Note: Descriptions are shown in the official language in which they were submitted.
1 3381 61
FIELD OF THE INVENTION
This invention relates to novel polymeric compositions which are useful for
water treatment. These novel compositions are comprised of polymers of a.~
ethylenically unsaturated monomer(s), preferably cont~ining carboxylic acid or
carboxylic amide functionalities, and amine-cont~ining allyl ether monomers.
BACKGROUND OF THE INVENTION
The present invention is directed to novel polymeric compositions cont:~lining
pendant functional groups. The polymers are useful for a broad range of water
treatment applications. They can be used to control the formation and deposition of
scale imparting compounds in water systems such as cooling, boiler, gas scrubbing, and
pulp and paper manufacturing systems. They will also find utility as corrosion
inhibitors, as well as functioning as chelating agents for various metallic ions in
solution.
-
1 3 3 8 1 6 1
As described comprehensively in U.S. Patent 4,497,713,
scaling and corrosion in cooling waters is a major problem. The
term "cooling water" is applied wherever water is circulated through
equipment to absorb and carry away heat. This definition includes
air conditioning systems, engine jacket systems, refrigeration
systems, as well as the multitude of industrial heat exchange
operations.
In a cooling water system employing a cooling tower, water
is circulated through the heat transfer equipment and subsequently
cooled by evaporation of a part of the circulating water as the
water is passed over the cooling tower. By virtue of the
evaporation which takes place in cooling, the dissolved and
suspended solids in the water become concentrated. The circulating
water becomes more concentrated than the make-up water due to this
evaporation loss.
The make-up water employed for recirculating systems is
obtained from surface or well water sources. These waters normally
contain a variety of dissolved salts, the abundance and composition
of which depend on the source of the water. Generally the make-up
water will contain a preponderance of the alkaline earth metal
cations, primarily calcium and magnesium, and sometimes iron, and
such anions as silicate, sulfate, bicarbonate, and carbonate. As
the water is concentrated by the evaporative process, precipitation
of a salt will occur whenever the solubility of the particular
cation/anion combination is exceeded. If the precipitation occurs
at a metal surface, and adheres to it, the resultant deposit is
referred to as scale. Some of the factors which affect scale are
temperature, rate of heat transfer, water velocity, dissolved solids
concentration, cycles of concentration, system retention, and pH of
the water.
3 1 3381 61
Preventing the corrosion and scaling of industrial heat
transfer equipment is essential to the efficient and economical
operation of a cooling system.
Excessive corrosion of metallic surfaces can cause the
premature failure of process equipment, necessitating downtime for
the repair or replacement.
In addition, the buildup of corrosion products on heat
transfer equipment impedes water flow and reduces heat transfer
efficiency, thereby limiting production or requiring downtime for
cleaning. Reduction in efficiency will also result from scaling
deposition which retards heat transfer and hinders water flow.
Scale can also cause rapid localized corrosion and
subsequent penetration of metallic surfaces through the formation of
differential oxygen concentration cells. The localized corrosion
resulting from differential oxygen cells originating from deposits
is commonly referred to as "under-deposit corrosion."
With regard to boiler systems, and as described
comprehensively in U.S. Patent 4,288,327, the formation of scale and
sludge deposits on boiler heating surfaces is the most serious water
problem encountered in steam generation. Although external
treatment is utilized in an attempt to remove calcium and magnesium
ions from the feed water, scale formation due to residual hardness
(calcium and magnesium salts) is normally experienced. Accordingly,
internal treatment is necessary to prevent, reduce, or inhibit
formation of the scale-imparting compounds and their deposition.
1 33 8 ~ 6 1
-- 4 --
Other scale-forming species (phosphate, sulfate, and
silicate salts, for example) can form complex insoluble salts,
depositing as boiler scale.
Therefore, there is a need in industrial water treatment
for materials which can prevent or inhibit the formation of scale
and deposits on heat transfer surfaces in boiler systems, and the
like.
DESCRIPTION OF THE PRIOR ART
~omba, U.S. Patent ~,g~ , descrlbes novel amino
acid-epihalohydrin copolymers with chelating properties. These
polymers differ chemically from those of the present invention. The
'636 polymers are of the condensation type, whereas the instant
polymers are prepared by addition polymerization. This results, in
the case of the instant invention, in polymeric chains containing a
carbon backbone, whereas in the '636 patent, a backbone containing
nitrogen atoms is produced. The molecular weights contemplated by
the '636 patent are furthermore well outside the molecular weight
range of the novel polymers of the present invention. In addition,
the instant polymers are significantly more effective as scale
control agents in boiler water treatment, since they reduce scale
more effectively than the '636 polymers at dosages far lower than
the specific '636 polymers described.
Quinlan, U.S. Patent 3,799,893, describes phosphorous
containing compounds which are described as useful for inhibiting
scale formation. The compounds described are methylene phosphonates
133~6l
of glycidyl reacted polyalkylene polyamines. These materials are chemically
significantly different from the instant polymers: the '893 compounds do not contain
carboxylic acid groups; the '893 best mode compounds are not polymers; and, the '893
compounds have nitrogen in the backbone of the structure. For these reasons, the '893
compounds are not considered to be pertinent prior art. Furthermore, although the test
conditions for detrrmining scale inhibition are substantially different in '893 and the
instant invention, the instant polymers appear to be more effective in scale inhibition at
substantially lower dosages than the best mode '893 compounds.
Boffardi, et. al., U.S. Patent 4,018,702 disclose scale and corrosion inhibitingcompositions which comprise amine adducts of polymaleic anhydride. The instant
invention differs from the '702 patent in a number of significant aspects. The '702
polymers are amides, whereas in the instant invention the amine group is attached to
the polymeric chain through a hydrogen-substituted carbon. The instant polymers are
significantly more stable in an aqueous environment than the '702 polymers, which
would be expected to lose the amine functionality from the polymer chain throughhydrolysis. Such hydrolysis is difficult with the instant polymers. Furthermore, the
best mode polymers of the '702 patent have a molecular weight of only about 200-300
(Example 1 of '702, the only disclosed example of the preparation of polymer). The
molecular weights of the present polymer fall within the range of about 1,000 to about
1,000,000, with the most preferred range being from about 1,500 to about 25,000.Thus, the instant polymers are well outside the range of the '702 polymers. Therefore,
the '702 polymers are not considered to be pertinent prior art to the instant invention.
-6- l 33~ 61
D'Alelio, et. al., Journal of Macromolecular Science-Chemistry, Vol. A6,
pp. 513-567 (1972) report on the synthesis and chelating properties of low molecular
weight poly (glycidyl methacrylate) reacted with iminoacetic and iminodiacetic acids.
Although the D'Alelio polymers have structural similarities to the instant polymers,
they are nonetheless chemically distinct. Significantly, the D'Alelio polymers are ester
derivatives and suffer from the same hydolytic instability as the '702 compounds.
Polymers of the instant invention are stable to hydrolysis in aqueous medium.
Therefore, the D'Alelio reference is not considered pertinent prior art to the instant
mventlon.
Lorenc, U.S. Patent 4,457,847, cites the use of carboxyl cont~inin~; sequestrantpolymers to treat hardness in boiler waters to prevent or remove scale formation on
heat transfer surfaces.
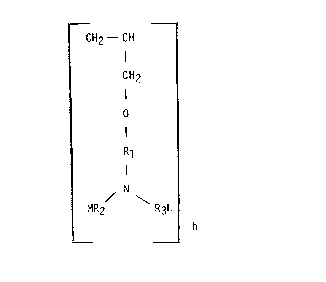
DETAILED DESCRIPTION OF THE INVENTION
This invention pertains to novel water-soluble copolymers which contain
pendant functional groups. Specifically, the novel copolymers of the invention have
the structure of Formula I:
1 338 1 6 ~
{ E } [CH2 _ CH }
g CH2
I
0
I FORMULA I
Rl
I
N
/ \
MR2 R3L
wherein E in the above formula is the repeat unit remaining after
polymerization of a polymerizable monomer, containing pendant
carboxylic acid or water-soluble salts thereof, carboxylic amide,
lower alkyl (Cl-C6) ester, or lower (Cl-C6) alkyl hydroxylated ester
of such carboxylic acids. Compounds encompassed by E in Formula I
include polymerized acrylic acid, methacrylic acid, acrylamide,
maleic acid or anhydride, itaconic acid, and the like.
It is contemplated that E in Formula I also encompasses
mixtures of monomers, provided that they fall within the definition
of E given above. One such preferred mixture of monomers is acrylic
acid/hydroxypropylacrylate.
Rl in Formula I is an unsubstituted linear or branched
lower alkylene group having from about 1 to about 6 carbon atoms, or
an hydroxyl substituted linear or branched lower alkylene group
1 338 1 6 1
having from about 1 to about 6 carbon atoms. R2 and R3 are chosen
independently from hydrogen, lower alkylene group containing from
about 1 to about 5 carbon atoms, hydroxyl substituted lower alkylene
group having from about 1 to about 5 carbon atoms, or carboxyl
substituted lower alkylene group having from about 1 to about 5
carbon atoms. The above substituents are preferred, but other
substituents on the nitrogen capable of chelation are also
contemplated. These groups include, but are not limited to,
phosphonic acid groups, sulfonic acid groups, and the like.
M and L independently denote hydrogen or a water-soluble
cation, e.g., ammonium, alkali metal, organic aminium ion, and the
l-,ke. It will De rea~ily apparent to tnose skllle~ ~n tne art that
M and L will be cations only when R2 and R3 contain groups requiring
a cation for electrical neutrality, such as carboxyl, phosphonic, or
sulfonic acid groups.
The molar ratio of monomers (g:h) in Formula I may fall
within the range of 20:1 to 1:10, with a molar ratio (g:h) of about
10:1 to 1:5 being preferred. It is to be understood that molecular
weight of the novel copolymers is less a key criterion than that the
copolymers be water-soluble. Nonetheless, the number-average
molecular weight of the novel water-soluble copolymers of Formula I
may fall within the range of 1,000 to 1,000,000, with the number
average molecular weight within the range of about 1,500 to about
500,000 being preferred, and the number-average molecular weight
within the range of about 1,500 to about 25,000 being most preferred.
The preparation of the monomer(s) designated as (g) in
Formula I may be in accordance with well known techniques. For
9 1 3381 61
instance, one such possible monomer, acrylic acid, may be prepared by hydrolysis of
acrylonitrile or by oxidation of acrolein.
The allyl ether monomer, represented by fragment (h) of Formula I, may be
prepared by a ring-opening reaction of an allylic glycidyl ether with ammonia,
primary, secondary, or tertiary amines. The ring-opening reaction of amines with the
epoxide group of the allylic glycidyl ether is analogous to the ring-opening reaction of
allylic glycidyl ethers with reagents such as bisulfites or phosphorous acid, to give
sulfonic acids, or salts thereof, or phosphites, respectively, as described thoroughly in
Chen, U.S. Patents 4,659,481 and 4,659,480. When Rl in Formula I is -CH2-CHOH-
CH2-, the allylic glycidyl ether precursor is allyl glycidyl ether (AGE), the preferred
allylic glycidyl ether. The reaction is illustrated with AGE and a secondary amine:
R2M R2M
CH2=CH-CH2-O-CH2-CH-CH2 + H-N ~ CH2=CH-CH2-O-CH2-CH-CH2-N
O R3L OH R3L
AGE will be used hereinafter as the illustrative allylic glycidyl ether for the
sake of simplicity, but its use hereinafter is not to be construed as limiting the
invention in any way. For example, a methallylic glycidyl ether will also be useful in
the present invention.
In the above equation, R2, R3, M, and L have the same meaning as
delineated in Formula I. The following amines, among others, may be employed
- lo 1 3 3 8 1 6 1
in the above reaction: ammonia, methylamine, ethylamine, dimethylamine, diethyla-
mine, propylamine, n-butylamine, isopropylamine, isobutylamine, ethanolamine,
propanolamine, etc. It is to be understood that the enumeration of the above amines in
no way limits the utility of the present invention. Those skilled in the art would
recognize the myriad amines which could be utilized to synthesize monomers that
would be useful for the present invention.
It is also to be understood that when a tertiary amine is used to synthesize theamine-cont~ining monomer in the above reaction, the third group attached to the
nitrogen will be either R2M or R3L, and one equivalent of an inorganic acid,
preferably hydrogen chloride, would be needed to achieve a stable product, which in
the case of a tertiary amine would be a qll~t~rn~ry ammonium salt. The quaternary
ammonium salt would have a permanent positive charge independent of pH. The
inorganic acid, preferably hydrogen chloride, needed when a tertiary amine is used
could be in any of its readily available forms, i.e., gaseous, aqueous solution, etc.
The carboxylate-cont~inin~ amines include, but are not limited to, aspartic acid,
glycine, sarcosine (n-methyl glycine), iminodiacetic acid (IDA), hydroxyethylglycine,
etc. The amines cont:~ining carboxylic acids can be utilized in the acid or the salt
form. If desired in the salt form, the carboxylic acids are preferably converted to their
water-soluble salts with ammonia, organic amines, caustic soda, and the like (asindicated by M and L in Formula I) prior to reaction with the AGE, but the
neutralization could also be conducted after the reaction with the AGE, or even after
the subsequent polymerization.
-11- 1 3381 61
The ring opening reaction may be carried out in the absence of solvent, or in a
suitable solvent, with water being preferred. The reaction temperature may range from
0 C to 80 C. Alkaline materials can be used in catalytic amounts to speed the
reaction, or to drive the reaction to completion. Preferred as the alkaline material is
caustic soda, caustic potash, or soda ash.
During the ring opening reaction, trace amounts of the glyceryl compound may
be formed. This can usually be controlled to less than 5 mole %, and is due to
hydrolysis of the AGE according to the equation:
CH2=CH-CH2-O-CH2-cH-cH2 + H20 ~ CH2=CH-CH2-O-CH2-CH-CH2
\ / I I
O OH OH
AGE GAE
If desired, the hydrolysis product (glyceryl allyl ether, GAE) may be removed
from the mixed monomer solution via the conventional techniques such as distillation,
solvent extraction, and the like. It is to be understood that the method of removal of
this, or other impurities, do not in any way limit the practice of our invention. In any
case, such methods will be known to those skilled in the art.
The present inventors prefer to utilize the monomer cont~inin~ the impurities, if
any, as it is produced. It may therefore contain minor amounts of GAE. When the
GAE is not separated prior to polymerization, it will be incorporated into the polymer
along with the primary amine component.
- 12 - l 3 3 8 1 6 1
It is to be understood that the above methods of synthesis of the amine
cont~ining monomer do not limit the methods of preparation of the said monomer.
After the desired monomers are produced, and isolated if desired,
polymerization is conducted. Radical initiation is the preferred means of initiation, and
the polymerization may be conducted in any of the media f~mili~r to those skilled in
the art, such as solution, suspension, bulk, or emulsion techniques. Any of the well
known initiators may be used to polymerize the monomers, such as azo compounds,
peroxides, redox couples, persulfates, and the like. Likewise, any of the chain transfer
agents familiar to those skilled in the art may be used to control molecular weight.
These include, but are not limited to, lower alkyl alcohols such as isopropanol, amines,
mercaptans, and the like. Accelerators such as bisulfite or ascorbic acid may also be
used. It is to be understood that the aforementioned polymerization methods do not in
any way limit the synthesis of polymers useful in our invention. Similarly, the tertiary
structure (tacticity, arrangement of monomers in the polymer chain, etc.) of thepolymers is not limiting to our invention.
The formation of polymers was confirmed by the following techniques:
viscosity increase; gel permeation chromatography; and carbon-13 nuclear magnetic
resonance (NMR) spectroscopy. The carbon-13 NMR spectra show the typical
broad, polymer-type backbone, with a complex C-C region (35-47 ppm) and C-O-C
region (70-75 ppm), with either a trace or no unreacted monomers. Preferred polymers
1 3381 61
- 13 -
according to our invention are copolymers of the sodium salt of
acrylic acid with allyloxyhydroxypropylamino components having the
structure of Formula II:
{ CH2-CH~ [CH2-CH }
S l l
C=O CH2
ONa O
9 CH2
I Formula II
CHOH
I
CH2
N
MR2 R3L h
wherein the identity of R2, R3, M and L for the preferred copolymers
are as shown in Table I.
TABLE 1 l 3 3 ~ 1 6 1
Structures of the Copolymers
Copolymer
Example R2 R3 M L Mn
8 CH2 CH2 CH2 COO/H* H Na/- 2,350
9 CH2 CH2 CH2 COO/H* H Na/- 3,200
CH2 CH2 H H 2,469
11 CH2 CH2 H H 2,350
I2 CH2 COO CH2 COO Na Na 4,450
13 CH2 CH2 COO H Na 4,200
14 CH2 CH2 COO CH2 CH2 COO Na Na 7,000
*Results from addition reaction of amino component to acrylic acid, giving
terpolymers. For details, see Exarnples 8 and 9.
Mn, number average molecular weight, was measured by gel permeation
chromatography (GPC) using Toyo Soda* G-2000 SW or G-4000 SW columns
calibrated with polystyrene sulfonate standards in sodium nitrate solution. Molecular
weight results from GPC depend on the type of column, conditions and standards used.
Also preferred are copolymers of sodium methacrylate with allyloxyhydroxy
propylamino components (Example 15).
Most preferred are copolymers of acrylic acid with glycine, N-(carboxymethyl)-
N-[2-hydroxy-3-(2-propenyloxy)propyl],
*Trademark
X
1 3381 ~1
- 15 -
disodium salt (wherein MR2 and R3L in Formula II are both
-CH2-COONa), and copolymers of methacrylic acid with 2-propanol,
l-(diethylamino)-3-(2-propenyloxy), (wherein MR2 and R3L in Formula
I are both -CH2-CH3).
The copolymers of the instant invention may be used alone
or in combination with other additives to inhibit corrosion and
control the formation and deposition of scale imparting compounds in
water systems. However, they are not limited to use in any specific
category of water system. For instance, in addition to boiler and
cooling water systems, the copolymers may also be effectively
utilized in scrubber systems and the like wherein corrosion and/or
tne formallon and aeposition ot scale Forming salts is a problem.
Other possible environments in which the inventive copolymers may be
used are for sea water desalinization and dust collecting systems in
iron and steel manufacturing. The copolymers are effective in
controlling iron-induced fouling in wells or other groundwater
systems. The copolymers will also be effective in scale control in
cooling systems containing high levels of alum or ferric chloride.
The copolymers may also be used to prevent precipitation
of calcium carbonate, calcium sulfate, calcium phosphate, calcium
phosphonate, calcium oxalate, barium sulfate, zinc hydroxide,
aluminum hydroxide, aluminum oxide, iron oxide, iron hydroxide,
ferric chloride, etc. in water systems. They will also be useful,
for èxample, as pigment dispersants, cement dispersants, builders in
detergents, and mineral beneficiation aids such as in iron, copper,
molybdate mining, etc.
The copolymers of the present invention can also be used
with other components in order to enhance the corrosion inhibition
1 33~1 61
- 16 -
and scale controlling properties thereof. For instance, the
copolymers may be used in combination with one or more kinds of
compounds selected from the group consisting of inorganic
phosphates, phosphonic acid salts, organic phosphoric acid esters,
and polyvalent metal salts such as those from zinc, chromate,
molybdate, and nickel.
/
The copolymers may be used in combination with
conventional corrosion inhibitors for iron, steel, copper, copper
alloys,or other metals, conventional scale and contamination
inhibitors, metal ion sequestering agents, and other conventional
water treating agents.
Exemplary corrosion inhibitors comprise chromates,
bichromates, tungstates, molybdates, nitrites, borates, silicates,
oxycarboxylic-acids, amino acids, catechols, aliphatic amino
surface-active agents, benzotriazole, and mercapto benzothiazole.
Scale and contamination inhibitors include lignin
derivatives, tannic acids, starches, polyacrylic acids, acrylic
acid/hydroxyalkylacrylate copolymers, and acrylic acid/allyloxy-
hydroxypropylsulfonate copolymers.
Metal ion sequestering agents include ethylenediamine,
diethylenetriamine, and the like, and polyaminocarboxylic acids
including nitrilotriacetic acid, ethylenediaminetetraacetic acid,
diethylenetriaminepentaacetic acid, and hydroxyethylethylene-
diaminetriacetic acid.
Synergistic effects may occur when the copolymers
disclosed in this invention are used in combination with the
reagents described above.
- 17 - 1 3381 61
The novel water soluble copolymers of our invention may
contain pendant functional amino carboxylic acid groups. These
functional groups are connected to the polymer backbone through the
hydrolytically and thermally stable ether linkage. These copolymers
have shown unique properties in controlling iron deposition and
preventing precipitation of calcium phosphate and calcium carbonate
in aqueous systems. The novel copolymers should also find
particularly useful application in boiler water treatment, whereby
their chelating abilities will allow a reduced dosage of chelating
agents, which are commonly used in boiler treatment programs.
Furthermore, the presence of the chelating group permanently
attached to a polymer chain will minimize the possible corrosion
caused by non-bonded cnelating agents in var~ous parts ot the boiler
systems, where corrosion caused by chelating agents could be a
problem. The invention is further illustrated by the following
specific, but not limiting, examples.
Examples
Examples 1-7 illustrate the synthesis of the monomers, and Examples
8-15 illustrate synthesis of the copolymers. The monomers of
Examples 3 and 4 have not been previously described, and thus have
no CAS Registry No. Likewise, the novel copolymers do not have CAS
Registry Nos. Examples of the efficacy of the copolymers in aqueous
systems are also given.
Example 1:
Preparation of 2-propanol, 1-(methylamino)-3-(2-propenyloxy)
[40987-35-7]
1 33 ~ ~ 6 ~
Allyl glycidyl ether (98.5% pure, 1969, 1.7 mole) was
added over a period of 130 minutes to methylamine (40% aqueous
solution, 1989, 2.55 mole), maintaining a reaction temperature of
35+ 4 C. After addition, the reaction mixture was stirred at 35+1
C for 30 minutes, then heated at 60+1 C for 90 minutes. The
reaction mixture was then cooled to room temperature. 2-Propanol,
l-(methylamino)-3-(2-propenyloxy) was collected via vacuum
distillation at about 95 C/3 mm Hg.
Example 2:
Preparation of 2-propanol,l-(dimethylamino)-3-(2-propenyloxy)
~7~75~
Allyl glycidyl ether (98.5% pure, 1159, 1.0 mole) was
added over a period of 130 minutes to dimethylamine (60% aqueous
solution, 909, 1.2 mole), maintaining a reaction temperature of 25+2
C. After addition, the reaction mixture was stirred at 30+3 C for
30 minutes, then heated at 50+2C for 120 minutes. The reaction
mixture was then cooled to room temperature. 2-Propanol,
l-(methylamino)-3-(2-propenyloxy) was collected via vacuum
distillation at about 73 C/4 mm Hg.
Example 3:
Preparation of Glycine, N-(carboxymethyl)-N-[2-hydroxy-3-
(2-propenyloxy) propyl], disodium salt
Iminodiacetic acid (98% pure, 349, 0.25 mole) was
dispersed in 104 ml DI water at room temperature. Sodium hydroxide
(50% aqueous solution, 409, 0.5 mole) was added over a period of 60
-
,9 1338151
minutes, maintaining a reaction temperature of 10+2 C. After
addition, the reaction mixture was stirred at room temperature for
60 minutes.
The resulting disodium iminodiacetate solution (24.9%,
169.39, 0.238 mole) was added over a period of 85 minutes to a
mixture of 34 ml DI water and allyl glycidyl ether (98.5% pure,
27.559, 0.238 mole), maintaining a reaction temperature of 27+3 C.
After addition, the reacton mixture was stirred at 30C for 70
minutes. Glycine, N-(carboxymethyl)-N-[2-hydroxy-3-(2-propenyloxy)
propyl], disodium salt was recovered as a 30% active solution.
Example 4:
Preparation of glycine, N-methyl-N-[2-hydroxy-3-(2-propenyloxy)-
propyl], monosodium salt
N-methyl glycine (98% pure, 329, 0.35 mole) was dispersed
in 64 ml DI water at room temperature. Sodium hydroxide (50%
aqueous solution, 289, 0.35 mole) was added over a period of 80
minutes, maintaining a reaction temperature of 5+2 C. After
addition, the reaction mixture was stirred at room temperature for
60 minutes.
The resulting sodium N-methyl glycinate solution (31.6%,
1239, 0.348 mole) was added over a period of 70 minutes to a mixture
of 33 ml of DI water and allyl glycidyl ether (98.5% pure, 40.39,
0.348 mole), maintaining a reaction temperature of 20+2 C. After
addition, the reaction mixture was stirred at 25C for 100 minutes.
Glycine, N-methyl-N-[2-hydroxy-3-(2-propenyloxy)propyl], monosodium
salt was recovered as a 40% active aqueous solution.
- 20 - 1 33~1 61
Example 5:
Preparation of 2-Propanol, l-amino-3-(2-propenyloxy)
[6967-44-8]
Allyl glycidyl ether (98.5% pure, 1859, 1.6 mole) was
added over a period of 225 minutes to ammonium hydroxide (26%
ammonia in water, 6299, 9.6 mole), maintaining a reaction
temperature of 9+3 C. After addition, the reaction mixture was
stirred at 10C for 20 minutes, then room temperature for 45
minutes. 2-Propanol, l-amino-3-(2-propenyloxy) was collected via
vacuum distillation at about 120C/10 mm Hg.
Example 6:
Preparation of Beta-alanine,N-(2-carboxyethyl)-N-[2-hydroxy-3-
(2-propenyloxy)propyl], disodium salt
[74988-14-0]
Methyl acrylate (99% pure, 44.29, 0.508 mole) was added
over a period of 180 minutes to a mixture of 33 ml methanol and
2-propanol, 1-amino-3-(2-propenyloxy) (99.8% pure, 33.49, 0.254
mole), maintaining a reaction temperature of 12+3 C. After
addition, the reaction mixture was stirred at room temperature for
11 hours.
48 ml of methanol was added to the resulting beta-alanine,
N-(2-carboxyethyl)-N-[2-hydroxy-3-(2-propenyloxy)propyl], dimethyl
ester (75%, 929, 0.228 mole), and the reaction mixture was cooled to
15 C. Sodium hydroxide (99% pure, 18.59, 0.456 mole) was then
dissolved in the reaction mixture, maintaining a reaction temperature
below 25 C. After dissolution, the batch was stirred at room
-21- 1 33~1 61
temperature for 135 minutes. 100 ml of DI water was then added and an exotherm to
31 C was observed. The reaction mixture was stirred at 20 C for 180 minutes,
before removing the methanol by vacuum distillation. Beta-alanine, N-(2-
carboxymethyl)-N-[2-hydroxy-3-(2-propenyloxy)propyl], disodium salt was recovered
as a 56% active aqueous solution.
Example 7:
Preparation of 2-propanol, l-(diethylamino)-3-(2-propenyloxv) [14112-80-2]
Allyl glycidyl ether (146g, 1.25 mole) was added over a period of 120 minutes
to a solution of diethylamine (97g, 1.3 mole) in DI water (33 ml), m~int~ining areaction temperature of 30+10 C. After addition, the batch was stirred at 35+5 C for
135 minutes, then room temperature overnight. The batch was then heated at 50_2 C
for 60 minutes before 2-propanol, 1-(diethylamino) 3-(2-propenyloxy) was collected
via vacuum distillation at 98 C/3mm Hg.
Syntheses of copolymers with acrylic acid are illustrated in Examples 8-14.
Example 8:
Preparation of acrylic acid/2-propanol. 1-(methylamino)-
3 -2-propenyloxy)/beta-alanine,
N-methyl-N-[2-hydroxy-3-(2-propenyloxy)propyl]. terpolymer
2-Propanol, l-(methylamino)-3-(2-propenyloxy) (Example 1,
- 22 - 1 33~1 6~
12.69), water (114.139), and isopropyl alcohol (32.099) were charged
to a suitable reactor and purged with nitrogen. Sodium persulfate
(22% aqueous solution, 15.739) and acrylic acid (36.779) were
simultaneously added over a 4 hour period, maintaining a reaction
temperature of 87+4 C. After addition, the reaction mixture was
held at 91 C for 1 hour. The residual isopropyl alcohol was then
removed by azeotropic distillation. Sodium hydroxide (50% aqueous
solution, 209) and 119 ml of water were then added, maintaining the
temperature below 40 C.
Under the polymerization conditions, some addition
reaction between the secondary amino hydrogen and the free acrylic
acid occurred. This was evidenced by the 13 C NMR spectroscopy
which indicated a mole ratio of acrylic acid/2-propanol,
l-(methylamino)-3-(2-propenyloxy)/beta-alanine, N-methyl-N-
[2-hydroxy-3-(2-propenyloxyl)propyl] of 15.6:1.0:1.9 respectively.
This corresponds to about 65% of the available amine forming the
adduct with acrylic acid.
Example 9:
Preparation of acrylic acid/2-propanol, l-(methylamino)-
3-(2-propenyloxy)/beta-alanine, N-methyl-N-[2-hydroxy-3-
(2-propenyloxy)propyl] terpolymer
Prepared as described in Example 8 except less isopropyl
alcohol (19.759) and less water (21.599) were utilized in the
polymerization. This resulted in a higher molecular weight. 13C
NMR analysis of the product was similar to that obtained for Example
8, confirming the terpolymer structure.
- 23 - 1 33~1 61
Example 10:
Preparation of acrylic acid/2-propanol, l-(dimethylamino)-
3-(2-propenyloxy) copolymer
Prepared as described in Example 8 utilizing 2-propanol,
1-(dimethylamino)-3-(2-propenyloxy) (Example 2, 13.689), water
(91.829), isopropyl alcohol (21.599), sodium persulfate (22% aqueous
solution, 16.059), and acrylic acid (36.769). The hold period after
addition was lengthened to 2 hours. After distillation, sodium
hydroxide (50% aqueous solution, 209) and 119 ml of water were added.
1~ FV~ p~
Preparation of acrylic acid/2-propanol, l-(dimethylamino)-
3-(2-propenyloxy) copolymer
Prepared as described in Example 10 utilizing 2-propanol,
l-(dimethylamino)-3-(2-propenyloxy) (Example 2, 20.529), water
(105.889), isopropyl alcohol (22.919), sodium persulfate (22%
aqueous solution, 18.239), and acrylic acid (36.769). After
distillation, sodium hydroxide (50% aqueous solution, 209) and 130
ml of water were added.
Example 12:
Preparation of acrylic acid/glycine,
N-(carboxymethyl)-N-[2-hydroxy-3-(2-propenyloxy) propyl], disodium
salt copolymer
Prepared as described in Example 10 utilizing glycine,
- 24 - 1 33~
N-(carboxymethyl)-N-[2-hydroxy-3-(2-propenyloxy)propyl], disodium
salt solution (Example 3, 97.049), water (105.459), isopropyl
alcohol (19.549), sodium persulfate (22.8% aqueous solution 20g),
and acrylic acid (36.779). One hour after addition,
tert-butylhydroperoxide (70% aqueous solution, 0.4569) was added.
After distillation, sodium hydroxide (50% aqueous solution, 129) and
71 ml of water were added.
Example 13:
Preparation of acrylic acid/glycine,
N-methyl-N-[2-hydroxy-3-(2-propenyloxy)propyl], monosodium salt
~:0;~ ynll~r
Prepared as described in Example 12 utilizing glycine,
N-methyl-N-[2-hydroxy-3(2-propenyloxy)propyl] monosodium salt
solution (Example 4, 56.299), water (106.22g), isopropyl alcohol
(31.329), sodium persulfate t20.5% aqueous solution, 209), acrylic
acid (36.779), and tert-butylhydroperoxide (70% aqueous solution,
0.4189). The addition period was lengthened to 5 hours. After
distillation, sodium hydroxide (50% aqueous solution, 169) and 110
ml of water were added.
Example 14:
Preparation of acrylic acid/beta-alanine, N-(2-carboxyethyl)-N-
[2-hydroxy-3-(2-propenyloxy)propyl], disodium salt copolymer
Prepared similarly to Example 10 utilizing beta-alanine,
N-(2-carboxyethyl)-N-[2-hydroxy-3-(2-propenyloxy)propyl], disodium
- 25 _ 1 338 ~ 6 1
salt solution (example 6, 30.009), water (63.179), isopropyl alcohol
(21.309), sodium persulfate (25% aqueous solution, 12.08g), acrylic
acid (23.34g), tert-butylhydroperoxide (70% aqueous solution,
0.589), and sodium hydroxide (50% aqueous solution, 8.479). For
this polymerization, the sodium hydroxide was charged simultaneously
with the acrylic acid and the sodium persulfate solution; the
addition period was shortened to 3 hours; the hold period was
lengthened to 3 hours; and the tert-butylhydroperoxide was added 2
hours after addition. After distillation, 95 ml of water was added.
Example 15 illustrates the preparation of a copolymer of
methacrylic acid with the product of Example 7.
Example 15:
Preparation of-methacrylic acid/2-propanol, 1-(diethylamino)-3-
(2-propenyloxy) copolymer
2-Propanol, l-(diethylamino)-3-(2-propenyloxy) (Example 7,
169, 0.083 mole) and 171 ml DI water were charged to a suitable
reactor and purged with nitrogen. Sodium persulfate (20.5% aqueous
solution, 209) and methacrylic acid (449, 0.5 mole) were
simultaneously added over a 4 hour period, maintaining a batch
temperature of 90+ 2 C. 50% aqueous sodium hydroxide (79 total)
was charged during the addition period as needed to maintain polymer
solubility. After addition, the batch was held at 90+ 2 C for 1.5
hours. After the hold period, 50% sodium hydroxide (299) was
charged, maintaining the batch temperature below 30 C.
Table II presents a summary of the physical properties of
the copolymers produced in accordance with Examples 8-15.
- 26 - 133~6~
TABLE II
Physical Properties of the Copolymers (g/h)
Charge
Co- Monomer Monomer Mole Ratio Brookfielda
polymer(g) (h) g:h Viscosity pH Mnb
Ex 8 Acrylic Ex 1 6:1 13.5 5.5 2,350
Acid
Ex 9 " Ex 1 6:1 15.6 5.5 3,200
Ex 10 " Ex 2 6:1 21.8 5.5 2,46g
Ex 11 " Ex 2 4:1 26.3 5.7 2,350
Ex 12 " Ex 3 5:1 21.5 4.9 4,450
Ex 13 " Ex 4 5:1 17.5 5.1 4,200
Ex 14 " Ex 6 6:1 13.4 5.2
Ex 15 Methacrylic Ex 7 6:1 38.4 9.65
Acid
a 25~ solutions @ 25C
b Number average molecular weight
Table III illustrates the excellent activity of the novel
copolymers for deposit control in aqueous systems containing high
levels of well water iron. The results are given as percent of
soluble iron remaining in solution after specified times. The
higher the percent soluble iron, the more effective the scale
control of the polymer.
-
- 27 _ 1 3381 61
TABLE III
Deposit Control Activity
Well Water Iron Results
Percent Soluble Iron
Conditions: 200 ppm Ca 2+ as CaC03; 100 ppm Mg2+ as CaC03;
8 ppm Fe+2; pH 8; 45 C; 0, 24, 48, 72 hour
equilibration
Treatment Treatment Conc
Copolymer(ppm active) 0 hour24 hour 48 hour 72 hour
Control 1.85 1.20 1.00 1.00
Example 810.00 8.80 1.60 1.00 3.70
20.00 96.20 96.30 81.60 96.20
40.00 97.40 95.50 83.70 97.50
Example 910.00 18.70 1.10 1.00 3.50
20.00 96.60 96.10 82.42 97.20
40.00 96.80 95.60 81.60 97.50
Example 1010.00 10.80 1.20 1.00 2.00
20.00 95.50 70.90 22.70 35.60
40.00 96.40 97.10 81.25 97.50
Example 1110.00 2.80 1.40 1.00 2.00
20.00 96.30 71.80 79.10 88.30
40.00 96.40 97.10 81.25 97.50
- 28 _ 1 3381 61
Table IV illustrates that the copolymers are effective in
inhibiting the formation of calcium phosphate, commonly encountered
in industrial water systems, such as cooling water systems.
TABLE IV
Calcium Phosphate Inhibition
Conditions: 600 ppm Ca2+ as CaC03, 12 ppm P04~3, 2 mM NaHC03,
pH 7.0, 70 C, 18 hour equilibration
% Inhibition
Treatment Treatment Concentrations (ppm active)
10Copolymer 5 10 20
Example 8 9.6 11.3 39.6
9 11.3 10.9 76.1
5.4 9.2 35.6
11 4.0 9.4 70.9
15 12 3.7 3.7 9.1
13 8.6 2.7 11.2
14 5.6 13.5 38.2
Table V demonstrates the excellent activity of the novel
copolymers in inhibiting the formation of calcium carbonate, another
commonly encountered scale-forming agent in various industrial water
systems.
1 338~ 61
- 29 -
TABLE V
Calcium Carbonate Inhibition
Conditions: 1105 ppm Ca2+ as CaC03, 1170 ppm C03-2 as CaC03
pH 9.0, 70 C, 18 hours equilibration, LSI = 3.67
% Inhibition
Treatment Treatment Concentrations (ppm active)
Copolymer 0.5 1.0 2.0
Example 8 0.0 23.4 37.5
q 5.8 2~ 9.4
10 10 0.0 18.0 34.6
11 0.0 10.5 33.6
12 6.3 31.8 44.6
13 8.3 31.0 43.0
14 8.5 35.9 50.2
Tables VI and VII show that the copolymers are less
effective in dispersing ferric oxide or montmorillonite clay.
1 33~1-bl
- 30 -
TABLE VI
Ferric Oxide Dispersion
Conditions: 300 ppm Fe203, 200 ppm Ca 2+ as CaC03, 1 mM NaCl,
10 mM NaHC03, pH 7.0, 45 C, 18 hours settling
% Transmittance
Treatment Treatment Concentrations (ppm active)
Copolymer 2.5 5.0 10.0
Example 8 1.5 2.5 4.3
9 3.0 4.5 5.0
n 10 ~,~ 1.5 '~
11 1.5 1.5 2.0
12 9.0 15.0 26.0
13 2.5 5.5 5.5
14 5.5 9.0 20.5
TABLE VII
Montmorillonite Dispersions
Conditions: 200 ppm Ca2+ as CaC03, pH 7.0, 1000 ppm
montmorillonite, 18 hours equilibration
% Transmittance
20 Treatment Treatment Concentrations (ppm active)
Copolymer 5 10 20
Example 8 0.0 0.0 0.0
9 0.0 0.0 0.0
0.0 0.0 0.0
25 11 0.0 0.0 0.0
_ 31 1 33 8 1 6 1
Table VIII demonstrates the scale control in a boiler
water system of a methacrylic acid copolymer (Example 15) in a
phosphate precipitation program. Details of typical boiler test
conditions can be found in U.S. Patent 4,659,481, col. 17.
TABLE VIII
Boiler Scale Reduction
Precipitating Phosphate Program
900 psig; 4 ppm Ca, 1 ppm Mg (as CaC03)
15 cycle
Deposit
Weight % Scale
Polymer Conc.(ppm) Density(g/ft2) Reduction
None - 8.15 --
Ex. 15 2.5 0.99 88
Ex. 15 5.0 0.22 97
% Scale reduction is calculated from the equation:
% Scale Reduction = DWD (control) - DWD (polymer)
DWD (control)
where DWD is Deposit Weight Density
control is the boiler test without polymer
13~8,6
It is to be understood that the above boiler studies in no
way limit the utility of the present invention for other boiler
treatment programs, such as polymer/phosphate/chelant, coordinated
phosphate, etc.
While this invention has been described with respect to
particular embodiments thereof, it is apparent that numerous other
forms and modifications of this invention will be obvious to those
skilled in the art. The appended claims and this invention
generally should be construed to cover all such obvious forms and
modifications which are within the true spirit and scope of the
present invention.