION. Preservation of the
highly specialized weight bearing cortical bone is allowed
and end plate perforation into the highly vascular
cancellous bone marrow with its attendant bleeding is
avoided. Such bleeding, when it occures, bears all the
risks of blood loss (e.g. hypoglycemic shock, transfusion
transmitted diseases such as hepatitis and acquired immune
deficiency syndrome, etc.), and all the complications
arising from the resultant impaired visualization of the
vital structures (e.g. nerves, blood vessels, and organs
due to such bleeding.
4. TECHNIQUE. The technique for insertion of
-7_ 1 337842
these implants is consistent with the established methods
of disc removal, and requires neither specialized
instrumentation nor specialized surgical technique.
5. EXTENT OF DISC REMOVAL. The extent of disc
removal can be determined by the surgeon at the time
surgery and can be individualized for each patient.
6. NO DRILLING. No drilling is involved with
the use of the present invention.
7. ELIMINATION OF INCORRECT IMPLANT SIZE
SELECTION. In those implant systems where a drill is used
and significant bone is removed then an estimate of the
implant size must first be made, and then, regardless of
the fit, an implant at least as large as the space created
by the drilling must be utilized, regardless of the quality
of that fit. With the present invention no significant
bone is removed, and the correct size implants are fitted
directly to the interspace eliminating the need to guess at
the correct implant size before the fact.
8. MODULAR DESIGN. The present implants are
available in varying lengths to accommodate the changing
depths of the interspace from central to lateral. The
devices are available in varying heights or are infinitely
adjustable as to the height within the physiological range.
The widths are standardized, and the various embodiments
can be used in any combination (e.g. in the lumbar spine
two auto-expanding implants could be used in conjunction
with two anchor deploying implants to completely fill the
interspace).
9. AVOIDANCE OF SIZE LIMITATIONS. Because in
one embodiment the system is modular, component parts can
be inserted through a very small opening until a much
larger implant is reconstituted completely filling the
available interspace; and yet much larger when assembled
than the opening through which the component modular
sections were introduced. For example, in the lumbar spine
four implants introduced one at a time and measuring 8mm in
width, would when reconstituted within the interspace
constitute a 32mm wide implant. Implantation of a single
implant of those dimensions from a posterior approach in 40
the lumbar spine would otherwise be impossible because of
-8- 1 3 3 7 8 4 2
_ the presence of the dural sac and spinal nerves.
10. THE AVOIDANCE OF INTERSPACE COLLAPSE. The
device is many times stronger than bone and will not
collapse. The implantation of the device allows
_ 5 preservation of the very strong vertebral cortex, which is
resistant to compression preventing the migration of the
implant into the vertebrae. The large surface area of the
assembled modular implant, minimizes the load per unit
area. For example, a reconstituted lumbar implant of four
modular components would have the weight distributed over
approximately 8 sq. cm. per vertebral interface.
11. REMOVABILITY. Because the present invention
is an interspace implant and not a "through vertebrae"
cross interspace implant, removal of the implant, should
that become necessary, would not result in iatrogenic
destruction of the adjacent vertebrae.
12. SELF-STABILIZING. The implant is
self-stabilizing without the use of threads. All of the
implants are surface configured to resist dislodgement and
the preferred embodiments contain active, mechanical means
to assure permanent anchoring. Long term stability begins
with the above and is further enhanced by surface treating
of the implant for bone ingrowth (by known conventional
means) and osteogenically loading the implants.
13. SPINE REDUCING. Various embodiments of the
present invention such as the ones with the 180 degree
opposed ratcheted surface, and the auto-expanding type, are
capable of reducing a vertebral listheses ( a forward or
backward translation of one vertebrae upon another).k
14. SPINAL STABILITY. These implants are
capable
of stabilizing a spial segment following disc removal, and
do so without the use of threads (threads would be design
need to violate the vertebrae themselves extensively).
15. SAFETY. The entire procedure is performed
under direct vision and with complete visualization of the
adjacent vital structures (e.g. organs, neural structures
and blood vessels).
In summary then, the present invention is an
interspace implant utilized to replace a damaged disc,
which unlike an artificial disc, seeks to permanently
1 337842
9 73486-3D
eliminate rather than to preserve spinal motion, and to do so by a
bony fusion. The present invention is clearly an improvement over
the prior art providing an interspace implant intrinsically
participating in the fusion process, self-stabilizing, stabilizing
to the spinal segment, consistent with conventional methods of
discectomy, and uniquely consistent with the preservation of the
integrity of the adjacent vertebrae.
BRIEF SUMMARY OF THE PRESENT INVFNTION
The present invention pertains to an artificial implant,
the purpose of which is to participate in, and directly cause bone
fusion across an intervertebral space following the excision of a
damaged disc, and to a driving member for inserting the implant.
Such implants are structurally load bearing devices, stronger than
bone, capable of withstanding the substantial forces generated
within the spinal interspace. Such devices have a plurality of
macro sized cells and openings of 1-3mm, which can be loaded with
fusion promoting materials, such as autogenous bone, for the
purpose of materially influencing the adjacent vertebrae to
perform a bony bond to the implants and to each other. The
implant casing may be surface textured or otherwise treated by any
of a number of known technologies to achieve a "bone ingrowth
surface" to further enhance the stability of the implant and to
expedite the fusion. Further, said devices are so configured and
designed so as to promote their own stability within the vertebral
interspace to resist dislodgement, and furthermore, to stabilize
the adjacent vertebrae.
To use the implant of the present invention a
conventional discectomy is performed and the vertebral endplates
1 337842
73486-3D
scraped, but not perforated. The appropriately sized implants are
loaded with autogenous bone and implanted within the interspace.
The present invention resides in a driving member for
driving a spinal implant comprising a first hollow tubular member,
said hollow member having an irregular end for conforming to the
external shape of a front surface of a spinal implant, and a
second rod member fitted within said hollow tubular member said
rod member having a threaded portion at one end and an enlarged
knob portion at the other end.
Objects of the Present Invention
It is an object of the present invention to provide for
means of achieving interspace fusion and stabilization as a single
procedure by a means consistent with the conventional method of
discectomy.
It is another object of the present invention to provide
for a means of achieving an interspace fusion and stabilization
that is quicker, safer, and entails less blood loss than by any
other known means.
It is another object of the present invention to provide
for means of achieving a one stage interspace fusion and
stabilization without significant violation or removal of the
adjacent vertebral bone stock.
It is another object of the present invention to provide
for method of intervertebral arthrodesis and stabilization of
enhanced safety where the entire procedure is performed under
direct vision.
It is another object of the present invention to provide
for a method of intervertebral arthrodesis and stabilization of
1 337842
lOa 73486-3D
greater simplicity and requiring minimal specialized
instrumentation or technique not already possessed by those doing
such procedures by conventional means.
It is another object of the present invention to provide
for modular prosthesis, allowing complimentary
1 337842
--11--
_ subunits to be inserted individually through a small
opening and to then be reassembled within the interspace,
so as to reconstitute an interspace occupying device much
larger than would be insertable as a whole.
~~ 5 It is another object of the present invention toto provide for a modular impant system such that it is
possible to precisely fit the contours of any interspace
without the need to sacrifice any vertebral bone to
accommodate the prosthesis. These and other objects of the
present invention will be apparent from review of the
following specifications and the accompanying drawings.
Brief description of the Drawings
Figure 1 is a top right perspective view of the
implant (cervical type).
Figure la is a front view of the implant of Fig.
1.
Figure lb is a rear view of the implant of Fig.
1.
Figure lc is a top view of the implant of Fig. 1.
Figure ld is a side view of the implant of Fig.
1.
Figure le is a bottom view of the implant of Fig.
1.
Figure 2 is a side sectional view of the implant
viewed along lines 2-2 of Fig. ld.
Figure 3 is the implant Figure 1 showing the
attachment to the driver and driver.
Figure 4 is a front perspective view showing the
implant being driven into the disc space.
Figure 4a is a front perspective view of the
implant located in the spine.
Figure 5 is a side view of the implant in the
spine attached to the driver.
Figure 5a is a close up partial sectional view of
the implant and driver.
Figure 6 is a perspective view of a series of
implants placed in the cervical intervertebral space.
Figure 6A is an alternative embodiment of a
rectangular solid implant.
-12- 1 337842 73486-3D
Figure 7 is a side sectional view of the
vertebrae and implant viewed along lines 7-7 of Figure 6.
Figure 7A is a side sectional view of the
vertebrae structure showing a third embodiment of the
rectangular solid implant in place.
Figure 8 is an exploded perspective view of
another embodiment of the implant.
Figure 9 is a side sectional view of the
vertebrae structure and implant viewed along lines 9-9 of
Fig. 8.
Figure 10 is a side sectional view of the implant
of Figure 8, in a contracted position.
Figure 11 is a side sectional view of the implant
of Figure 10, in an expanded position.
Figure 12 is a perspective view of an alternative
embodiment of the implant of Figure 9.
Figure 13 is an alternative embodiment of a
hollow rectangular solid implant.
Figure 14 is a cross sectional view of the hollow
rectangular solid implant of Figure 13 viewed along lines
14-14 of Fig. 13.
Figure 15 is an alternative embodiment of an
expandable implant in its extended position.
Figure 16 is an expandable implant of Figure 15
in its retracted position.
Figure 17 is an expandable implant of Figure 16
located in the disc space.
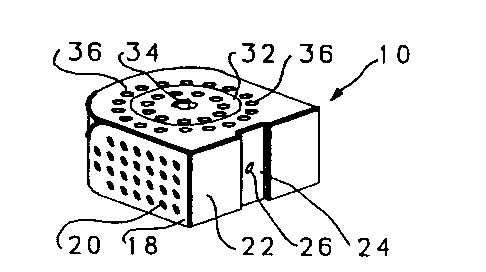
Referring to Figures 1 through 5 an implant for
use in the disc space and associated apparatus used for
inserting the implant 10 is shown. The implant 10 is shown
as a substantially rectangular hollow configuration, having
a tapered forward portion.
The implant 10 has an upper surface 12 and a
parallel lower surface 14. The two side walls 16 and 18
are parallel to one another and have a series of small
sized openings 20 of lmm-3mm through the side walls 16 and
18.
The front wall 22 is slightly convex and has a
depressed portion 24 with a central threaded opening 26 for
receiving the engaging end 28 of a driving member 30.
1 337842
-13-
The upper surface 12 has a threaded cap 32, which
has opening 33 there through, with a central allen wrench
opening 34 for engagement with an allen wrench A of Figure
3. The cap 32 covers the opening into the hollow implant
~- 5 10 and permits the insertion of autogenous bone material
into the hollow portion of the implant 10. The cap 32 is
surrounded by a series of small sized openings 36 of lmm to
3mm passing through the upper surface and into the central
hollow portion of the implant 10.
The rear wall 38 is convex so as to conform to
the rear of the disc space.
The driving member 30, shown in figure 3,
comprises a substantially hollow tubular member 40 having a
long internal rod 42 having a turning knob 44 at one end
and a thLeaded portion 46 at the other end for threadably
engaging the threaded opening 26 of the implant 10. The
engaging end 28 of the driving member 30 has a slightly
convex surface to complement the slightly convex surface of
the front wall 22. The engaging end 28 has an extension 48
for fitting within the depressed portion 24 on the front
wall 22 of the implant 10. The engaging end 28 also has
restriction members 47 and 49 to restrict the depth of
penetration of the driver 30.
In use, the cap 32 is removed from the implant 10
and autogenous bone material is inserted into the hollow
portion of the implant 10. The cap is then replaced.
Various methods of packing the implant 10 with the
autogenous bone material may be used to obtain a completely
packed implant 10.
Referring to Figures 4, 4a, 5 and 5a, the method
of inserting the implant is shown. The threaded end 46 of
the internal rod 42 of the driving member 30 is attached to
the threaded opening 26 of the implant 10 by turning of the
knob 44. Once the engaging end 28 is in place, the fitting
of the extended portion 48 into the depressed portion 24
prevents movement of the implant 10 in relationship to the
driving member 30.
The implant is then placed at the entrance to the
disc space between the two adjacent vertebrae V. The knob
44 is then tapped with hammer H sufficiently hard enough to
-14- 1 3 3 7 8 4 2
_ drive the implant 10 into the disc space. The restriction
members 47 and 49 which are wider than the disc space,
prevent over penetration of the implant.
The size of the implant 10 is substantially the
~- 5 same size as the disc space that it is replacing and thus
will be larger or smaller depending on the disc space in
which it is to be used. In the preferred embodiment the
implant 10 is approximately 32mm wide.
Referring to Figures 4A and 5 the implant 10 is
shown in place in the disc space after removal of the
driving member once the implant was inserted in place.
The autogenous bone material that was packed
within the hollow portion of the implant 10 serves to
promote bone ingrowth between the implant and the adjacent
vertebrae. Once the bone ingrowth occurs, the implant 10
will be a permanent fixture preventing dislodgement of the
implant as well as preventing any movement between the
adjacent vertebrae.
Referring to Figure 6 an alternative embodiment
of the implant is disclosed. The implant 61 comprises a
substantially rectangular member having a series of ridges
62 on the upper and lower surfaces of the implant 60. One
or more grooves 64 are placed on the upper and lower
surfaces as well. As indicated in Figure 6, a series of
such implants 61 are used as the interbody spinal implant,
each placed closely adjacent one another to approximate the
size of the removed disc. A series of micro sized opening
63 perforate the implant 61, to promote bone ingrowth.
The implant ~f Figure 6 is inserted as follows:
the disc is substantially removed by conventional means.
The implants 61 are then inserted in the intervertebral
space between the two vertebrae.
The size of the implant 61 of Figure 6 is
approximately 26 millimeters in length and is wide enough
so that four of them will substantially fill the
intervertebral space, depending on which vertebras are
fused.
In Figure 6a a "bullet nosed" implant 67 having a
open front portion 69 to facilitate insertion of implant 67
is shown.
-15- l 337842
_ Referring to Figures 7 and 7a alternative
embodiments of the implant 61 of Figure 6 is shown in place
between two vertebrae V.
In Figure 7 the implant 70 is shown with the
~- 5 ridges 62 shown in the form of teeth facing the anterior.
These ridges serve to prevent the implant 60 from 'walking'
out of the space between the vertebrae.
In Figure 7a an embodiment of the implant 70 of
Figure 6 is shown having opposed ridges 72 and 74. This
serves to maintain the alignment of the vertebrae when the
two vertebrae V are improperly aligned with respect to one
another.
Referring to Figure 8 an adjustable implant 81
having means for adjusting the width of the implant 81 is
shown. The implant 81 comprises a lower member 82 and an
upper member 84 which when fitted together form an
essentially rectangular implant. The upper member 84 and
the lower member 82 have hollow portions that face one
another and receive tapered wedges 86 and 88 that fit
within the hollow portion of the upper and lower members 82
and 84. The wedges 82 and 84 are such that at their large
and they are higher than the combined hollow space between
the upper and lower members 84 and 82, and shallower at the
other end than the hollow space between the upper and lower
members.
The wedges 86 and 88 have a central threaded
opening 90 and 92 in alignment with each other for
receiving threaded screw 94. Deformable burrs 95 on the
head 98 of the screw 94 are used for locking the screw in
place. The implant has a series of holes 100 throughout
the body of the implant to assist in the ingrowth process.
Referring to Figures 9 through 11 the expandable
implant 81 is shown positioned between the two vertebrae V.
In Figure 10 the expandable implant 81 is illustrated in
its contracted position. The wedges 86 and 88 abutt the
interior sloped surfaces 104 of the upper and lower members
82 and 84.
As the screw 94 is turned, as shown in Figure 11,
the wedges 86 and 88 are drawn together, and the sloped
portions of the wedges force the upper member 82 away from
-16- 1 3 3 7 8 4 2
the lower member 84. Once the screw 94 has been turned
sufficiently, the screw head 98 is hit, causing the
deformable burrs to be crimped so as to prevent the reverse
rotation of the screw 94.
In Figure 12, another alternative embodiment of
the expandable implant 81 is illustrated with spike
projections 106 extending from the top and bottom members
to dig into the vertebrae and assist in maintaining it in
place.
In use, the disc is removed, and the implant 81
is placed between the vertebrae. The screw 94 is then
turned expanding the implant. In the preferred embodiment,
the width is from 8 millimeters to 18 millimeters.
Referring to Figures 13 and 14, another
alternative embodiment of the invention is shown in which
the implant 200 comprises a rectangular hollow member
having a slightly tapered forward section 202. The cross
section, shown in Figure 14, shows the rectangular
configuration of the implant.
In use of the implant the interior of the implant
is filled with a paste made of autogenous bone, and
inserted in the place of the former disc. The strength of
the material used to make the implant is such that, even
though it is substantially hollow, it does have sufficient
strength to withstand the forces of the vertebrae
compressing the implant.
Referring to Figures 15-17, another alternative
embodiment is shown in which the implant has movable
projections which are movable from a first positon within
the implant to a second position extending outside of the
implant.
The implant 300 is of a generally rectangular
configuration. The top surface 302 and the bottom surface
304 of the implant have slots 306 for permitting pivotal
member 307 having spikes 308 at their ends to project
through said slots 306. The spikes 308 are pinned at one
end 310 within the implant 300.
Opposing wedge shaped members 312 and 314 having
a central threaded opening 316 for receiving a threaded
screw 318 having a head 320 and a slot 322. The wedges are
1 337842
-17-
facing each other so that upon turning of the screw will
draw the two wedges together, causing the wedges to cause
the spikes 308 to pivot about their end 310 and cause the
spikes to project out of the implant through the aligned
slots 306. The depressions 329 in the pivotal member 307
engage the wedges 314 and 312 to lock the pivotal members
307 in place. A series of holes 324 for promoting bone
ingrowth and fusion are provided in the implant 300.
In use, after the removal of the disc material,
the implants with the spikes 308 in their withdrawn
position, are inserted into the disc space. Then the screw
318 is turned until the spikes 308 are forced to enter the
vertebrae material, as shown in Figure 17. The implant 300
is thus held firmly in place.
These implants have a surface configuration so as
to induce bone ingrowth through the implant, and into the
wall of the vertebrae in effect inducing fusion from one
vertebrae V joint to the other, thereby eventually making
the implant itself superfluous as the bone would do the
work.
The implant itself, because of its being made of
stronger material than bone, would provide structural
support to the two vertebrae while awaiting bone ingrowth.
Once the bone ingrowth occurred, however, the implant would
be firmly and permanently fixed in place.
While the invention has been described with
regards to the preferred embodiment and a number of
alternative embodiments, it is recognized that other
embodiments of the present invention may be devised which
would not depart from the scope of the present invention.